Abstract
Research examining medication effects on set shifting in teens with attention deficit/hyperactivity disorder (ADHD) is lacking. An animal model of ADHD may be useful for exploring this gap. The Spontaneously Hypertensive Rat (SHR) is a commonly used animal model of ADHD. SHR and two comparator strains, Wistar-Kyoto (WKY) and Wistar (WIS), were evaluated during adolescence in a strategy set shifting task under conditions of a 0-sec or 15-sec delay to reinforcer delivery. The task had three phases: initial discrimination, set shift and reversal learning. Under 0-sec delays, SHR performed as well as or better than WKY and WIS. Treatment with 0.3 mg/kg/day atomoxetine had little effect, other than to modestly increase trials to criterion during set shifting in all strains. Under 15-sec delays, SHR had longer lever press reaction times, longer latencies to criterion and more trial omissions than WKY during set shifting and reversal learning. These deficits were not reduced systematically by 1.5 mg/kg/day methylphenidate or 0.3 mg/kg/day atomoxetine. Regarding learning in SHR, methylphenidate improved initial discrimination, whereas atomoxetine improved set shifting but disrupted initial discrimination. During reversal learning, both drugs were ineffective in SHR, and atomoxetine made reaction time and trial omissions greater in WKY. Overall, WIS performance differed from SHR or WKY, depending on phase. Collectively, a genetic model of ADHD in adolescent rats revealed that neither methylphenidate nor atomoxetine mitigated all deficits in SHR during the set shifting task. Thus, methylphenidate or atomoxetine monotherapy may not mitigate all set shift task-related deficits in teens with ADHD.
Keywords: Atomoxetine, Attention Deficit/Hyperactivity Disorder, Methylphenidate, Spontaneously Hypertensive Rat, Strategy Set Shifting Task
1. Introduction
Clinical studies in children, teens, and adults indicate that attention deficit/hyperactivity disorder (ADHD) involves structural and functional abnormalities in frontostriatal and cerebellar circuits [1–3]. Individuals of all ages afflicted with ADHD exhibit executive function impairments that are related to abnormal activity of prefrontal cortex (PFC). Specifically, deficits in attention, inhibitory control, working memory and set shifting have been reported [4, 5]. Regarding set shifting, which is a measure of behavioral flexibility, the stimulant drug methylphenidate has been shown to improve performance in children [6–8], but see [9] and [10], but not in adults [5]. The non-stimulant drug atomoxetine has differential effects on set shifting performance as well, with children responding better than adults to treatment [8, 11–13]. Though research examining medication effects on set shifting performance in teens with ADHD is lacking, the above data suggest that ADHD medications may differ in effectiveness across developmental stages for improving set shifting. An adolescent animal model of ADHD may be useful for exploring the gap in the literature with respect to teens.
Since 1990, the inbred Spontaneously Hypertensive Rat (SHR) has been used as an animal model of ADHD [11–14]. Behaviorally, adult SHR display impulsivity [15, 16], inattention [17, 18], working memory impairment [19, 20] and set shifting deficits [19] relative to Wistar-Kyoto (WKY) rats, the inbred comparator strain. It is important to note, however, that these behavioral deficits are not observed consistently in adult SHR [21–23]. These conflicting reports may relate to several factors, including the specific task used, whether task requirements were easy or difficult, and whether the criterion accuracy levels were set low or high.
Only a handful of studies have investigated the ADHD phenotype of adolescent SHR as it pertains to performance on various PFC-related neurocognitive tasks. In a T-maze visual discrimination task, adolescent SHR required more trials than WKY or Wistar (WIS), a genetically-related outbred comparator strain, for forming an initial attentional set [24]. Performance in this task by the SHR was significantly improved after chronic treatment with 1.5 mg/kg oral (po) methylphenidate. In a more complex visual signal detection task, adolescent SHR were not different from WKY with respect to accuracy across increasing cue light durations when untreated [25]. After acute intraperitoneal (ip) treatment with 5.0 and 10 mg/kg methylphenidate, the SHR were significantly less impaired than WKY on several measures in the signal detection task. Impulsivity also was greater in adolescent SHR compared to WKY, and chronic treatment with 3.0 mg/kg (twice daily, ip) methylphenidate significantly reduced the expression of this trait in the SHR [26]. In a recent investigation of set shifting involving juvenile/periadolescent rats (P21–P35), untreated SHR performed more poorly than WKY across all phases of the task, and chronic treatment with 2.5 mg/kg methylphenidate (ip) significantly improved performance of the SHR [27]. To date, set shifting in SHR when treated and tested exclusively throughout adolescence (P28–P56) has not been evaluated. The adolescent age range is important to target because there are dramatic surges in the development of brain structure and function during this period, and therefore, the response to pharmacological agents may not be the same during adolescence as compared to juvenile and adult developmental stages [28]. Therefore, the present study examined performance of adolescent SHR, WKY and WIS strains using an automated strategy set shifting task similar in concept to the Wisconsin Card Sorting Test [29]. The effects of chronic methylphenidate and atomoxetine treatments on performance were evaluated.
During the set shift and reversal learning phases of the task, behavioral flexibility is required for reaching criterion levels of learning [29]. For set shifting, rats must shift attention from one stimulus dimension (e.g., visual cue) to another (e.g., egocentric spatial cue). Previous research indicates that the medial PFC, but not the orbital PFC, contributes to this form of behavioral flexibility [29–32]. For reversal learning, the stimulus dimension does not change, but rats must reverse an established response rule (e.g., respond on left rather than right lever). This simpler form of behavioral flexibility requires the active functioning of the orbital PFC, but not the medial PFC [30, 33, 34]. Given this dichotomy, it is possible that the strategy set shifting task can be used to assess the functioning of different PFC subregions in SHR relative to WKY and WIS comparator strains and to assess how stimulant and non-stimulant drugs may alter the functioning of different PFC subregions during adolescence.
2. Materials and Methods
2.1. Subjects
Male rats of the WKY/Cr, WIS/Cr, and SHR/Cr strains (Charles River Laboratories, Wilmington, MA) arrived on postnatal day 25 (P25). They were individually housed in clear plastic cages (24 cm × 22 cm × 20 cm) in a light (on at 08:00 and off at 20:00) and temperature (21–23 °C) controlled vivarium. Access to water was provided ad libitum between experimental sessions and rats were weighed daily Monday through Friday. Beginning on P28, food was restricted to approximately 10–16 grams per day in order to maintain body weight at no less than 85% of a growth adjusted ad libitum body weight specific for each strain. Food restriction was used to motivate lever responding during the strategy set shifting task that used food as the reinforcer. All experimental procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals as well as specific national laws.
2.2 Apparatus
The strategy set shifting task was conducted in operant chambers (model ENV-008CT, Med Associates, St. Albans, VT). Each chamber was outfitted with two retractable levers, a stimulus light above each lever, a food receptacle, a pellet dispenser and a house light. A sound-attenuating cubicle, with an exhaust fan for ventilation, enclosed each chamber. A computer that was programmed in Medstate Notation and connected to an interface (Med Associates) controlled the experimental events.
2.3. Drugs
From P28 until the end of the study at P56, groups from each strain received drug or vehicle treatment Monday through Friday to mimic the weekend “medication holiday” often recommended for individuals with ADHD [35]. (±)-Methylphenidate hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in tap water (1.5 mg/ml concentration) and injected into an oyster cracker to attain a dose of 1.5 mg/kg for oral consumption. Oyster crackers containing tap water (1.0 ml/kg) were used for vehicle control. The amount of time to consume daily oyster crackers averaged < 3 min. A 1.5 mg/kg po dose of methylphenidate ensured that therapeutically relevant plasma drug levels were achieved [36]. Atomoxetine hydrochloride (Tocris Biosciences, Ellisville, MO) was dissolved in 0.9% sterile saline (0.3 mg/ml concentration) and injected ip to attain a dose of 0.3 mg/kg. The vehicle control consisted of 0.9% sterile saline (1 ml/kg). A 0.3 mg/kg dose of atomoxetine was chosen to selectively augment extracellular norepinephrine concentrations in PFC; doses higher than 0.3 mg/kg also augment extracellular dopamine concentrations in PFC [37]. An ip route of administration was utilized due to poor bioavailability of oral atomoxetine in the rat [38]. On days that the strategy set shifting task was conducted, the different groups of rats from each strain were treated either with po methylphenidate or po vehicle 30 min before the start of sessions or with ip atomoxetine or ip vehicle 45 min before the start of sessions. These pretreatment times ensured that brain monoamine levels peaked before the start of behavioral sessions [36, 37].
2.4. Strategy Set Shifting Task Procedures
The operant version of the strategy set shifting task was used [29]. The task was divided into four phases: habituation, initial discrimination, set shift and reversal learning. The habituation phase was conducted between P31-P41 (Monday-Friday), and was used to train rats to lever press for food reward within 10 sec of lever insertion and to establish a lever position bias as previously described in detail [29]. Stimulus lights above the levers were not illuminated during the habituation phase.
During the three experimental phases of the strategy set shifting task, which took place between P42 and P56, each session was 2 hr in duration (Monday-Friday). Fixed session lengths were used to standardize the amount of time that performance was evaluated after drug or vehicle was administered and to reduce the influence of food satiation on motivation to respond. For the initial discrimination, each trial was initiated with a 20 sec timeout period in a darkened chamber. A randomly selected stimulus light was then illuminated, starting 3 sec before the house light was turned on and the levers inserted into the chamber. Within 10 sec of lever insertion, rats were required to press the lever that had the stimulus light illuminated above it, regardless of the lever position bias, to earn a food pellet (visual cue discrimination). Levers were retracted after a lever was pressed (correct or incorrect) or if 10 sec elapsed without a lever press being made (trial omission). Following a correct lever press and pellet delivery, the stimulus light remained illuminated for 4 sec. After an incorrect lever press or an omitted trial, the stimulus light and house light were extinguished immediately. The daily 2 hr sessions continued until the criterion was reached (eight consecutive correct lever choices [29]). The next day, rats underwent a shift to a discrimination that required the rat to press the lever that was opposite its lever position bias, regardless of which stimulus light was illuminated to earn a food pellet (egocentric spatial response discrimination). The daily 2 hr sessions continued until the criterion was reached (ten consecutive correct choices [29]). The following day, reversal learning was examined that required the rat to press the lever that was the same as its lever position bias, regardless of which stimulus light was illuminated to earn a food pellet (reversal of egocentric spatial response discrimination). The daily (Monday-Friday) 2 hr sessions continued until the criterion was reached (ten consecutive correct choices [29]). The same trial contingencies as outlined for the initial discrimination phase were in effect for the set shift and reversal learning phases.
These procedures were used under two conditions for reinforcer delivery: a condition for which a 0-sec delay was imposed between making a correct lever choice and food pellet delivery and a condition for which a 15-sec delay was imposed between making a correct lever choice and food pellet delivery. All other contingencies were identical, except that the house light terminated with the stimulus light after a correct lever press under the 0-sec delay and the house light remained illuminated until 4 sec following food pellet delivery under the 15-sec delay. The 0-sec delay was used in Experiment 1 that examined the effects of atomoxetine and its vehicle in WKY, WIS and SHR (n=8 per treatment and strain). Experiment 2 was conducted under the 15-sec delay condition for which the effects of methylphenidate (n=8–9 per strain) and its vehicle (n=8–10 per strain) and the effects of atomoxetine (n=5–8 per strain) and its vehicle (n=7 per strain) were examined in WKY, WIS and SHR. It was anticipated that the 15-sec delay version of the task would be more difficult, with SHR performing worse than comparator strains [39]. The 15-sec delay might also better reveal improvements in performance after treatment with methylphenidate or atomoxetine.
2.5. Data Analyses
The primary measures evaluated for each phase of the strategy set shifting task in Experiments 1 and 2 were: trials to criterion, ratio of correct to incorrect lever choices, number of omitted trials, latency to reach criterion, and average lever press reaction time. The first and last measures did not include the trials for which the lever press was omitted in order not to skew the data. Separate analyses for the number of correct and incorrect lever choices were not performed because each measure is linearly related to the number of trials conducted. The number of trials conducted depended on how many trials were needed by individual rats to reach criterion. Thus, these two measures do not reflect the accuracy of learning, as does the ratio of correct to incorrect lever choices. In experiment 2, a number of secondary measures also were evaluated that pertain to the subtypes of errors made. Errors during the set shift phase were examined in blocks of eight trials and consisted of perseverative, regressive and never-reinforced errors [29, 40, 41]. A perseverative error was recorded when a rat pressed the incorrect lever on six or more trials per block of eight trials. Once a rat made five or fewer incorrect choices in a block of eight trials, the incorrect lever choices were then scored as regressive errors. Never-reinforced errors were counted when a rat pressed the incorrect lever on trials for which the correct lever had the stimulus light illuminated above it. Errors during the reversal learning phase were examined in blocks of sixteen trials and consisted of perseverative and regressive errors [34, 42]. After the first block of 16 trials, if a rat made ten or more errors within the next block of sixteen trials, a perseverative error was recorded. Following a pattern of fewer than ten errors within a block of sixteen trials, errors were scored as regressive.
As there were no statistical differences between po vehicle and ip vehicle within each strain for any of the primary measures across the three phases in Experiment 2, the data from the vehicle conditions were combined to form a single vehicle control group for each strain. Each dependent measure in both experiments was analyzed by a two-factor (strain x treatment) ANOVA followed by post-hoc Tukey tests. Due to high variability in trials to criterion, latency to criterion and trial omissions in Experiment 2, these data were square root transformed prior to analysis.
3. Results
3.1. Experiment 1: Strategy Set Shifting with a 0-sec Delay
During the initial discrimination phase, strains differed in trials to criterion (F [2, 43] = 5.3, p ≤ 0.01), trial omissions (F [2, 43] = 3.4, p ≤ 0.04) and latency to reach criterion (F [2, 43] = 5.3, p ≤ 0.01), but not in accuracy ratio or reaction time (Table 1). Post-hoc tests for strain indicated that SHR (p ≤ 0.01) and WKY (p ≤ 0.05) required fewer trials to learn the visual cue discrimination than WIS. SHR and WKY also had shorter latencies to reach criterion than WIS (p ≤ 0.01 and 0.04, respectively) during the initial discrimination phase. In addition, SHR made fewer trial omissions than WIS (p ≤ 0.05) and did not differ from WKY for the trials to criterion, latency to reach criterion or trial omissions measures. During the set shift phase, significant main effects of strain (F [2, 43] = 4.3, (p ≤ 0.02) and treatment (F [2, 43] = 4.2, (p ≤ 0.05) were found for trials to criterion (Table 1). Also, a significant main effect of strain was found for latency to reach criterion (F [2, 43] = 3.7, (p ≤ 0.03). Post-hoc tests for strain indicated that fewer trials were required by SHR compared to WKY (p ≤ 0.03) and WIS (p ≤ 0.05). In addition, SHR had a shorter latency to reach criterion than WIS (p ≤ 0.05). Post-hoc tests for treatment indicated that, overall, more trials were required with atomoxetine treatment compared to vehicle treatment for learning the egocentric spatial response discrimination (p ≤ 0.05). No significant strain or treatment differences were found for accuracy ratio, reaction time or trial omissions during the set shift phase. Lastly, during the reversal learning phase, strains differed in trial omissions (F [2, 43] = 5.5, p ≤ 0.01), but not in trials to criterion, accuracy ratio, reaction time or latency to reach criterion (Table 1). Post-hoc tests for strain indicated that SHR and WKY made fewer trial omissions than WIS (p ≤ 0.01 and 0.03, respectively). SHR did not differ from WKY for trial omissions.
Table 1.
Strategy set shifting performance under the 0-sec delay condition after vehicle (VEH) and atomoxetine (ATO) treatment in adolescent WKY, WIS and SHR. Values are the mean ± SEM.
| Phase | Strain | Treatment | Trials to Criterion | Accuracy Ratio | Reaction Time (sec) | Trial Omissions | Latency to Criterion (min) |
|---|---|---|---|---|---|---|---|
| Initial Discrimination | WKY | VEH | 194 ± 57 | 1.5 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.6 | 86 ± 25 |
| ATO | 192 ± 63 | 1.4 ± 0.01 | 1.0 ± 0.1 | 2.3 ± 0.8 | 87 ± 28 | ||
| WIS | VEH | 268 ± 97 ** | 1.6 ± 0.3 | 1.2 ± 0.1 | 15 ± 10 * | 127 ± 47 ** | |
| ATO | 484 ± 120 ** | 1.4 ± 0.3 | 0.8 ± 0.2 | 9 ± 6 * | 214 ± 53 ** | ||
| SHR | VEH | 156 ± 38 | 1.8 ± 0.2 | 1.1 ± 0.1 | 0.3 ± 0.2 | 70 ± 17 | |
| ATO | 134 ± 37 | 1.5 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.4 | 60 ± 17 | ||
| Set Shift | WKY | VEH | 57 ± 7 | 3.1 ± 0.8 | 0.5 ± 0.2 | 0.4 ± 0.2 | 25 ± 3 |
| ATO | 70 ± 7 ^ | 2.0 ± 0.3 | 0.9 ± 0.1 | 1.0 ± 0.7 | 31 ± 3 | ||
| WIS | VEH | 59 ± 7 | 2.0 ± 0.3 | 1.2 ± 0.4 | 4.5 ± 2.2 | 29 ± 4 * | |
| ATO | 66 ± 8 ^ | 1.9 ± 0.3 | 0.7 ± 0.2 | 0.3 ± 0.3 | 29 ± 4 * | ||
| SHR | VEH | 36 ± 7 ** | 3.8 ± 1.0 | 0.9 ± 0.4 | 2.5 ± 2.2 | 17 ± 3 | |
| ATO | 53 ± 7 ^** | 2.2 ± 0.3 | 1.1 ± 0.1 | 0.1 ± 0.1 | 24 ± 3 | ||
| Reversal Learning | WKY | VEH | 74 ± 12 | 1.5 ± 0.1 | 0.7 ± 0.1 | 0.2 ± 0.1 | 33 ± 5 |
| ATO | 79 ± 14 | 2.4 ± 1.4 | 0.9 ± 0.1 | 0.1 ± 0.1 | 34 ± 6 | ||
| WIS | VEH | 81 ± 10 | 1.4 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.3 ** | 36 ± 4 | |
| ATO | 72 ± 14 | 1.5 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.7 ** | 32 ± 6 | ||
| SHR | VEH | 47 ± 6 | 2.1 ± 0.4 | 0.8 ± 0.1 | 0.1 ± 0.1 | 21 ± 3 | |
| ATO | 59 ± 9 | 1.7 ± 0.2 | 0.9 ± 0.1 | 0.0 ± 0.0 | 26 ± 4 |
p ≤ 0.05 compared to both other strains overall;
p ≤ 0.05 compared to the SHR strain overall.;
p ≤ 0.05 compared to vehicle treatment overall.
3.2. Experiment 2: Strategy Set Shifting with a 15-sec Delay
Across the three phases, adolescent rats of each strain found the 15-sec delay version of the strategy set shifting task more difficult to learn than the 0-sec delay version of the task (231 vs. 108 trials to criterion, on average), and omitted more trials with the 15-sec delay than the 0-sec delay (135 vs. 2 trial omissions, on average). Furthermore, all rats found the egocentric spatial response discrimination and its reversal easier to learn than the visual cue discrimination under the 0-sec (56 and 69 vs. 206 trials to criterion, on average) and 15-sec (100 and 125 vs. 428 trials to criterion, on average) delay conditions.
3.2.1. Initial Discrimination Phase
Data obtained during the initial discrimination phase are displayed in Figure 1. Analysis of trials to criterion showed a significant strain X treatment interaction (F [4, 82] = 5.4, p ≤ 0.001). Post-hoc tests between strains for each treatment revealed that in vehicle-treated rats, SHR and WIS required fewer trials to reach criterion than WKY (p ≤ 0.001 and 0.03, respectively). In methylphenidate-treated rats, SHR reached criterion in fewer trials than WKY (p ≤ 0.02) and WIS (p ≤ 0.01). In atomoxetine-treated rats, strains were not significantly different for this measure, though there was a trend for atomoxetine-treated SHR to require more trials than atomoxetine-treated WIS (p ≤ 0.08). Post-hoc tests between treatments for each strain revealed that atomoxetine-treated WKY required fewer trials than vehicle-treated WKY (p ≤ 0.01), atomoxetine-treated WIS required fewer trials than methylphenidate-treated WIS (p ≤ 0.03), and atomoxetine-treated SHR required more trials than methylphenidate-treated SHR (p ≤ 0.04). For the accuracy ratio, the strain X treatment interaction also was significant (F [4, 82] = 2.6, p ≤ 0.05). Post-hoc tests between strains for each treatment revealed that in methylphenidate-treated rats, SHR had a higher accuracy ratio than WKY (p ≤ 0.05) and WIS (p ≤ 0.03). In vehicle-treated and atomoxetine-treated rats, strains did not differ significantly for this measure. Post-hoc tests between treatments for each strain revealed that atomoxetine-treated SHR had a lower accuracy ratio than methylphenidate-treated SHR (p ≤ 0.05) and a trend for vehicle-treated SHR to have a lower accuracy ratio than methylphenidate-treated SHR (p ≤ 0.06). For the reaction time measure, there was a significant main effect of strain (F [2, 82] = 7.0, p ≤ 0.002). Post-hoc tests showed that, overall, SHR and WIS had longer reaction times than WKY (p ≤ 0.002 and 0.01, respectively). Analysis of trial omissions revealed a significant strain X treatment interaction (F [4, 82] = 2.9, p ≤ 0.03). Post-hoc tests between strains for each treatment showed no significant strain differences in vehicle-treated rats, but revealed fewer trial omissions in methylphenidate-treated SHR than methylphenidate-treated WIS (p ≤ 0.02) and more trial omissions in atomoxetine-treated SHR than atomoxetine-treated WKY (p ≤ 0.05). Within each strain, post-hoc tests showed that treatments were not significantly different, though trends were evident between atomoxetine and methylphenidate treatments. Specifically, in SHR there were fewer omissions with methylphenidate than atomoxetine treatment (p ≤ 0.08) and in WIS there were fewer omissions with atomoxetine than methylphenidate treatment (p ≤ 0.0 8). Lastly, analysis of latency to reach criterion revealed a significant strain X treatment interaction (F [4, 82] = 4.3, p ≤ 0.0 03). Post-hoc tests (Table 3) showed that vehicle-treated SHR required less time than vehicle-treated WKY (p ≤ 0.05), methylphenidate-treated SHR required less time than methylphenidate-treated WIS (p ≤ 0.01) or atomoxetine-treated SHR (p ≤ 0.04), and atomoxetine-treated WKY required less time than vehicle-treated WKY (p ≤ 0.03) to reach criterion.
Figure 1.
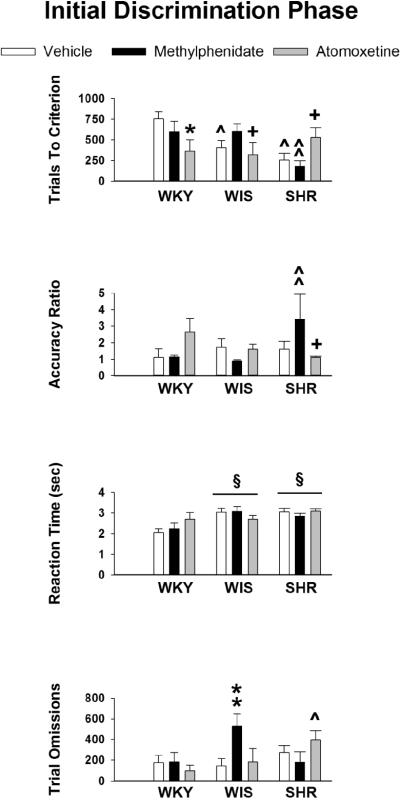
Mean ± SEM trials to criterion, accuracy ratio, reaction time (sec) and trial omissions during the initial discrimination phase in adolescent WKY, WIS and SHR treated with vehicle, methylphenidate or atomoxetine under conditions of a 15-sec delay for reinforcer delivery. § p ≤ 0.05 compared to WKY overall; ^ p ≤ 0.05 compared to identical treatment in WKY; ** p ≤ 0.05 compared to identical treatment in SHR; ^^ p ≤ 0.05 compared to identical treatment in both other strains; * p ≤ 0.05 compared to vehicle treatment in the same strain; + p ≤ 0.05 compared to methylphenidate treatment in the same strain.
Table 3.
Latency (min) to reach criterion during the strategy set shifting task under the 15-sec delay condition after vehicle (VEH), methylphenidate (MPH) and atomoxetine (ATO) treatment in adolescent WKY, WIS and SHR. Values are the mean ± SEM.
| Strain | Treatment | Initial Discrimination | Set Shift | Reversal Learning |
|---|---|---|---|---|
| WKY | VEH | 537 ±79 | 71 ± 17 | 94 ± 28 |
| MPH | 449 ± 99 | 62 ± 12 | 56 ± 6 | |
| ATO | 270 ± 170* | 75 ± 11 | 165 ± 45 | |
| WIS | VEH | 407 ± 64 | 114 ± 14 | 117 ± 23 |
| MPH | 645 ± 106 | 46 ± 12 * | 144 ± 29 | |
| ATO | 293 ± 158 | 99 ± 17 | 139 ± 36 | |
| SHR | VEH | 308 ± 74 ^ | 132 ± 16 ^ | 189 ± 26 # |
| MPH | 209 ± 91 ^^§ | 108 ± 25 ^^ | 110 ± 15 # | |
| ATO | 536 ± 117 | 50± 15 ** | 195 ± 57 # |
p < 0.05 compared to VEH in same strain
p < 0.05 compared to ATO treatment in same strain
p < 0.05 compared to both other treatments in same strain
p < 0.05 compared to same treatment in WKY
p < 0.05 compared to same treatment in WIS
p < 0.05 compared to WKY overall
3.2.2. Set Shift Phase
Data obtained during the set shift phase are displayed in Figure 2. Analysis of trials to criterion revealed no strain or treatment differences, indicating that the speed of learning the egocentric spatial response discrimination was the same in WKY, WIS and SHR, as well as after vehicle, methylphenidate and atomoxetine treatments. There was a trend for a strain X treatment interaction for the accuracy ratio (F [4, 82] = 2.4, p ≤ 0.06), and Tukey tests revealed that atomoxetine-treated SHR performed more accurately than vehicle-treated (p ≤ 0.03) and methylphenidate-treated (p ≤ 0.02) SHR. For reaction time, there was a significant main effect of strain (F [4, 82] = 13.4, p ≤ 0.001). Post-hoc tests showed that, overall, SHR had longer reaction times than WKY (p ≤ and WIS (p ≤ 0.003). Analysis of trial omissions revealed a significant strain X treatment interaction (F [4, 82] = 4.3, p ≤ 0.003). Post-hoc tests between strains for each treatment showed that in vehicle-treated rats, SHR and WIS had more trial omissions than WKY (p ≤ 0.001 and 0.01, respectively). In methylphenidate-treated rats, SHR had more trial omissions than WKY (p ≤ 0.01) and a trend for more trial omissions than WIS (p ≤ 0.06). In atomoxetine-treated rats, no strain differences in trial omissions were apparent. However, post-hoc tests between treatments for each strain revealed that there were fewer trial omissions in atomoxetine-treated SHR than vehicle-treated SHR (p ≤ 0.001) and methylphenidate-treated SHR (p ≤ 0.04). Lastly, analysis of latency to reach criterion revealed a significant strain X treatment interaction (F [4, 82] = 3.0, p ≤ 0.02). Post-hoc tests (Table 3) showed that vehicle-treated SHR required more time than vehicle-treated WKY (p ≤ 0.02), methylphenidate-treated SHR required more time than methylphenidate-treated WIS (p ≤ 0.05), atomoxetine-treated SHR required less time than vehicle-treated SHR (p ≤ 0.01) or methylphenidate-treated SHR (p ≤ 0.05), and methylphenidate-treated WIS required less time than vehicle-treated WIS (p ≤ 0.03) to reach criterion.
Figure 2.
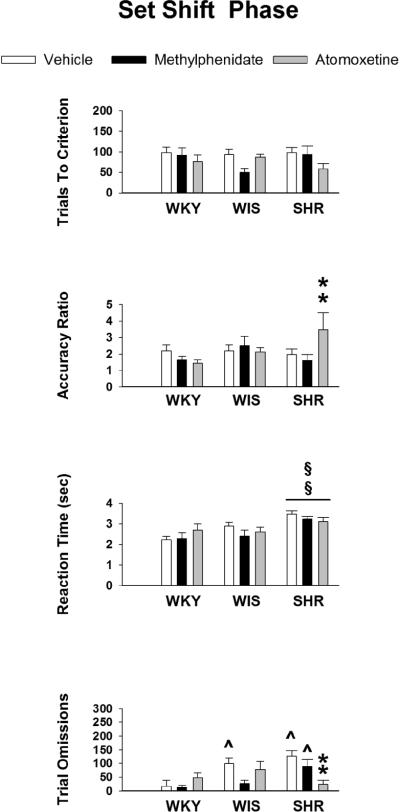
Mean ± SEM trials to criterion, accuracy ratio, reaction time (sec) and trial omissions during the set shift phase in adolescent WKY, WIS and SHR treated with vehicle, methylphenidate or atomoxetine under conditions of a 15-sec delay for reinforcer delivery. §§ p ≤ 0.05 compared to both other stains overall; ^ p ≤ 0.05 compared to identical treatment in WKY; ** p ≤ 0.05 compared to both other treatments in the same strain.
The analysis of error subtypes during the set shift phase revealed no significant strain or treatment differences in the number of perseverative, regressive or never-reinforced errors (Table 2). This suggests than the higher accuracy ratio (correct to incorrect lever choices) in atomoxetine-treated SHR during the set shift phase was not due to proportionately fewer errors being made, but to a proportionately greater number of correct lever choices being made. Overall, rats made more regressive and never-reinforced errors than perseverative errors during the set shift phase.
Table 2.
Subtypes of errors made during the strategy set shifting task under the 15-sec delay condition after vehicle (VEH), methylphenidate (MPH) and atomoxetine (ATO) treatment in adolescent WKY, WIS and SHR. Values are the mean ± SEM.
| Set Shift Phase | Reversal Learning Phase | |||||
|---|---|---|---|---|---|---|
| Strain | Treatment | Perseverative | Regressive | Never Reinforced | Perseverative | Regressive |
| WKY | VEH | 2.5 ± 0.6 | 19.6 ± 8.7 | 21.5 ± 4.8 | 40.9 ± 10.0 | 31.1 ± 14.5 |
| MPH | 2.5 ± 0.5 | 23.4 ± 2.4 | 24.6 ± 3.3 | 19.4 ± 4.3 | 28.6 ± 7.5 | |
| ATO | 2.5 ± 0.4 | 14.83 ± 2.6 | 18.5 ± 3.5 | 40.8 ± 13.7 | 24.5 ± 6.7 | |
| WIS | VEH | 2.6 ± 0.8 | 14.9 ± 3.0 | 18.9 ± 3.8 | 44.9 ± 12.8 | 32.3 ± 6.9 |
| MPH | 2.0 ± 0.4 | 12.4 ± 3.3 | 12.3 ± 2.3 | 48.1 ± 12.0 | 23.4 ± 8.8 | |
| ATO | 2.2 ± 1.0 | 14.8 ± 2.5 | 17.8 ± 1.7 | 53.0 ± 10.4 | 27.2 ± 5.1 | |
| SHR | VEH | 3.1 ± 0.6 | 19.8 ± 3.6 | 10.9 ± 4.2 | 43.2 ± 14.4 | 39.5 ± 6.4 |
| MPH | 2.2 ± 0.6 | 18.9 ± 3.9 | 22.6 ± 5.0 | 24.3 ± 8.1 | 33.6 ± 5.8 | |
| ATO | 2.3 ± 0.5 | 14.4 ± 3.1 | 15.9 ± 4.0 | 27.6 ± 7.8 | 35.5 ± 5.6 | |
3.2.3. Reversal Learning Phase
Data obtained during the reversal learning phase are displayed in Figure 3. Although analysis of trials to criterion revealed no strain or treatment differences, analysis of accuracy ratio revealed a significant main effect of strain (F [2, 82] = 7.1, p ≤ 0.001). Post-hoc tests showed that, overall, SHR had higher accuracy ratios than WKY (p ≤ 0.001) and WIS (p ≤ 0.003). For reaction time, there was a significant strain X treatment interaction (F [2,82] = 3.4, p ≤ 0.01). Post-hoc tests between strains for each treatment revealed that in vehicle-treated rats, SHR had longer reaction times than WKY (p ≤ 0.001) and WIS (p ≤ 0.02), and in methylphenidate-treated rats, SHR had longer reaction times than WKY (p ≤ 0.05). There were no strain differences in atomoxetine-treated rats. Post-hoc tests between treatments for each strain revealed that atomoxetine-treated WKY had longer reaction times than vehicle-treated and methylphenidate-treated WKY (p ≤ 0.003 and 0.01, respectively). In SHR and WIS, the effects of methylphenidate and atomoxetine on reaction time did not significantly differ from vehicle. Analysis of trial omissions revealed a significant main effect of strain (F [2, 82] = 3.9, p ≤ 0.02). Post-hoc tests showed that, overall, SHR had more trial omissions than WKY (p ≤ 0.02). Though the strain X treatment interaction was not significant (F [2, 82] = 1.9, p ≤ 0.11), Tukey tests revealed that atomoxetine-treated WKY had more trial omissions than vehicle-treated and methylphenidate-treated WKY (p ≤ 0.03 and 0.02, respectively). Furthermore, vehicle-treated SHR had more trial omissions than vehicle-treated WKY (p ≤ 0.001). Lastly, analysis of latency to reach criterion revealed a trend for strain differences (F [4, 82] = 2.8, p ≤ 0.06). Tukey tests revealed that, overall, SHR required more time than WKY (p ≤ 0.05) to reach criterion (Table 3).
Figure 3.
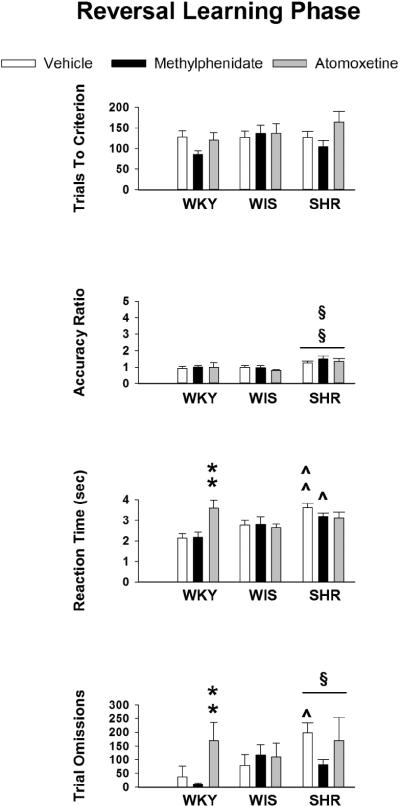
Mean ± SEM trials to criterion, accuracy ratio, reaction time (sec) and trial omissions during the reversal learning phase in adolescent WKY, WIS and SHR treated with vehicle, methylphenidate or atomoxetine under conditions of a 15-sec delay for reinforcer delivery. § p ≤ 0.05 compared to WKY overall; §§ p ≤ 0.05 compared to both other stains overall; ^ p ≤ 0.05 compared to identical treatment in WKY; ^^ p ≤ 0.05 compared to identi cle treatment in both other strains; ** p ≤ 0.05 compared to both other treatments in the same strain.
Analysis of error subtypes during the reversal learning phase revealed no significant strain or treatment differences in the number of perseverative or regressive errors (Table 2). This suggests than the higher accuracy ratio in the SHR during the reversal learning phase was likely due to a proportionately greater number of correct lever choices being made. Overall, rats made more perseverative errors than regressive errors during the reversal learning phase.
4. Discussion
4.1. Strain and Age Comparisons in the Strategy Set Shifting Task
In the current investigation, the order of discrimination difficulty as measured by trials to criterion (initial visual cue discrimination > reversal of the response discrimination > response discrimination) is similar to that reported in adult Sprague Dawley (SD) rats [43], but different from the order of discrimination difficulty (reversal of the response discrimination > response discrimination > initial visual cue discrimination) reported in adult Long Evans rats [29, 44] using the same operant version of the strategy set shifting task. Long Evans rats have been suggested to have a preexisting bias toward using a visual cue strategy [29]. These strain differences in the order of discrimination difficulty may reflect visual acuity disparity in pigmented (Long Evans) vs. albino (SHR, WKY, WIS and SD) rats, which can impact behavioral performance in vision-dependent tasks [45].
During the set shift and reversal learning phases, the number of trials to criterion under the 0-sec delay condition in adolescent rats (56 and 69 trials, respectively) was within the range reported for adult SD rats also tested under a 0-sec delay (approximately 65 and 80 trials, respectively; [43]). Under the 15-sec delay condition, the number of trials to criterion during these two phases in adolescent rats (100 and 125 trials, respectively) was within the range reported for the adult Long Evans rats that were tested under a 0-sec delay (approximately 85 and 110 trials, respectively; [29, 44]). The only robust age difference was during the initial visual cue discrimination for which the number of trials to criterion was approximately 2 to 3.5-fold greater in adolescent rats than adult Long Evans and SD rats under identical 0-sec delay conditions. In all phases, imposing a 15-sec delay between making a correct lever choice and reinforcer delivery was associated with higher numbers of trial omissions, particularly in the SHR. When the 0-sec delay was used in adolescent rats, very few omissions were observed, as in adult rats of other strains. Of relevance to age differences is a recent study in adult and adolescent Long Evans rats involving an attentional set shifting task (digging in a scented-medium cup for a hidden reward). Adolescents required more trials than adults to reach the learning criterion during the extradimensional shift and reversal learning phases in addition to the initial compound discrimination phase [46]. Thus, how age and strain impact set shifting task performance in rodent models may depend in part on the specific task design and task requirements.
In the present investigation, performance of the WIS strain in the strategy set shifting task warrants separate consideration. Under the 0-sec delay condition, the performance of WIS was poorer than WKY and SHR for several measures. Under the more difficult 15-sec delay condition, vehicle-treated WIS performed similarly to vehicle-treated SHR during the initial discrimination and set shift phases, and performed similarly to vehicle-treated WKY during the reversal learning phase. Past research in adult rats has shown that WIS performed similar to WKY and different from SHR when reinforcement rates were low in a task used to measure operant hyperactivity [47]. In a test of non-habituated open-field behavior, however, WIS performed similar to SHR and different from WKY [48]. Other studies report no differences between WIS and SHR, or better performance in SHR than WIS, in an attentional set shifting task that involved digging in a scented-medium cup for a hidden reward [22]. Lastly, in adolescent rats, WIS performance in a T-maze visual discrimination task was at a level between that of SHR and WKY [24]. Collectively, these findings suggest that WKY may be a more consistent and appropriate comparator strain than WIS for evaluating strategy set shifting task performance in SHR and for exploring the impact of ADHD medications.
4.2. Strain Differences in the Strategy Set Shifting Task under Vehicle Treatment
Under the 0-sec delay condition, no behavioral deficits were detected across the three discrimination phases of the strategy set shifting task in vehicle-treated SHR. In fact, SHR performed as well as or better than (e.g., during the set shift phase) the vehicle-treated WKY and WIS. These findings are consistent with studies showing that operant performance in SHR is similar to or better than WKY when reinforcers are immediate or frequent [39, 49]. Our findings also are in line with a laboratory study in children with ADHD showing that inattention is not apparent if reinforcers are given frequently [50]. When reinforcer delivery is delayed, operant performance deteriorates more rapidly in SHR than WKY [39,49] and attentional impairment is more obvious in children with ADHD [50].
Under the 15-sec delay condition in the present investigation, strain differences emerged. The deficits observed in vehicle-treated SHR were not of the type related to speed or accuracy of learning, but were related to reaction time, trial omissions and latency to reach criterion. In the vehicle-treated SHR, reaction time was longer during all three discrimination phases, and trial omissions and latency to reach criterion were greater during the set shift and reversal learning phases compared to vehicle-treated WKY. Thus, deficits were more extensive when SHR were required to behave flexibly under the 15-sec delay condition. During the initial (visual cue) discrimination phase with the 15-sec delay, vehicle-treated SHR reached criterion in fewer trials and with a shorter latency than vehicle-treated WKY. It is unlikely that SHR have a preexisting bias toward using a visual cue strategy compared to WKY, as SHR have poorer visual acuity than WKY [51] and do not perform better than WKY during acquisition of an initial attentional set in a T-maze visual discrimination task [24]. Rather, these findings may reflect a tendency for SHR, under discrete trial/delayed reinforcement conditions, to more readily select a lever associated with reinforcement than WKY during a phase of testing that does not require a complex form of behavioral flexibility [29]. This idea is supported by findings showing that SHR are less likely than WKY to extinguish previously reinforced lever responses [52].
Under the 0-sec delay condition, reaction time, trial omissions and latency to reach criterion in vehicle-treated SHR were not different from vehicle-treated WKY across the discrimination phases, suggesting that the deficits in these measures during the set shift and reversal learning phases under the 15-sec delay condition do not reflect a general motor impairment in SHR. Moreover, discrete trials were used during testing, allowing only a single lever press. Thus, an opportunity to display operant hyperactivity (high frequency responding) or impulsivity (responses with short inter-response times) was eliminated by this design under both delay conditions. It is more likely that longer reaction times, greater numbers of trial omissions, and longer latencies to reach criterion during set shift and reversal learning phases under the more difficult 15-sec delay condition are indicative of attentional or executive function weaknesses in SHR, when required to behave flexibly. In tests of sustained attention, SHR have robust and substantial deficits compared to WKY under more difficult testing conditions [17, 53]. Furthermore, poorer sustained attention under more difficult testing conditions was accompanied by longer reactions times and greater numbers of trial omissions in Long Evans rats [54]. Compared to controls, children with ADHD were shown to exhibit longer reaction time or increased reaction time variability across cognitive tasks measuring a range of attentional and executive function domains [55]. Longer reaction times in children with ADHD are suggested to represent attentional lapses [56] or dysfunctional reward-related decision-making [57], but not motivational deficits [58]. During the Wisconsin Card Sorting Test, teens with ADHD complete fewer categories than controls, consistent with a profile of attentional-executive impairment [4], [59]. Sagvolden [60] argues that the core symptoms of ADHD, including inattention, hyperactivity and impulsivity, may be related to a shorter delay-of-reinforcement gradient, as observed in SHR and individuals with ADHD [49, 61]. A reward has a smaller reinforcing effect as the delay between a response and reward delivery increases, and such dynamics may contribute to the emergence of attentional and executive function impairments associated with ADHD.
4.3. Medication Effects in the Strategy Set Shifting Task
4.3.1. Atomoxetine
Under the 0-sec delay condition, atomoxetine had little effect except to increase the number of trials needed to reach criterion in all strains during the set shift phase, though the difference from vehicle treatment was modest (63 vs. 51 trials, on average). Effects of atomoxetine were more striking under the 15-sec delay condition. Relative to vehicle or methylphenidate, treatment with atomoxetine increased the speed of learning in WKY and WIS, but slowed the speed of learning in SHR during the initial discrimination phase. During this initial phase of testing, atomoxetine also caused SHR to omit more trials and did not reverse the reaction time deficits in SHR. During the set shift phase, atomoxetine had no influence on the speed of learning in any strain. However, in SHR, atomoxetine improved the accuracy of learning, reduced the number of trial omissions and decreased the latency to reach criterion compared to vehicle and methylphenidate treatments. Atomoxetine had no effects on any measure in WKY and WIS during this second phase of testing. During the reversal learning phase, atomoxetine was ineffective in SHR, as it did not reverse the longer reaction time, the longer latency to reach criterion and the greater number of trial omissions observed in vehicle-treated SHR. The fact that not every measure that was impaired in vehicle-treated SHR was improved after atomoxetine treatment should not be construed as a weakness in the animal model. Even in children and adults with ADHD, not every executive function deficit is improved by atomoxetine treatment [12, 62]. Also, during the reversal learning phase, atomoxetine lengthened reaction time and increased the number of trial omissions in WKY compared to vehicle or methylphenidate treatment. These effects in WKY are in line with the lengthened reaction time and increased delay aversion observed in Long Evans rats chronically treated with atomoxetine during adolescence and given an acute atomoxetine challenge [63].
As previous research indicates that the medial PFC, but not the orbital PFC, is required for set shifting [29–32], our findings suggest that atomoxetine has greater capacity for improving the functioning of the medial than orbital PFC in SHR. In an analysis of PFC subregions of adolescent rats, however, mRNA for the norepinephrine transporter and several markers of synaptic plasticity (e.g., BDNF mRNA) have been shown to change in orbital, but not medial PFC, after chronic atomoxetine treatment [63]. Furthermore, imaging studies in adult rats showed that changes in the BOLD response within the PFC were restricted to the orbital subregion after acute treatment with atomoxetine [64]. An important distinction regarding these studies is that the rats (SD or Long Evans) were not strains exhibiting an ADHD phenotype. As indicated by Heal and colleagues [65, 66], no in vivo or in vitro analyses of medial vs. orbital PFC have yet been reported in SHR after acute or chronic atomoxetine treatment. Biochemical or imaging studies are needed to confirm if there is a greater influence of atomoxetine in medial vs. orbital PFC of adolescent SHR and if this profile differs from that in adolescent WKY.
4.4.2. Methylphenidate
The beneficial effects of methylphenidate on learning in SHR were restricted to the initial discrimination phase when methylphenidate improved the speed and accuracy of learning relative to WKY and WIS under the 15-sec delay. This result is consistent with our earlier findings showing methylphenidate treatment in adolescent SHR improved acquisition of an initial attentional set in a T-maze visual discrimination task [24]. Acquiring an attentional set is thought to depend on catecholamine projections to the PFC [67]. Methylphenidate may improve learning during the initial discrimination phase in SHR by blocking norepinephrine and dopamine reuptake into nerve terminals in PFC. Relative to comparator strains, untreated SHR have smaller basal release of norepinephrine [66] and smaller electrically-stimulated release of dopamine [68] in PFC. Thus, methylphenidate treatment may normalize these neurochemical deficits to improve learning [69]. The learning improvement in SHR, but not WKY or WIS, during the initial discrimination phase in response to chronic methylphenidate treatment may be related to the opposing strain differences we reported for maximal velocity (Vmax) of dopamine uptake by the dopamine transporter in whole PFC [24]. Vmax was significantly decreased in SHR and significantly increased in WKY and WIS after chronic methylphenidate treatment. Notably, the improvement in initial discrimination learning in SHR was observed without methylphenidate treatment significantly impacting the deficit in reaction time, suggesting some attentional or executive function impairment remains after treatment. During the set shift and reversal learning phases in SHR, methylphenidate did not prevent the deficits in reaction time, latency to reach criterion, and trial omissions. Nor did methylphenidate enhance the speed or accuracy of learning in SHR. In children with ADHD, an effective response to methylphenidate in the domains of attention and executive function can be variable, and in some cases, not demonstrated [70].
One possible explanation for the differential effects of chronic methylphenidate on learning during initial vs. latter phases is the development of drug tolerance later in the testing sequence. In support of this view, methylphenidate challenge after chronic methylphenidate treatment has been shown to induce tolerance to drug-induced increases in the firing rates of PFC neurons in rats [71]. However, tolerance may not be an issue, as acute methylphenidate does not consistently improve performance during set shifting assessments in children with ADHD [9]. Recently, it has been suggested that set shifting in rats displays a narrow sensitivity to the beneficial actions of acute methylphenidate, with an intermediate dose (2.0 mg/kg ip) improving performance to a greater extent than a low (0.5 mg/kg ip) or high (4.0 mg/kg ip) dose [72]. Thus, another possible explanation for the differential effects of chronic methylphenidate during initial vs. latter phases of the strategy set shifting task in SHR relates to the dose of methylphenidate employed. Although 1.5 mg/kg po methylphenidate is a clinically relevant dose [36], it may be too low for improving behavioral flexibility in SHR, while it is a sufficient dose for improving working memory and initial discrimination learning in SHR [19, 24, present findings].
In imaging studies in adult SD rats, acute methylphenidate treatment was shown to increase the BOLD response in orbital PFC, but to decrease it in medial PFC [73]. This result is interesting because the profile for the acute effects of methylphenidate on neural activity within the PFC is different from the acute effects of atomoxetine [64]. These differences in neural activation may explain the contrasting effects of methylphenidate vs. atomoxetine during the strategy set shifting task, at least in the adolescent WKY that does not exhibit an ADHD phenotype. Other studies measuring BDNF mRNA in whole PFC of adolescent SHR vs. WKY showed chronic atomoxetine increased and chronic methylphenidate decreased expression, suggesting these two ADHD medications would have different effects on neuroplasticity and PFC-related neurocognitive functions in SHR [74]. As with atomoxetine, neurochemical and imaging analyses of medial vs. orbital PFC after chronic methylphenidate in SHR are lacking. Such studies are needed to explain more fully the differences between these two ADHD medications in adolescent SHR during different phases of the strategy set shifting task.
5. Conclusions
Using a genetic model of ADHD in adolescent rats, neither methylphenidate nor atomoxetine mitigated all deficits in SHR during the strategy set shifting task with the 15-sec delay. Longer reaction times, longer latencies to reach criterion and greater numbers of trial omissions were still evident after drug treatments. In addition, the profile for learning improvements in SHR after methylphenidate (during the initial discrimination phase) vs. atomoxetine (during the set shift phase) was different. Moreover, the influence of drug treatment in SHR was in opposite directions during the initial discrimination phase (improvement in learning after methylphenidate and disruption in learning after atomoxetine). These findings suggest that teens with ADHD may not receive complete symptomatic relief of attentional and executive function impairment with either methylphenidate or atomoxetine monotherapy. Instead, these findings suggest that combined treatment with atomoxetine and methylphenidate may provide additional advantages, a possibility that has been suggested by open label clinical trials [75] and case studies [76, 77]. Moreover, whether the alternative stimulant drug amphetamine produces the same profile as methylphenidate during the strategy set shifting task in adolescent SHR is unknown. Although both amphetamine and methylphenidate increase extracellular concentrations of dopamine and norepinephrine, the underlying mechanism for this effect is different, with amphetamine reversing plasma membrane monoamine transporters and methylphenidate blocking the transporters [78–80]. Consequently, the effects of chronic treatment with amphetamine and methylphenidate on strategy set shifting in adolescent SHR might be the same or potentially different. Along these lines, previous research in peri-adolescent rhesus monkeys showed that chronic treatment with either methylphenidate or amphetamine failed to improve performance during the extradimensional shift phase of an attentional set shifting task [81]. One caveat of this study is that the animals were healthy normal monkeys not intended to model the ADHD population. Thus, studies evaluating amphetamine monotherapy as well as combination treatments warrant investigation in the adolescent SHR model of ADHD. This preclinical work may help inform treatment decisions for teens with ADHD. Our current and past research suggests that methylphenidate monotherapy may not be the best choice. Although adolescent treatment with methylphenidate improved some aspects of the strategy set shifting task in SHR, vulnerability to cocaine addiction was further increased during adulthood in SHR, but not in WKY or WIS, after methylphenidate treatment was discontinued [24].
Highlights
Unmedicated SHR took longer to reach criterion during set shifting and reversal learning
Methylphenidate increased learning speed in SHR during the initial discrimination
Atomoxetine slowed learning speed in SHR during the initial discrimination
Atomoxetine increased learning accuracy in SHR during set shifting
Neither drug modified learning speed or accuracy in SHR during reversal learning
Acknowledgments
This study was supported by DA011716. We thank Meghan Michalski, Britahny Baskin and Malak El Quessny for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors' contribution KK and LD were responsible for the study concept and design. RH, CJ, DT and KM collected data and analyzed results. RH and KK wrote the manuscript and all authors critically reviewed and edited the content and approved the final version for publication.
References
- [1].Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–72. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- [2].Castellanos FX. Anatomic magnetic resonance imaging studies of attention-deficit/hyperactivity disorder. Dialogues Clin Neurosci. 2002;4:444–8. doi: 10.31887/DCNS.2002.4.4/fxcastellanos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- [4].Seidman LJ, Biederman J, Faraone SV, Weber W, Ouellette C. Toward defining a neuropsychology of attention deficit-hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J Consult Clin Psychol. 1997;65:150–60. doi: 10.1037/0022-006X.65.1.150. [DOI] [PubMed] [Google Scholar]
- [5].Chamberlain SR, Robbins TW, Winder-Rhodes S, Muller U, Sahakian BJ, Blackwell AD, et al. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biol Psychiatry. 2011;69:1192–203. doi: 10.1016/j.biopsych.2010.08.019. [DOI] [PubMed] [Google Scholar]
- [6].Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med. 1999;29:527–38. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- [7].Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- [8].Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B. Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry Hum Dev. 2011;42:257–69. doi: 10.1007/s10578-010-0212-3. [DOI] [PubMed] [Google Scholar]
- [9].Tannock R, Schachar R. Methylphenidate and cognitive perseveration in hyperactive children. J Child Psychol Psychiatry. 1992;33:1217–28. doi: 10.1111/j.1469-7610.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- [10].Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2006;30:1225–45. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- [11].Gau SS, Shang CY. Improvement of executive functions in boys with attention deficit hyperactivity disorder: an open-label follow-up study with once-daily atomoxetine. Int J Neuropsychopharmacol. 2010;13:243–56. doi: 10.1017/S1461145709990836. [DOI] [PubMed] [Google Scholar]
- [12].Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155:693–5. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- [13].Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–84. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [14].Wultz B, Sagvolden T, Moser EI, Moser MB. The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: effects of methylphenidate on exploratory behavior. Behav Neural Biol. 1990;53:88–102. doi: 10.1016/0163-1047(90)90848-z. [DOI] [PubMed] [Google Scholar]
- [15].Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–12. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- [16].Wooters TE, Bardo MT. Methylphenidate and fluphenazine, but not amphetamine, differentially affect impulsive choice in spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Brain Res. 2011;1396:45–53. doi: 10.1016/j.brainres.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jentsch JD. Impaired visuospatial divided attention in the spontaneously hypertensive rat. Behav Brain Res. 2005;157:323–30. doi: 10.1016/j.bbr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- [18].Sagvolden T, Xu T. l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD) Behav Brain Funct. 2008;4:3. doi: 10.1186/1744-9081-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, et al. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–57. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- [20].Meneses A, Ponce-Lopez T, Tellez R, Gonzalez R, Castillo C, Gasbarri A. Effects of d-amphetamine on short- and long-term memory in spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Behav Brain Res. 2011;216:472–6. doi: 10.1016/j.bbr.2010.08.035. [DOI] [PubMed] [Google Scholar]
- [21].van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–90. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- [22].Chess AC, Raymond BE, Gardner-Morse IG, Stefani MR, Green JT. Set shifting in a rodent model of attention-deficit/hyperactivity disorder. Behav Neurosci. 2011;125:372–82. doi: 10.1037/a0023571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Robertson BA, Clements KM, Wainwright PE. The working memory capabilities of the spontaneously hypertensive rat. Physiol Behav. 2008;94:481–6. doi: 10.1016/j.physbeh.2008.02.016. [DOI] [PubMed] [Google Scholar]
- [24].Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36:837–47. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thanos PK, Ivanov I, Robinson JK, Michaelides M, Wang GJ, Swanson JM, et al. Dissociation between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention, impulsivity and hyperactivity in a Visual Stimulus Position Discrimination Task. Pharmacol Biochem Behav. 2010;94:374–9. doi: 10.1016/j.pbb.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- [27].Cao AH, Yu L, Wang YW, Wang JM, Yang LJ, Lei GF. Effects of methylphenidate on attentional set-shifting in a genetic model of attention-deficit/hyperactivity disorder. Behav Brain Funct. 2012;8:10. doi: 10.1186/1744-9081-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- [29].Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- [30].Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–94. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–37. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- [33].Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [34].Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–73. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [35].Medication for children with attentional disorders American Academy of Pediatrics Committee on Children With Disabilities and Committee on Drugs. Pediatrics. 1996;98:301–4. [PubMed] [Google Scholar]
- [36].Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–71. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- [38].Mattiuz EL, Ponsler GD, Barbuch RJ, Wood PG, Mullen JH, Shugert RL, et al. Disposition and metabolic fate of atomoxetine hydrochloride: pharmacokinetics, metabolism, and excretion in the Fischer 344 rat and beagle dog. Drug Metab Dispos. 2003;31:88–97. doi: 10.1124/dmd.31.1.88. [DOI] [PubMed] [Google Scholar]
- [39].Hand DJ, Fox AT, Reilly MP. Response acquisition with delayed reinforcement in a rodent model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2006;175:337–42. doi: 10.1016/j.bbr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [40].Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–57. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- [42].Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–33. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn Affect Behav Neurosci. 2010 doi: 10.3758/s13415-012-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62:1299–307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Prusky GT, Harker KT, Douglas RM, Whishaw IQ. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res. 2002;136:339–48. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- [46].Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hill JC, Herbst K, Sanabria F. Characterizing operant hyperactivity in the Spontaneously Hypertensive Rat. Behav Brain Funct. 2012;8:5. doi: 10.1186/1744-9081-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Langen B, Dost R. Comparison of SHR, WKY and Wistar rats in different behavioural animal models: effect of dopamine D1 and alpha2 agonists. Atten Defic Hyperact Disord. 2011;3:1–12. doi: 10.1007/s12402-010-0034-y. [DOI] [PubMed] [Google Scholar]
- [49].Johansen EB, Sagvolden T, Kvande G. Effects of delayed reinforcers on the behavior of an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2005;162:47–61. doi: 10.1016/j.bbr.2005.02.034. [DOI] [PubMed] [Google Scholar]
- [50].Aase H, Sagvolden T. Infrequent, but not frequent, reinforcers produce more variable responding and deficient sustained attention in young children with attention-deficit/hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2006;47:457–71. doi: 10.1111/j.1469-7610.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- [51].Rogers LJ, Bolden SW, Patrech AS, Ehrlich D. Visual dysfunction in the spontaneously hypertensive rat. Physiol Behav. 1993;54:903–7. doi: 10.1016/0031-9384(93)90300-5. [DOI] [PubMed] [Google Scholar]
- [52].Johansen EB, Aase H, Meyer A, Sagvolden T. Attention-deficit/hyperactivity disorder (ADHD) behaviour explained by dysfunctioning reinforcement and extinction processes. Behav Brain Res. 2002;130:37–45. doi: 10.1016/s0166-4328(01)00434-x. [DOI] [PubMed] [Google Scholar]
- [53].De Bruin NM, Kiliaan AJ, De Wilde MC, Broersen LM. Combined uridine and choline administration improves cognitive deficits in spontaneously hypertensive rats. Neurobiol Learn Mem. 2003;80:63–79. doi: 10.1016/s1074-7427(03)00024-8. [DOI] [PubMed] [Google Scholar]
- [54].Jentsch JD, Aarde SM, Seu E. Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology (Berl) 2009;202:497–504. doi: 10.1007/s00213-008-1181-0. [DOI] [PubMed] [Google Scholar]
- [55].Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, et al. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–41. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–40. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- [57].Drechsler R, Rizzo P, Steinhausen HC. Decision making with uncertain reinforcement in children with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2010;16:145–61. doi: 10.1080/09297040903190774. [DOI] [PubMed] [Google Scholar]
- [58].Desman C, Petermann F, Hampel P. Deficit in response inhibition in children with attention deficit/hyperactivity disorder (ADHD): impact of motivation? Child Neuropsychol. 2008;14:483–503. doi: 10.1080/09297040701625831. [DOI] [PubMed] [Google Scholar]
- [59].Reeve WV, Schandler SL. Frontal lobe functioning in adolescents with attention deficit hyperactivity disorder. Adolescence. 2001;36:749–65. [PubMed] [Google Scholar]
- [60].Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- [61].Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J Abnorm Child Psychol. 2001;29:541–56. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- [62].Wehmeier PM, Schacht A, Wolff C, Otto WR, Dittmann RW, Banaschewski T. Neuropsychological outcomes across the day in children with attention-deficit/hyperactivity disorder treated with atomoxetine: results from a placebo-controlled study using a computer-based continuous performance test combined with an infra-red motion-tracking device. J Child Adolesc Psychopharmacol. 2011;21:433–44. doi: 10.1089/cap.2010.0142. [DOI] [PubMed] [Google Scholar]
- [63].Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology (Berl) 2011;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- [64].Easton N, Marshall F, Fone K, Marsden C. Atomoxetine produces changes in cortico-basal thalamic loop circuits: assessed by phMRI BOLD contrast. Neuropharmacology. 2007;52:812–26. doi: 10.1016/j.neuropharm.2006.09.024. [DOI] [PubMed] [Google Scholar]
- [65].Heal DJ, Smith SL, Findling RL. ADHD: Current and Future Therapeutics. Curr Top Behav Neurosci. 2012;9:361–90. doi: 10.1007/7854_2011_125. [DOI] [PubMed] [Google Scholar]
- [66].Heal DJ, Smith SL, Kulkarni RS, Rowley HL. New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacol Biochem Behav. 2008;90:184–97. doi: 10.1016/j.pbb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- [67].Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–26. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- [68].Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Brain Res. 1995;676:343–51. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- [69].Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- [70].Blum NJ, Jawad AF, Clarke AT, Power TJ. Effect of osmotic-release oral system methylphenidate on different domains of attention and executive functioning in children with attention-deficit-hyperactivity disorder. Dev Med Child Neurol. 2011;53:843–9. doi: 10.1111/j.1469-8749.2011.03944.x. [DOI] [PubMed] [Google Scholar]
- [71].Salek RL, Claussen CM, Perez A, Dafny N. Acute and chronic methylphenidate alters prefrontal cortex neuronal activity recorded from freely behaving rats. Eur J Pharmacol. 2012;679:60–7. doi: 10.1016/j.ejphar.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, et al. Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic alpha(1) - and alpha(2)-receptors. Biol Psychiatry. 2012;71:467–73. doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Easton N, Marshall FH, Marsden CA, Fone KC. Mapping the central effects of methylphenidate in the rat using pharmacological MRI BOLD contrast. Neuropharmacology. 2009;57:653–64. doi: 10.1016/j.neuropharm.2009.08.018. [DOI] [PubMed] [Google Scholar]
- [74].Fumagalli F, Cattaneo A, Caffino L, Ibba M, Racagni G, Carboni E, et al. Sub-chronic exposure to atomoxetine up-regulates BDNF expression and signalling in the brain of adolescent spontaneously hypertensive rats: comparison with methylphenidate. Pharmacol Res. 2010;62:523–9. doi: 10.1016/j.phrs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- [75].Wilens TE, Hammerness P, Utzinger L, Schillinger M, Georgiopoulous A, Doyle RL, et al. An open study of adjunct OROS-methylphenidate in children and adolescents who are atomoxetine partial responders: I. Effectiveness. J Child Adolesc Psychopharmacol. 2009;19:485–92. doi: 10.1089/cap.2008.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Brown TE. Atomoxetine and stimulants in combination for treatment of attention deficit hyperactivity disorder: four case reports. J Child Adolesc Psychopharmacol. 2004;14:129–36. doi: 10.1089/104454604773840571. [DOI] [PubMed] [Google Scholar]
- [77].Jaworowski S, Benarroch F, Gross-Tsur V. Concomitant use of atomoxetine and OROS-methylphenidate in a 10-year-old child suffering from attention-deficit/hyperactivity disorder with comorbid bipolar disorder and Tourette syndrome. J Child Adolesc Psychopharmacol. 2006;16:365–70. doi: 10.1089/cap.2006.16.365. [DOI] [PubMed] [Google Scholar]
- [78].Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Richelson E, Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol. 1984;104:277–86. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- [80].Patrick KS, Caldwell RW, Ferris RM, Breese GR. Pharmacology of the enantiomers of threo-methylphenidate. J Pharmacol Exp Ther. 1987;241:152–8. [PubMed] [Google Scholar]
- [81].Soto PL, Wilcox KM, Zhou Y, Ator NA, Riddle MA, Wong DF, et al. Long-Term Exposure to Oral Methylphenidate or dl-Amphetamine Mixture in Peri-Adolescent Rhesus Monkeys: Effects on Physiology, Behavior, and Dopamine System Development. Neuropsychopharmacology. 2012;37:2566–79. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]


