Abstract
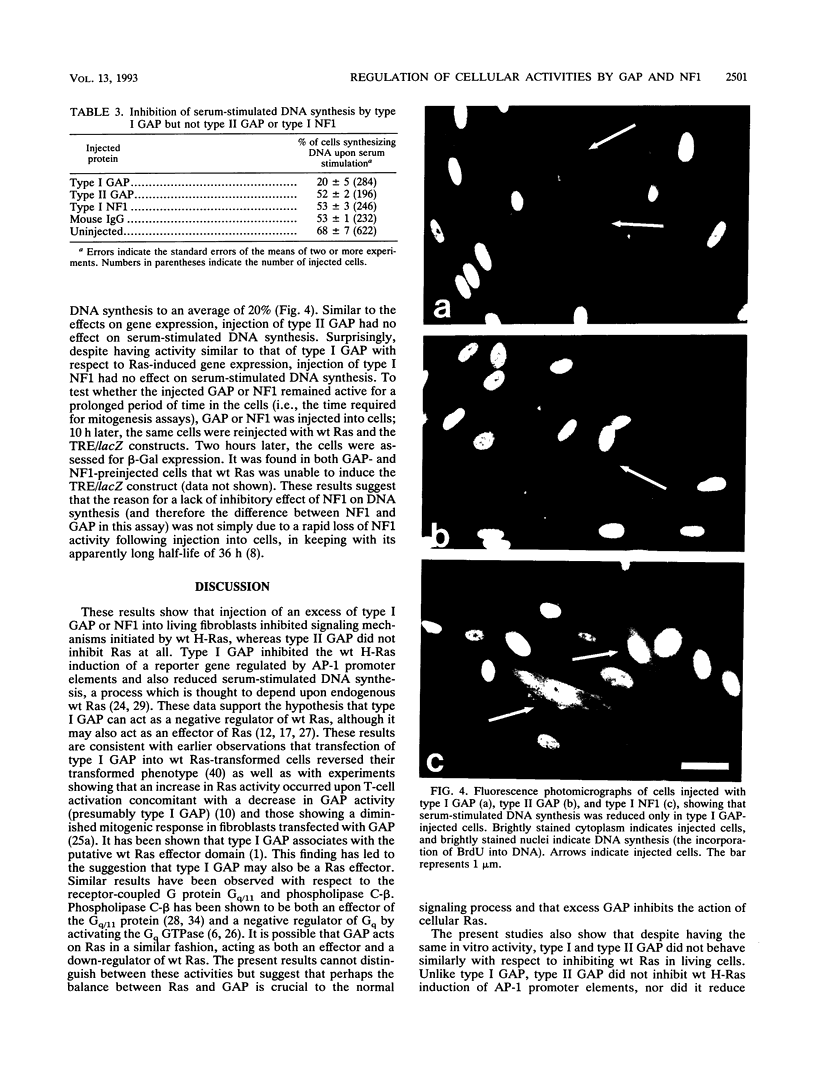
The regulation of the GTPase activity of the Ras proteins is thought to be a key element of signal transduction. Ras proteins have intrinsic GTPase activity and are active in signal transduction when bound to GTP but not following hydrolysis of GTP to GDP. Three cellular Ras GTPase-activating proteins (Ras-gaps) which increase the GTPase activity of wild-type (wt) Ras but not activated Ras in vitro have been identified: type I and type II GAP and type I NF1. Mutations of wt Ras resulting in lowered intrinsic GTPase activity or loss of response to cellular Ras-gap proteins are thought to be the primary reason for the transforming properties of the Ras proteins. In vitro assays show type I and type II GAP and the GAP-related domain of type I NF1 to have similar biochemical properties with respect to activation of the wt Ras GTPase, and it appears as though both type I GAP and NF1 can modulate the GTPase function of Ras in cells. Here we report the assembling of a full-length coding clone for type I NF1 and the biological effects of microinjection of Ras and Ras-gap proteins into fibroblasts. We have found that type I GAP, type II GAP, and type I NF1 show markedly different biological activities in vivo. Coinjection of type I GAP or type I NF1, but not type II GAP, with wt Ras abolished the ability of wt Ras to induce expression from an AP-1-controlled reporter gene. We also found that serum-stimulated DNA synthesis was reduced by prior injection of cells with type I GAP but not type II GAP or type I NF1. These results suggest that type I GAP, type II GAP, and type I NF1 may have different activities in vivo and support the hypothesis that while type I forms of GAP and NF1 may act as negative regulators of wt Ras, they may do so with differential efficiencies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Ballester R., Marchuk D., Boguski M., Saulino A., Letcher R., Wigler M., Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990 Nov 16;63(4):851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- Ballester R., Michaeli T., Ferguson K., Xu H. P., McCormick F., Wigler M. Genetic analysis of mammalian GAP expressed in yeast. Cell. 1989 Nov 17;59(4):681–686. doi: 10.1016/0092-8674(89)90014-7. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Basu T. N., Gutmann D. H., Fletcher J. A., Glover T. W., Collins F. S., Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992 Apr 23;356(6371):713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- Berstein G., Blank J. L., Jhon D. Y., Exton J. H., Rhee S. G., Ross E. M. Phospholipase C-beta 1 is a GTPase-activating protein for Gq/11, its physiologic regulator. Cell. 1992 Aug 7;70(3):411–418. doi: 10.1016/0092-8674(92)90165-9. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Cohen B. D., Lowy D. R. Identification and characterization of the neurofibromatosis type 1 protein product. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9914–9918. doi: 10.1073/pnas.88.22.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Papageorge A. G., Fletcher J. A., Diehl S. R., Ratner N., Vass W. C., Lowy D. R. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992 Apr 17;69(2):265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Evans T., Hart M. J., Cerione R. A. The Ras superfamilies: regulatory proteins and post-translational modifications. Curr Opin Cell Biol. 1991 Apr;3(2):185–191. doi: 10.1016/0955-0674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Cheng S. H., Rich D. P., Marshall J., Paul S., Hehir K., Ostedgaard L., Klinger K. W., Welsh M. J., Smith A. E. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990 Sep 27;347(6291):382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Gross M., Sweet R. W., Sathe G., Yokoyama S., Fasano O., Goldfarb M., Wigler M., Rosenberg M. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985 May;5(5):1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann D. H., Wood D. L., Collins F. S. Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9658–9662. doi: 10.1073/pnas.88.21.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halenbeck R., Crosier W. J., Clark R., McCormick F., Koths K. Purification, characterization, and western blot analysis of human GTPase-activating protein from native and recombinant sources. J Biol Chem. 1990 Dec 15;265(35):21922–21928. [PubMed] [Google Scholar]
- Haubruck H., McCormick F. Ras p21: effects and regulation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):215–229. doi: 10.1016/0304-419x(91)90015-d. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Pearlman J. A., Chamberlain J. S., Caskey C. T. Expression of recombinant dystrophin and its localization to the cell membrane. Nature. 1991 Jan 24;349(6307):334–336. doi: 10.1038/349334a0. [DOI] [PubMed] [Google Scholar]
- Lu K. H., Levine R. A., Campisi J. c-ras-Ha gene expression is regulated by insulin or insulinlike growth factor and by epidermal growth factor in murine fibroblasts. Mol Cell Biol. 1989 Aug;9(8):3411–3417. doi: 10.1128/mcb.9.8.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D. A., Saulino A. M., Tavakkol R., Swaroop M., Wallace M. R., Andersen L. B., Mitchell A. L., Gutmann D. H., Boguski M., Collins F. S. cDNA cloning of the type 1 neurofibromatosis gene: complete sequence of the NF1 gene product. Genomics. 1991 Dec;11(4):931–940. doi: 10.1016/0888-7543(91)90017-9. [DOI] [PubMed] [Google Scholar]
- Martin G. A., Viskochil D., Bollag G., McCabe P. C., Crosier W. J., Haubruck H., Conroy L., Clark R., O'Connell P., Cawthon R. M. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990 Nov 16;63(4):843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Alberts A. S., Feramisco J. R. Construction of mammalian cell lines with indicator genes driven by regulated promoters. Ciba Found Symp. 1990;150:47–56. doi: 10.1002/9780470513927.ch4. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Nishi T., Lee P. S., Oka K., Levin V. A., Tanase S., Morino Y., Saya H. Differential expression of two types of the neurofibromatosis type 1 (NF1) gene transcripts related to neuronal differentiation. Oncogene. 1991 Sep;6(9):1555–1559. [PubMed] [Google Scholar]
- Nori M., L'Allemain G., Weber M. J. Regulation of tetradecanoyl phorbol acetate-induced responses in NIH 3T3 cells by GAP, the GTPase-activating protein associated with p21c-ras. Mol Cell Biol. 1992 Mar;12(3):936–945. doi: 10.1128/mcb.12.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P., Rubinfeld B., McCormick F. Phosphorylation of rap1GAP in vivo and by cAMP-dependent kinase and the cell cycle p34cdc2 kinase in vitro. J Biol Chem. 1992 May 25;267(15):10780–10785. [PubMed] [Google Scholar]
- Schweighoffer F., Barlat I., Chevallier-Multon M. C., Tocque B. Implication of GAP in Ras-dependent transactivation of a polyoma enhancer sequence. Science. 1992 May 8;256(5058):825–827. doi: 10.1126/science.1317056. [DOI] [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Feig L. A., Gibbs J. B. Dominant inhibitory Ras mutants selectively inhibit the activity of either cellular or oncogenic Ras. Mol Cell Biol. 1991 Aug;11(8):4053–4064. doi: 10.1128/mcb.11.8.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Watson T., Kung H. F., Curran T. Microinjection of transforming ras protein induces c-fos expression. Mol Cell Biol. 1987 Jan;7(1):523–527. doi: 10.1128/mcb.7.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki H., Kayama T., Yoshimoto T., Shibahara S. Brain tumors predominantly express the neurofibromatosis type 1 gene transcripts containing the 63 base insert in the region coding for GTPase activating protein-related domain. Biochem Biophys Res Commun. 1991 Dec 31;181(3):955–961. doi: 10.1016/0006-291x(91)92029-j. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nakafuku M., Satoh T., Marshall M. S., Gibbs J. B., Matsumoto K., Kaziro Y., Toh-e A. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell. 1990 Mar 9;60(5):803–807. doi: 10.1016/0092-8674(90)90094-u. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Trahey M., Wong G., Halenbeck R., Rubinfeld B., Martin G. A., Ladner M., Long C. M., Crosier W. J., Watt K., Koths K. Molecular cloning of two types of GAP complementary DNA from human placenta. Science. 1988 Dec 23;242(4886):1697–1700. doi: 10.1126/science.3201259. [DOI] [PubMed] [Google Scholar]
- Wiesmüller L., Wittinghofer A. Expression of the GTPase activating domain of the neurofibromatosis type 1 (NF1) gene in Escherichia coli and role of the conserved lysine residue. J Biol Chem. 1992 May 25;267(15):10207–10210. [PubMed] [Google Scholar]
- Xu G. F., Lin B., Tanaka K., Dunn D., Wood D., Gesteland R., White R., Weiss R., Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990 Nov 16;63(4):835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- Xu G. F., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990 Aug 10;62(3):599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- Zhang K., DeClue J. E., Vass W. C., Papageorge A. G., McCormick F., Lowy D. R. Suppression of c-ras transformation by GTPase-activating protein. Nature. 1990 Aug 23;346(6286):754–756. doi: 10.1038/346754a0. [DOI] [PubMed] [Google Scholar]