Abstract
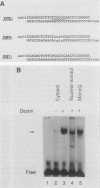
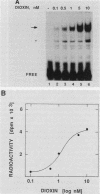
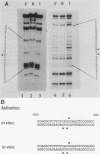
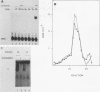
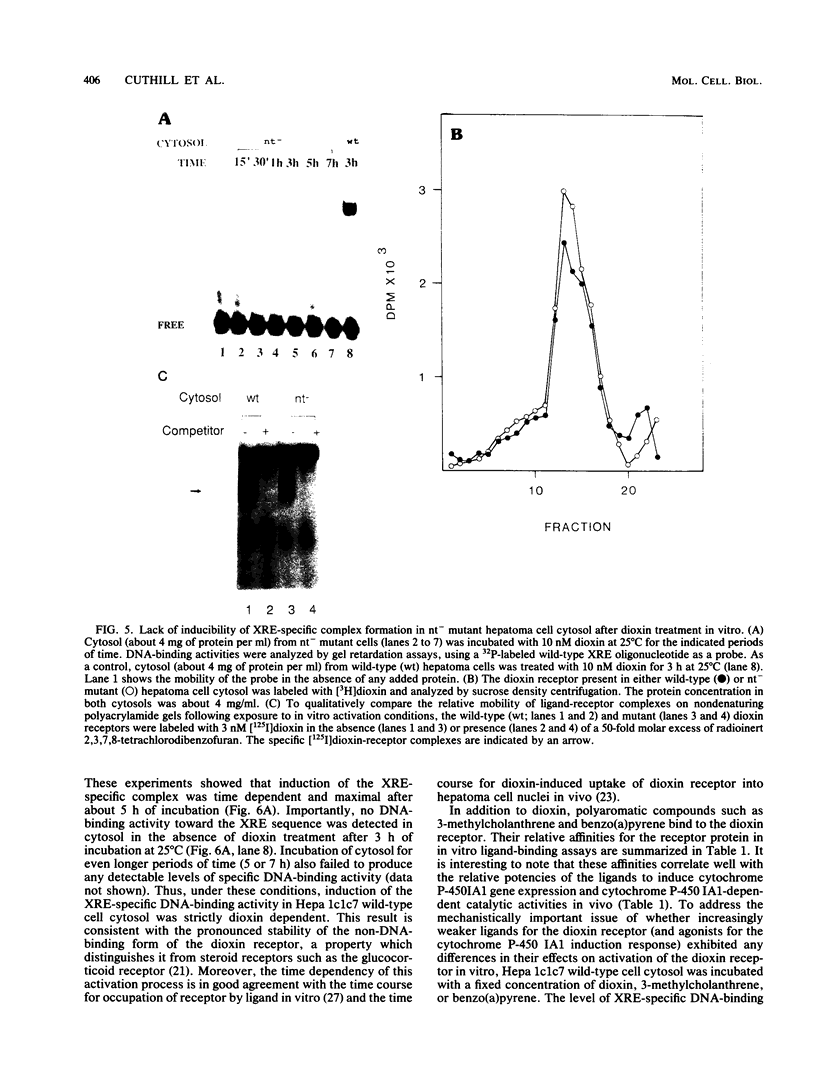
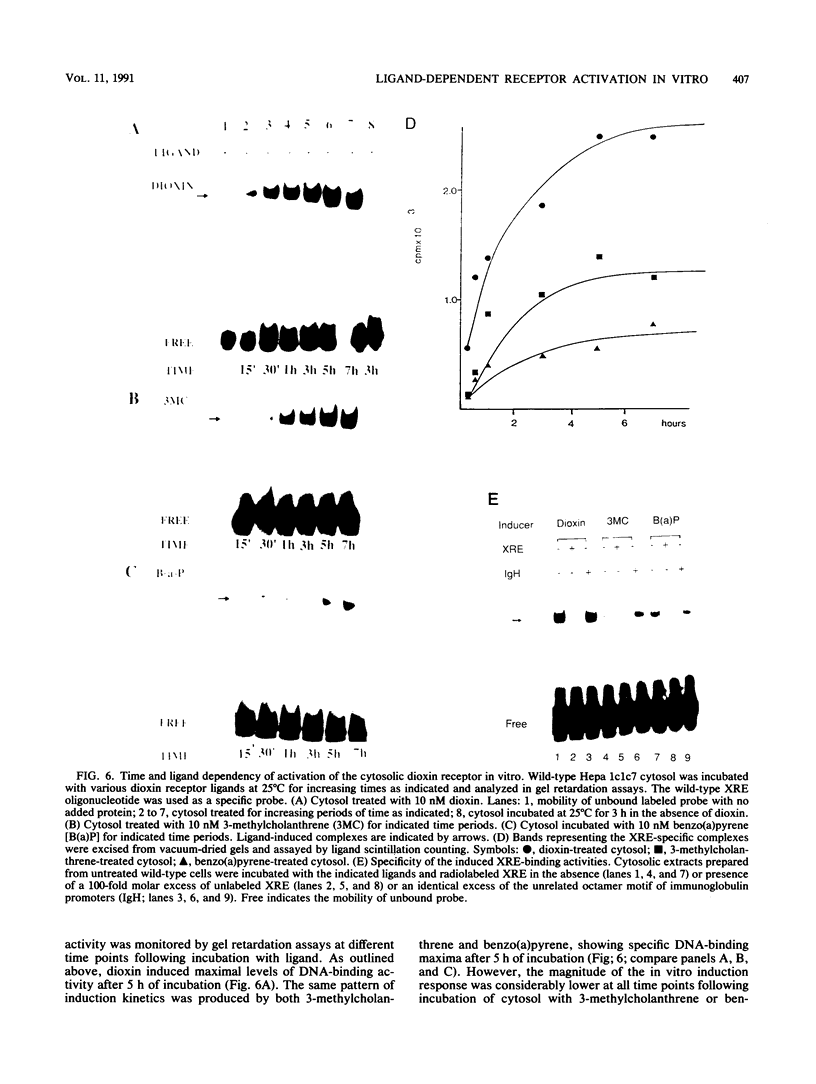
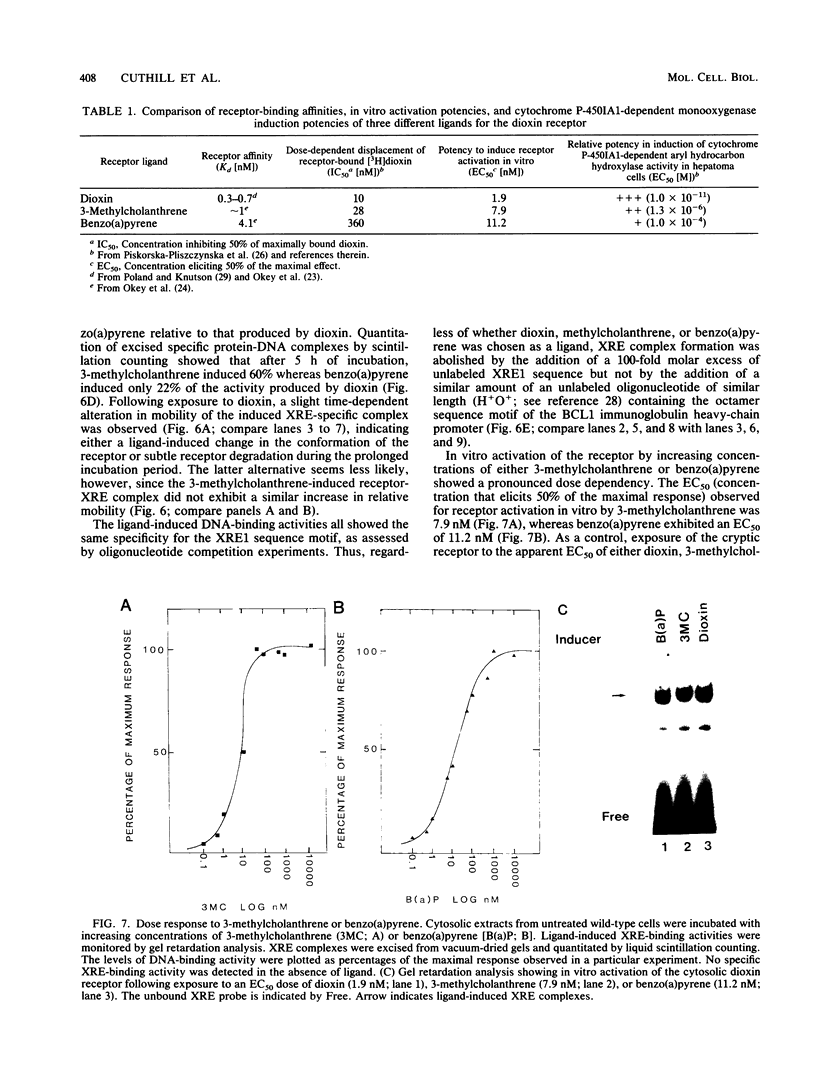
To reconstitute the molecular mechanisms underlying the cellular response to soluble receptor ligands, we have exploited a cell-free system that exhibits signal- (dioxin-)induced activation of the latent cytosolic dioxin receptor to an active DNA-binding species. The DNA-binding properties of the in vitro-activated form were qualitatively indistinguishable from those of in vivo-activated nuclear receptor extracted from dioxin-treated cells. In vitro activation of the receptor by dioxin was dose dependent and was mimicked by other dioxin receptor ligands in a manner that followed the rank order of their relative affinities for the receptor in vitro and their relative potencies to induce target gene transcription in vivo. Thus, in addition to triggering the initial release of inhibition of DNA binding and presumably allowing nuclear translocation, the ligand appears to play a crucial role in the direct control of the level of functional activity of a given ligand-receptor complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Gloss B., Schmid W., Strähle U., Schütz G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature. 1986 Dec 18;324(6098):686–688. doi: 10.1038/324686a0. [DOI] [PubMed] [Google Scholar]
- Bradfield C. A., Kende A. S., Poland A. Kinetic and equilibrium studies of Ah receptor-ligand binding: use of [125I]2-iodo-7,8-dibromodibenzo-p-dioxin. Mol Pharmacol. 1988 Aug;34(2):229–237. [PubMed] [Google Scholar]
- Cuthill S., Poellinger L. DNA binding properties of dioxin receptors in wild-type and mutant mouse hepatoma cells. Biochemistry. 1988 Apr 19;27(8):2978–2982. doi: 10.1021/bi00408a047. [DOI] [PubMed] [Google Scholar]
- Cuthill S., Poellinger L., Gustafsson J. A. The receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in the mouse hepatoma cell line Hepa 1c1c7. A comparison with the glucocorticoid receptor and the mouse and rat hepatic dioxin receptors. J Biol Chem. 1987 Mar 15;262(8):3477–3481. [PubMed] [Google Scholar]
- Denis M., Cuthill S., Wikström A. C., Poellinger L., Gustafsson J. A. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988 Sep 15;155(2):801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- Denis M., Poellinger L., Wikstöm A. C., Gustafsson J. A. Requirement of hormone for thermal conversion of the glucocorticoid receptor to a DNA-binding state. Nature. 1988 Jun 16;333(6174):686–688. doi: 10.1038/333686a0. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2528–2532. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5859–5863. doi: 10.1073/pnas.85.16.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O. Dominant and recessive aryl hydrocarbon hydroxylase-deficient mutants of mouse hepatoma line, Hepa-1, and assignment of recessive mutants to three complementation groups. Somatic Cell Genet. 1983 Jul;9(4):497–514. doi: 10.1007/BF01543050. [DOI] [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Denis M., Poellinger L., Gustafsson J. A. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Legraverend C., Hannah R. R., Eisen H. J., Owens I. S., Nebert D. W., Hankinson O. Regulatory gene product of the Ah locus. Characterization of receptor mutants among mouse hepatoma clones. J Biol Chem. 1982 Jun 10;257(11):6402–6407. [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Mason G. G., Wilhelmsson A., Cuthill S., Hapgood J., Gustafsson J. A., Poellinger L. Activation of the dioxin and glucocorticoid receptors to a DNA binding state under cell-free conditions. J Biol Chem. 1990 Feb 5;265(4):2269–2277. [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey A. B., Bondy G. P., Mason M. E., Nebert D. W., Forster-Gibson C. J., Muncan J., Dufresne M. J. Temperature-dependent cytosol-to-nucleus translocation of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in continuous cell culture lines. J Biol Chem. 1980 Dec 10;255(23):11415–11422. [PubMed] [Google Scholar]
- Okey A. B., Dubé A. W., Vella L. M. Binding of benzo(a)pyrene and dibenz(a,h)anthracene to the Ah receptor in mouse and rat hepatic cytosols. Cancer Res. 1984 Apr;44(4):1426–1432. [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J., Keys B., Safe S., Newman M. S. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol Lett. 1986 Nov;34(1):67–74. doi: 10.1016/0378-4274(86)90146-3. [DOI] [PubMed] [Google Scholar]
- Poellinger L., Lund J., Dahlberg E., Gustafsson J. A. A hydroxylapatite microassay for receptor binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3-methylcholanthrene in various target tissues. Anal Biochem. 1985 Feb 1;144(2):371–384. doi: 10.1016/0003-2697(85)90130-7. [DOI] [PubMed] [Google Scholar]
- Poellinger L., Roeder R. G. Octamer transcription factors 1 and 2 each bind to two different functional elements in the immunoglobulin heavy-chain promoter. Mol Cell Biol. 1989 Feb;9(2):747–756. doi: 10.1128/mcb.9.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Meshinchi S., Tienrungroj W., Schlesinger M. J., Toft D. O., Pratt W. B. Relationship of the 90-kDa murine heat shock protein to the untransformed and transformed states of the L cell glucocorticoid receptor. J Biol Chem. 1987 May 25;262(15):6986–6991. [PubMed] [Google Scholar]
- Schauer M., Chalepakis G., Willmann T., Beato M. Binding of hormone accelerates the kinetics of glucocorticoid and progesterone receptor binding to DNA. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1123–1127. doi: 10.1073/pnas.86.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E. S., Whitlock J. P., Jr The potential role of DNA methylation in the response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1989 Oct 25;264(30):17754–17758. [PubMed] [Google Scholar]
- Tukey R. H., Hannah R. R., Negishi M., Nebert D. W., Eisen H. J. The Ah locus: correlation of intranuclear appearance of inducer-receptor complex with induction of cytochrome P1-450 mRNA. Cell. 1982 Nov;31(1):275–284. doi: 10.1016/0092-8674(82)90427-5. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Cuthill S., Denis M., Wikström A. C., Gustafsson J. A., Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990 Jan;9(1):69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson A., Wikström A. C., Poellinger L. Polyanionic-binding properties of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. A comparison with the glucocorticoid receptor. J Biol Chem. 1986 Oct 15;261(29):13456–13463. [PubMed] [Google Scholar]
- Willmann T., Beato M. Steroid-free glucocorticoid receptor binds specifically to mouse mammary tumour virus DNA. Nature. 1986 Dec 18;324(6098):688–691. doi: 10.1038/324688a0. [DOI] [PubMed] [Google Scholar]