Abstract
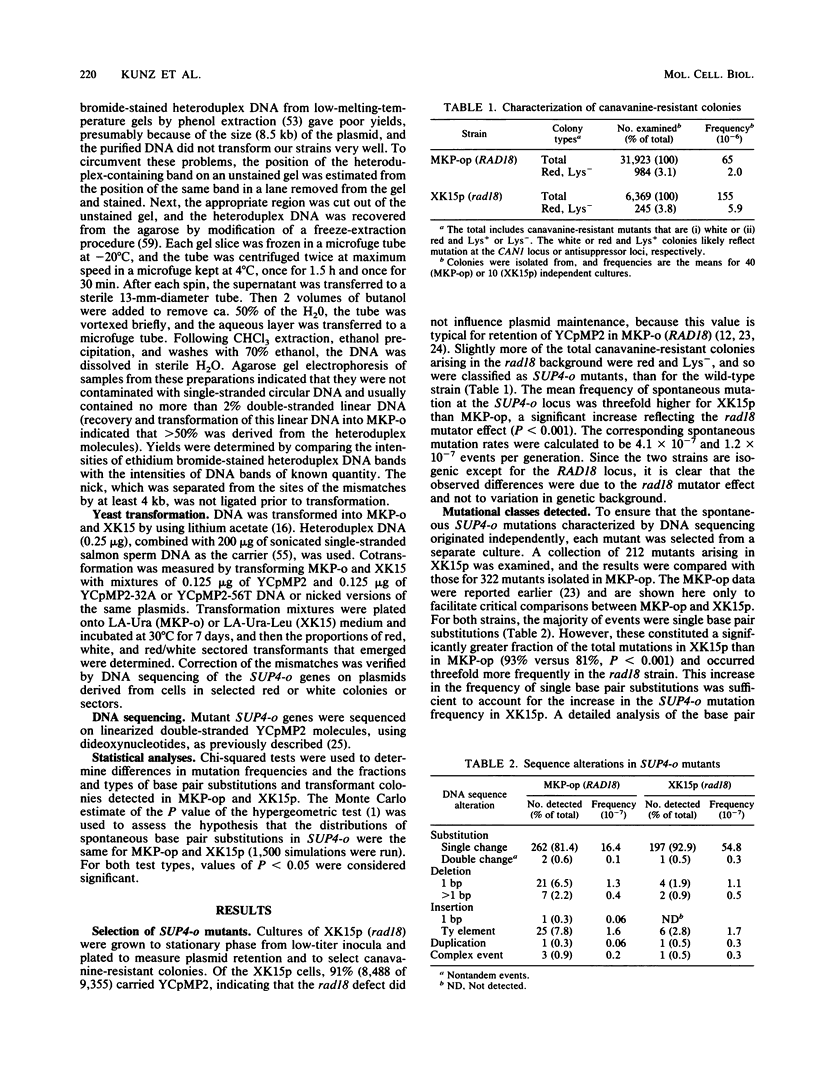
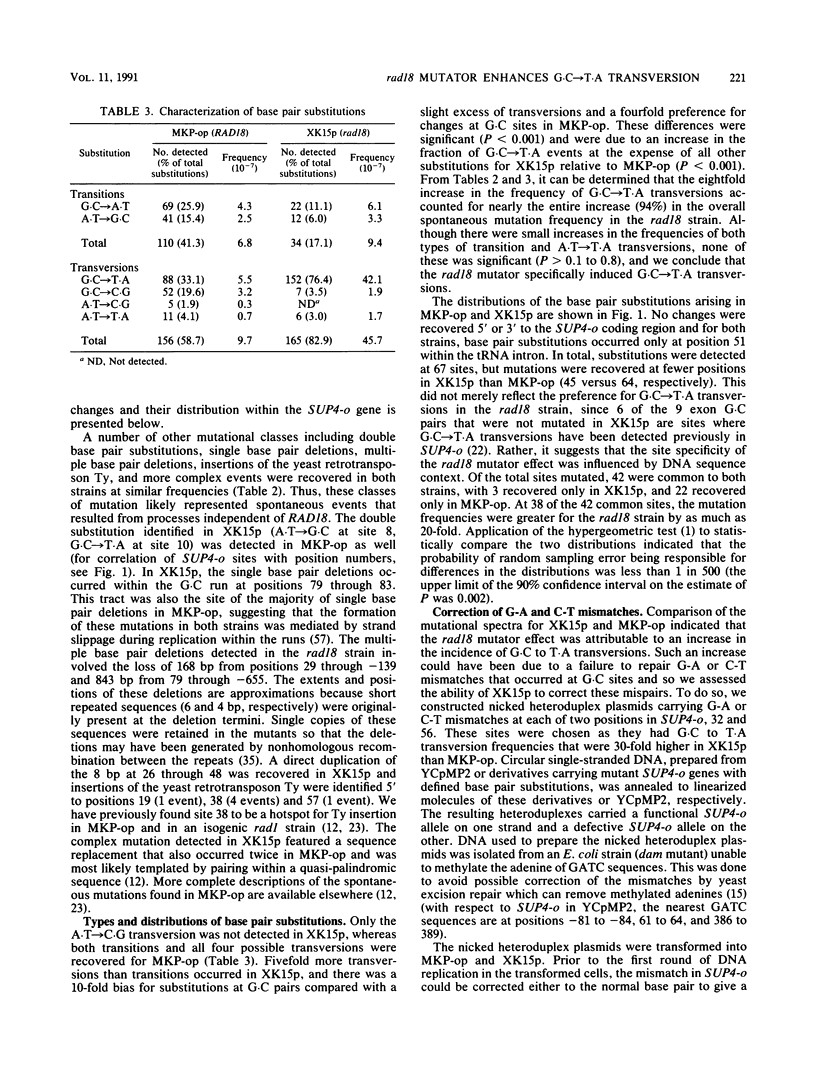
Inactivation of the Saccharomyces cerevisiae RAD18 gene confers a mutator phenotype. To determine the specificity of this effect, a collection of 212 spontaneous SUP4-o mutants arising in a rad18 strain was characterized by DNA sequencing. Comparison of the resulting mutational spectrum with that for an isogenic wild-type (RAD18) strain revealed that the rad18 mutator specifically enhanced the frequency of single base pair substitutions. Further analysis indicated that an increase in the frequency of G.C----T.A transversions accounted for the elevated SUP4-o mutation frequency. Thus, rad18 is the first eucaryotic mutator found to generate only a particular base pair substitution. The majority of G.C pairs that were not mutated in the rad18 background were at sites where G.C----T.A events can be detected in SUP4-o, suggesting that DNA sequence context influences the rad18 mutator effect. Transformation of heteroduplex plasmid DNAs into the two strains demonstrated that the rad18 mutator did not reduce the efficiency of correcting G-A or C-T mismatches to G.C pairs or preferentially correct the mismatches to A.T pairs. We propose that the RAD18 gene product might contribute to the fidelity of DNA replication in S. cerevisiae by involvement in a process that serves to limit the formation of G-A and C-T mismatches at template guanine and cytosine sites during DNA synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. T., Skopek T. R. Statistical test for the comparison of samples from mutational spectra. J Mol Biol. 1987 Apr 5;194(3):391–396. doi: 10.1016/0022-2836(87)90669-3. [DOI] [PubMed] [Google Scholar]
- Akiyama M., Maki H., Sekiguchi M., Horiuchi T. A specific role of MutT protein: to prevent dG.dA mispairing in DNA replication. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3949–3952. doi: 10.1073/pnas.86.11.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au K. G., Clark S., Miller J. H., Modrich P. Escherichia coli mutY gene encodes an adenine glycosylase active on G-A mispairs. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8877–8881. doi: 10.1073/pnas.86.22.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel M., Khan N. A., Haynes R. H. Common steps in the repair of alkylation and radiation damage in yeast. Mol Gen Genet. 1970;106(4):289–295. doi: 10.1007/BF00324046. [DOI] [PubMed] [Google Scholar]
- Chanet R., Magana-Schwencke N., Fabre F. Potential DNA-binding domains in the RAD18 gene product of Saccharomyces cerevisiae. Gene. 1988 Dec 30;74(2):543–547. doi: 10.1016/0378-1119(88)90187-4. [DOI] [PubMed] [Google Scholar]
- Chlebowicz E., Jachymczyk W. J. Endonuclease for apurinic sites in yeast. Comparison of the enzyme activity in the wild type and in rad mutants of Saccharomyces cerevisiae to MNS. Mol Gen Genet. 1977 Jul 20;154(2):221–223. doi: 10.1007/BF00330841. [DOI] [PubMed] [Google Scholar]
- Cooper A. J., Waters R. A complex pattern of sensitivity to simple monofunctional alkylating agents exists amongst the rad mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1987 Aug;209(1):142–148. doi: 10.1007/BF00329849. [DOI] [PubMed] [Google Scholar]
- Fabre F., Magana-Schwencke N., Chanet R. Isolation of the RAD18 gene of Saccharomyces cerevisiae and construction of rad18 deletion mutants. Mol Gen Genet. 1989 Feb;215(3):425–430. doi: 10.1007/BF00427039. [DOI] [PubMed] [Google Scholar]
- Game J. C., Little J. G., Haynes R. H. Yeast mutants sensitive to trimethoprim. Mutat Res. 1975 May;28(2):175–182. doi: 10.1016/0027-5107(75)90094-9. [DOI] [PubMed] [Google Scholar]
- Giroux C. N., Mis J. R., Pierce M. K., Kohalmi S. E., Kunz B. A. DNA sequence analysis of spontaneous mutations in the SUP4-o gene of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Feb;8(2):978–981. doi: 10.1128/mcb.8.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin G. N., Capecchi M. R., Adams J. M., Argetsinger J. E., Tooze J., Weber K., Watson J. D. Protein synthesis directed by DNA phage messengers. Cold Spring Harb Symp Quant Biol. 1966;31:157–171. [PubMed] [Google Scholar]
- Hoekstra M. F., Malone R. E. Excision repair functions in Saccharomyces cerevisiae recognize and repair methylation of adenine by the Escherichia coli dam gene. Mol Cell Biol. 1986 Oct;6(10):3555–3558. doi: 10.1128/mcb.6.10.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. S., Weber S., Prakash L. The Saccharomyces cerevisiae RAD18 gene encodes a protein that contains potential zinc finger domains for nucleic acid binding and a putative nucleotide binding sequence. Nucleic Acids Res. 1988 Jul 25;16(14B):7119–7131. doi: 10.1093/nar/16.14.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp G., Beckmann J. S., Johnson P. F., Fuhrman S. A., Abelson J. Transcription and processing of intervening sequences in yeast tRNA genes. Cell. 1978 Jun;14(2):221–236. doi: 10.1016/0092-8674(78)90109-5. [DOI] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Williamson M. S., Fogel S. Heteroduplex DNA correction in Saccharomyces cerevisiae is mismatch specific and requires functional PMS genes. Mol Cell Biol. 1989 Oct;9(10):4432–4440. doi: 10.1128/mcb.9.10.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Hamatake R. K., Motto-Fox J., Fitzgerald M. P., Sugino A. Fidelity of DNA polymerase I and the DNA polymerase I-DNA primase complex from Saccharomyces cerevisiae. Mol Cell Biol. 1989 Oct;9(10):4447–4458. doi: 10.1128/mcb.9.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Armstrong J. D., Glattke M., Kohalmi S. E., Mis J. R. The SUP4-o system for analysis of mutational specificity in yeast. Prog Clin Biol Res. 1990;340A:337–346. [PubMed] [Google Scholar]
- Kunz B. A., Kohalmi L., Kang X. L., Magnusson K. A. Specificity of the mutator effect caused by disruption of the RAD1 excision repair gene of Saccharomyces cerevisiae. J Bacteriol. 1990 Jun;172(6):3009–3014. doi: 10.1128/jb.172.6.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Peters M. G., Kohalmi S. E., Armstrong J. D., Glattke M., Badiani K. Disruption of the RAD52 gene alters the spectrum of spontaneous SUP4-o mutations in Saccharomyces cerevisiae. Genetics. 1989 Jul;122(3):535–542. doi: 10.1093/genetics/122.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Pierce M. K., Mis J. R., Giroux C. N. DNA sequence analysis of the mutational specificity of u.v. light in the SUP4-o gene of yeast. Mutagenesis. 1987 Nov;2(6):445–453. doi: 10.1093/mutage/2.6.445. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Hall B. D. Mutations at the Saccharomyces cerevisiae SUP4 tRNA(Tyr) locus: isolation, genetic fine-structure mapping, and correlation with physical structure. Mol Cell Biol. 1982 Dec;2(12):1501–1513. doi: 10.1128/mcb.2.12.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. B. Ultraviolet-induced reversion of cyc1 alleles in radiation-sensitive strains of yeast. III. rev3 mutant strains. Genetics. 1979 Jun;92(2):397–408. doi: 10.1093/genetics/92.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics. 1976 Feb;82(2):207–232. doi: 10.1093/genetics/82.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W. Mutagenesis in Saccharomyces cerevisiae. Adv Genet. 1982;21:173–254. doi: 10.1016/s0065-2660(08)60299-0. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Stewart J. W., Sherman F., Christensen R. Specificity and frequency of ultraviolet-induced reversion of an iso-1-cytochrome c ochre mutant in radiation-sensitive strains of yeast. J Mol Biol. 1974 May 5;85(1):137–162. doi: 10.1016/0022-2836(74)90134-x. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Preston B. D. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- Mayer V. W., Goin C. J. Semidominance of rad18-2 for several phenotypic characters in Saccharomyces cerevisiae. Genetics. 1984 Apr;106(4):577–589. doi: 10.1093/genetics/106.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee R. H., Lawrence C. W. Genetic analysis of gamma-ray mutagenesis in yeast. I. Reversion in radiation-sensitive strains. Genetics. 1979 Oct;93(2):361–373. doi: 10.1093/genetics/93.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mis J. R., Kunz B. A. Analysis of mutations induced in the SUP4-o gene of Saccharomyces cerevisiae by cis-diammine dichloroplatinum(II). Carcinogenesis. 1990 Apr;11(4):633–638. doi: 10.1093/carcin/11.4.633. [DOI] [PubMed] [Google Scholar]
- Moore C. W. Responses of radiation-sensitive mutants of Saccharomyces cerevisiae to lethal effects of bleomycin. Mutat Res. 1978 Aug;51(2):165–180. doi: 10.1016/s0027-5107(78)80016-5. [DOI] [PubMed] [Google Scholar]
- Morrison A., Christensen R. B., Alley J., Beck A. K., Bernstine E. G., Lemontt J. F., Lawrence C. W. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J Bacteriol. 1989 Oct;171(10):5659–5667. doi: 10.1128/jb.171.10.5659-5667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat M. R., Jachymczyk W. J., Hastings P. J., von Borstel R. C. Repair of gamma-ray induced DNA strand breaks in the radiation-sensitive mutant rad18-2 of Saccharomyces cerevisiae. Mol Gen Genet. 1983;189(2):256–262. doi: 10.1007/BF00337814. [DOI] [PubMed] [Google Scholar]
- Newlon C. S. Yeast chromosome replication and segregation. Microbiol Rev. 1988 Dec;52(4):568–601. doi: 10.1128/mr.52.4.568-601.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem Y., Cabrera M., Cupples C. G., Miller J. H. The mutY gene: a mutator locus in Escherichia coli that generates G.C----T.A transversions. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picologlou S., Brown N., Liebman S. W. Mutations in RAD6, a yeast gene encoding a ubiquitin-conjugating enzyme, stimulate retrotransposition. Mol Cell Biol. 1990 Mar;10(3):1017–1022. doi: 10.1128/mcb.10.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M. K., Giroux C. N., Kunz B. A. Development of a yeast system to assay mutational specificity. Mutat Res. 1987 Apr;182(2):65–74. doi: 10.1016/0165-1161(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Popoff S. C., Spira A. I., Johnson A. W., Demple B. Yeast structural gene (APN1) for the major apurinic endonuclease: homology to Escherichia coli endonuclease IV. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4193–4197. doi: 10.1073/pnas.87.11.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol Gen Genet. 1981;184(3):471–478. doi: 10.1007/BF00352525. [DOI] [PubMed] [Google Scholar]
- Prakash L. Effect of Genes Controlling Radiation Sensitivity on Chemically Induced Mutations in SACCHAROMYCES CEREVISIAE. Genetics. 1976 Jun;83(2):285–301. doi: 10.1093/genetics/83.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah S. K., von Borstel R. C., Hastings P. J. The origin of spontaneous mutation in Saccharomyces cerevisiae. Genetics. 1980 Dec;96(4):819–839. doi: 10.1093/genetics/96.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall S. K., Eritja R., Kaplan B. E., Petruska J., Goodman M. F. Nucleotide insertion kinetics opposite abasic lesions in DNA. J Biol Chem. 1987 May 15;262(14):6864–6870. [PubMed] [Google Scholar]
- Resnick M. A. Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics. 1969 Jul;62(3):519–531. doi: 10.1093/genetics/62.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J., Friedberg E. C. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: incision of ultraviolet-irradiated deoxyribonucleic acid in vivo. J Bacteriol. 1981 May;146(2):692–704. doi: 10.1128/jb.146.2.692-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki T., Machida I., Nakai S. Genetic control of diploid recovery after gamma-irradiation in the yeast Saccharomyces cerevisiae. Mutat Res. 1980 Dec;73(2):251–265. doi: 10.1016/0027-5107(80)90192-x. [DOI] [PubMed] [Google Scholar]
- Sargentini N. J., Smith K. C. Spontaneous mutagenesis: the roles of DNA repair, replication, and recombination. Mutat Res. 1985 Jul;154(1):1–27. doi: 10.1016/0165-1110(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Von Borstel R. C., Cain K. T., Steinberg C. M. Inheritance of spontaneous mutability in yeast. Genetics. 1971 Sep;69(1):17–27. doi: 10.1093/genetics/69.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S. S. AP endonucleases and DNA glycosylases that recognize oxidative DNA damage. Environ Mol Mutagen. 1988;12(4):431–477. doi: 10.1002/em.2860120411. [DOI] [PubMed] [Google Scholar]
- Wang S. S., Hopper A. K. Isolation of a yeast gene involved in species-specific pre-tRNA processing. Mol Cell Biol. 1988 Dec;8(12):5140–5149. doi: 10.1128/mcb.8.12.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Cox E. C., Horn V. The unusual mutagenic specificity of an E. Coli mutator gene. Proc Natl Acad Sci U S A. 1966 Feb;55(2):274–281. doi: 10.1073/pnas.55.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Caprio L., Cox B. S. DNA synthesis in UV-irradiated yeast. Mutat Res. 1981 Jun;82(1):69–85. doi: 10.1016/0027-5107(81)90139-1. [DOI] [PubMed] [Google Scholar]


