Abstract
Impaired brain energy metabolism and oxidative stress are implicated in cognitive decline and the pathological accumulations of amyloid β-peptide (Aβ) and hyperphosphorylated Tau (p-Tau) in Alzheimer's disease (AD). To determine whether improving brain energy metabolism will forestall disease progress in AD, the impact of the NAD+ precursor nicotinamide on brain cell mitochondrial function and macroautophagy, bioenergetics-related signaling and cognitive performance were studied in cultured neurons and in a mouse model of AD. Oxidative stress resulted in decreased mitochondrial mass, mitochondrial degeneration and autophagosome accumulation in neurons. Nicotinamide preserved mitochondrial integrity and autophagy function, and reduced neuronal vulnerability to oxidative/metabolic insults and Aβ toxicity. NAD+ biosynthesis, autophagy and PI3K signaling were required for the neuroprotective action of nicotinamide. Treatment of 3xTgAD mice with nicotinamide for 8 months resulted in improved cognitive performance, and reduced Aβ and p-Tau pathologies in hippocampus and cerebral cortex. Nicotinamide treatment preserved mitochondrial integrity, and improved autophagy-lysosome procession by enhancing lysosome/autolysosome acidification to reduce autophagosome accumulation. Treatment of 3xTgAD mice with nicotinamide resulted in elevated levels of activated neuroplasticity-related kinases (Akt and ERKs) and the transcription factor cyclic AMP response element-binding protein in the hippocampus and cerebral cortex. Thus, nicotinamide suppresses AD pathology and cognitive decline in a mouse model of AD by a mechanism involving improved brain bioenergetics with preserved functionality of mitochondria and the autophagy system.
Keywords: NAD+, nicotinamide, mitochondria, DLP1, autophagy, lysosome, 3xTgAD, p-Akt, CREB, learning and memory
1. Introduction
Compromised brain energy metabolism and oxidative stress are implicated in the etiology of Alzheimer's disease (AD), contributing to the neurodegenerative process and associated cognitive deficits (Kennedy et al., 1995; Nunomura et al., 2001; Blass et al., 2002; Mattson, 2004; Kapogiannis and Mattson, 2011; Dumont and Beal, 2011). Age-related free radical production damages proteins, lipids and nucleic acids, and impairs cellular organelle functions. The progressive accumulation of the two major AD pathological hallmark proteins, amyloid β-peptide (Aβ) and tau, may exacerbate oxidative stress (Mattson et al., 2004, Rottkamp et al., 2007, De Felice et al., 2007). On the other hand, Aβ neurotoxicity is enhanced under conditions of mitochondrial dysfunction and cellular energy deficits (Arias et al., 2002).
Mitochondria are the major organelle for energy production in neurons, and are also a major source of free radicals and a target of oxidative damage. Growing evidence indicates that oxidative mitochondrial damage and dysfunction are critical factors in age-related neurodegenerative diseases including AD (Gibson et al., 2010, Sultana et al., 2010, Moreira et al., 2006, Mattson et al., 2004, Hirai et al., 2001). Mitochondria are capable of dividing and growing (biogenesis), and are also subject to degradation and removal when they become dysfunctional and damaged; mitochondria turnover every 2-4 weeks in neurons (Menzies et al., 1971). Accordingly, one approach for counteracting the adverse effects of aging and AD is to preserve mitochondrial quality and homeostatic dynamics, which would be particularly important for long-lived postmitotic cells such as neurons (Terman et al., 2010).
In neurons, as in other cell types, the macroautophagy - lysosome system is the major degradation pathway responsible for turnover of defective organelles and aggregated proteins (Simonsen et al., 2008; Cherra et al., 2010). Autophagy is also a recycling process that is regulated by cellular energy state and oxidative stress signaling (Scherz-Shouval et al., 2007; Hsu et al., 2009); it is activated by nutrient deprivation (Mizushima et al., 2004; Scott et al., 2007; Miwa et al., 2008) and is inhibited by the kinase mammalian target of rapamycin (mTOR) (Yu et al., 2010). When nutrients are lacking, mTOR repression shifts cellular metabolism towards autophagy. The links between bioenergetic state, oxidative stress and autophagy in aging and age-related neurodegenerative diseases remain to be established.
Suppression of autophagy can result in neurodegeneration that involves the accumulation of aggregated proteins and damaged organelles (Cherra et al., 2010, Hara et al., 2006, Komatsu et al., 2006, Batlevi et al., 2011). However, in physiological and pathological settings, the accumulation of autophagosomes may represent either an adaptive response to stress or a defective autophagylysosomal process that triggers cell death (Scherz-Shouval et al., 2007; Banerjee et al., 2010). Autophagy can be stimulated by a range of mild stressors including caloric restriction (Egan et al., 2011), oxidative stress (Scherz-Shouval et al., 2007; Karna et al., 2010) and inhibition of mTOR (Ravikumar et al., 2004). Perturbations of autophagy occur during normal aging and may be exacerbated or dysregulated in neurodegenerative disorders (Rubinsztein et al., 2011). It was recently observed that autophagosomes are abundant in neurons in AD, where aberrations in macroautophagy may occur (Li et al., 2010; Moreira et al., 2010). In addition, both Aβ accumulation and tau neurofibrillary tangles (NFTs) have been observed to be associated with the autophagic pathway (Yu et al., 2005; Hung et al., 2009). Such observations have provoked controversial viewpoints, such as whether autophagosome accumulation indicates an increase in autophagic activity or rather is a consequence of impaired autophagic degradation, and whether autophagy promotes neuronal cell death or is neuroprotective (Banerjee et al., 2010).
It was recently reported that mitochondrial biogenesis and axonal transport of mitochondria are impaired in association with synaptic degeneration in a mouse model of AD, by a mechanism involving cytotoxic actions of Aβ (Calkins and Reddy, 2011; Calkins et al., 2011). In addition, Manczak et al. (2011) provided evidence for an abnormal interaction between Drp1 and Aβ related to mitochondrial abnormalities in neurons in the brains of AD patients. Drp1 (dynamin-related protein 1), which is also called DLP1 (dynamin-like protein 1), plays a critical role in mitochondrial biogenesis. Thus, there is reason to believe that impaired mitochondrial bioenergetics and accumulation of damaged, dysfunctional mitochondria occurs in vulnerable neurons in AD. Therapeutic interventions that sustain mitochondrial bioenergetics may therefore protect synapses and neurons against dysfunction and degeneration in AD.
In the present study we determined whether improving neuronal bioenergetics via provision of the nicotinamide (NAM) would enhance autophagy and ameliorate neuronal dysfunction and degeneration in experimental models of AD. NAM, an amide of vitamin B3, is the essential precursor of β-nicotinamide adenine dinucleotide (NAD+) in mammalian cells (Belenky et al., 2006). NAM is converted to NAD+ through the activity of nicotinamide phosphoribosyltransferase (NAMPT), a rate-limiting enzyme in NAD+ biosynthesis. As an energy substrate and cofactor for electron transfer, NAD+ is essential in multiple steps of aerobic energy metabolic reactions and diverse biological processes (Belenky et al., 2006). NAD+ can serve as an adenosine donor and source of high energy phosphate for the synthesis of ATP. Depletion of NAD+ impairs glycolysis, the tricarboxylic acid cycle and mitochondrial oxidative phosphorylation (Sheline et al., 2000). Moreover, the NADH:NAD+ redox state influences mitochondrial free radical production and anti – oxidant capacity (Starkov et al., 2003; Ghosh et al., 2012). NAM modulates NAD+ and NADH redox levels. In mitochondria, NAD+ and NADH are in a dynamic state, as NADH generation in the tricarboxylic acid cycle requires NAD+. NADH, the reduced form of NAD+, is the substrate and electron donor in complex I of the mitochondrial electron transport chain (ETC), and is oxidized to NAD+ during mitochondrial respiration (Liu et al., 2008).
Accumulating evidence reveals roles for NAD+ in regulating bioenergetics, cell survival and the involvement of perturbed NAD+-dependent processes in age-related neurological diseases and diabetes (Sauve A.A., 2008). NAD+-dependent signaling pathways are involved in the aging process and lifespan determination in model organisms (Houtkooper et al., 2009). For example, NAM modulates sirtuin activity, both as a precursor of the NAD+ required several members of sirtuin family, and as an inhibitor of histone deacetylases. In addition, NAM prevents PARP1 activation and subsequent NAD+ depletion induced by DNA strand breaks (Alano et al., 2010). NAM enhances glycolysis and may reduce the accumulation of abnormal protein aggregates and glycation end products associated with neurodegenerative disorders (Hipkiss et al., 2009). NAD+ can also stimulate lysosomal acidification by modulating the proton gradient, which is essential for autophagosomelysosome fusion and the optimum function of lysosomal proteases (Hsu et al., 2009).
NAM moves rapidly (within minutes) across the blood-brain barrier by facilitated diffusion and is converted to NAD+ (Spector et al., 2007). Green et al. (2008) found that treatment of 3xTgAD mice (an animal model of AD) with NAM results in amelioration of cognitive deficits, and provided evidence for a role for sirtuin 1 activation in this cognition-preserving action of NAM. We previously found that NAM preserves cellular NAD+ levels and improves cell survival under conditions of metabolic and excitotoxic stress in neurons and in a rodent model of stroke (Liu et al., 2009). Here we report that oral administration of NAM ameliorates cognitive deficits, and Aβ and p-tau pathologies, in 3xTgAD mice. We show that the benefit of NAM in AD mice involves elevation of NAD+ levels and increased resistance of mitochondria to oxidative stress, and enhancement of the autophagy-lysosome process. NAM treatment results in activation of signaling pathways critical for neuronal survival and synaptic plasticity including Akt and extracellular signal-regulated kinases (ERKs), and the transcription factor cyclic AMP response element-binding protein (CREB).
2. Methods
2.1. Primary neuronal cell cultures
Methods for the preparation and maintenance of dissociated cultures of embryonic rat cerebral cortical cells have been described previously (Mattson et al., 1995). Dissociated neurons were seeded onto polyethylenimine-coated plastic culture dishes (for biochemical assays) in MEM medium with 15% fetal bovine serum for 4 h, and the medium was then changed to serum-free Neurobasal Medium containing B-27 supplements (Invitrogen), 1 mM HEPES and 2 mM gentamycin (Sigma). The medium contained 0.8 mM Mg2+. Cultures were maintained at 37 C in a 6%CO2/94% air atmosphere. All experiments were performed using 7 to 9 day-old cultures. All treatments were done in the same culture medium for a duration of 6 hours, unless stated otherwise.
2.2. Measurements of mitochondrial mass, morphology, membrane potential and content of acidic organelles
Mitochondrial mass and membrane potential (MMP) were evaluated with the fluorescent probes MitoTrackerTM Green (Molecular Probes) and tetramethylrhodamine ethyl ester (TMRE), respectively, as described previously (Liu et al., 2006). MitoTracker™ Green accumulates in all mitochondria independent of mitochondrial membrane potential, while TMRE is an indicator of mtΔψ. Briefly, cultured neurons were exposed to different treatments for designated time periods, and were then loaded with MitoTrackerTM Green or TMRE (25 nM); MitoTrackerTM Green was removed by washing with fresh medium prior to imaging, whereasTMRE was maintained in the medium throughout the imaging period. Images of labeled cells were acquired at excitation and emission wavelengths of 490 nm and 516 nm for MitoTrackerTM Green and 549 nm and 574 nm for TMRE (Zeiss LSM 510). LysoSensor™ Green (pH range 4.5-6, Molecular Probes), which selectively concentrated in acidic organelles, was used as a fluorescent pH indicator of acidic organelles in live cells. Cells were incubated with 1 μM LysoSensor G and time-lapse imaging following treatment was performed on live cells at 2 sec intervals using a Zeiss 510 confocal microscope, and the average pixel intensity of fluorescence (cell body) was quantified in at least 20 neurons per culture, in 3-4 separate cultures for each treatment group using software supplied by the manufacturer (Zeiss).
2.3. Cell viability assay
Cell viability was quantified using the dye Alamar blue (resazurine) as described previously (Liu et al., 2009, White et al., 1996). Briefly, neurons were cultured in 24-well plates (1×105/well) for 7 days, and then exposed to experimental treatments. After the designated time periods, cells were incubated in medium with Alamar blue at 37°C, and the intensity of fluorescence was measured using a fluorescence plate reader at excitation wavelength of 540 nm and emission of 590 nm. Values were normalized to the mean value for vehicle-treated control cultures. Aβ1-42 was purchased from Bachem; and 1 mM stock solutions of the peptide were prepared in sterile deionized water and incubated at 37°C overnight to initiate the peptide aggregation process.
2.4. NAD+ assay
The total intracellular level of NAD+ was measured using a modified enzymatic cycling colorimetric method. Briefly, NAD+ was extracted from cells with 0.5N HClO4, neutralized with 3.0 N KOH in Gly-Gly buffer, and centrifuged at 10,000 × g for 5 min. NAD+ in the supernatant was converted to NADH by enzymatic cycling with alcohol dehydrogenase. The rate of reduction is proportional to the concentration of coenzyme. The optical absorbance was measured at 560 nm using a plate reader after incubation at 37°C; a standard curve was generated using pure β-NAD (Sigma, MO) diluted and detected under the same conditions as the experimental samples.
2.5. Electron microscopy
Cells grown on Thermomax coverslips were fixed in a mixture of 2.5% paraformaldehyde and 2.0% glutaraldehyde in PBS for 1 h followed by extensive washing in PBS. The samples were then rinsed in 0.1 M sodium cacodylate buffer (SCB; pH = 7.4) and postfixed in 1.0% osmium tetroxide in the SCB for 60 min. After several rinses in SCB, the samples were dehydrated by incubation in increasing concentrations of ethanol (30%, 50%, 75%, 95% for 5 min and 100% for 30 min with 3 changes) and infiltrated with Epon-Aradite (Ted Pella, Redding, CA) over a 2 day period (30% Epon-Aradite in ethanol for 2 h, 50% for 4 h, 75% overnight, and 100% for 1 day with 2 changes). Samples were polymerized at 600°C for one day. Ultrathin sections (about 80 nm) were cut with a Reichert Ultracut E Microtome and collected on copper slot grids. Sections were counter-stained with uranyl acetate and lead citrate, and examined under a FEI Tecnai12 transmission electron microscope operating with a beam energy of 120 keV. Images were acquired using a Gatan 2k × 2k cooled CCD camera.
2.6. Immunoblots
These methods were similar to those described previously (Liu et al., 2010). Protein samples from cell cultures or brain tissue were separated on bis-Tris gels or glycine gels (Invitrogen), with acrylamide concentrations appropriate for the molecular weight of the protein of interest. After electrophoretic transfer to a nitrocellulose membrane, the membrane was blotted with one of the following primary antibodies: LC3B (co-purified with microtubule-associated protein 1B, Sigma); DLP1 (Cell signaling); Aβ (6E10, Signet Laboratories); total Tau (T46, Invitrogen); phosphorylated Tau (AT180, AT8, Pierce), phospho-mTOR (Cell Signaling); p-ERK1/2 (Santa Cruz); p-Akt (Upstate); p-CREB (Cell Signaling); SIRT1 (Upstate); SIRT3 (Cell Signaling); and β-actin (Sigma). HRP-conjugated secondary antibodies were used in all immunoblots (Vector Laboratories) and were visualized by enhanced chemiluminescence (Amersham). The densities of protein bands were normalized to β-actin band intensities.
2.7. 3xTgAD mice and NAM administration
3xTgAD mice were generated from a presenilin-1 mutant (PS1M146V) mouse embryo that was transfected with two expression plasmids, one containing a cDNA encoding an AD-linked Swedish double mutation of APP (APPKM670/671NL) and the other a cDNA encoding a tau mutation (taup301L) that causes frontotemporal lobe dementia, both under the control of the Thy-1 promoter (Oddo et al., 2003). The 3xTgAD mice were backcrossed for 8 generations onto a C57BL/6 background; the congenic 3xTgAD mice develop Aβ and tau pathologies, and behavioral deficits, over a protracted time course compared to the original 3xTgAD mice (Liu et al., 2010). 3xTgAD mice were administered NAM provided in the drinking water (40 μg/g body weight/day) or water alone ad libitum beginning at 4 months of age. There were 10 more mice in each group. At 12 months of age behavioral tests of spatial learning and memory (Morris Water Maze) and spontaneous locomotor activity (open field) were performed. One month after behavioral tests, brains were removed and prepared for biochemical and histological analyses. All procedures using live mice were approved by the National Institute on Aging Animal Care and Use Committee, and complied with NIH guidelines.
2.8. Behavioral tests
These methods were similar to those described previously (Okun et al., 2010; Kawamoto et al., 2012). For Morris water maze testing, a circular tank was filled with water (23 + 1°C) to a depth of 100 cm. Spatial visual cues (each was a different shape) were applied to the walls of each quadrant. A clear circular platform (10 cm in diameter) was submerged about 1 cm below the surface and was placed at one of the four quadrant locations. Each mouse was placed in the pool in one of the four starting locations (North, South, East & West) which were randomly changed between trials. A video camera was mounted on the ceiling in the center of the pool and connected to a computerized tracking/image analyzer system. The swimming path length was monitored with a Videomex tracking system and data was collected using Videomex Water Maze Software (Columbus Instruments, Ohio, USA). Each day of testing the mice performed four trials; each trial lasted until the mouse found the platform or until 60 seconds had elapsed. If the mouse did not find the platform within 60 seconds it was placed on the platform for 30 seconds. A probe trial in which the platform was removed was performed after training on day 6; the mice were allowed to swim freely for 60 seconds and the amount of time spent in the target quadrant was calculated. Spontaneous locomotor activity in the open field was assessed using a plexiglass apparatus equipped with infrared light-sensitive photo cells. The apparatus was placed in a lit, ventilated and quiet testing room. Distance traveled, ambulatory counts, vertical counts and stereotypic counts were recorded for a 30 minute period.
2.9. Immunohistology
Mice were euthanized , their brains were removed, and one half of the brain was frozen immediately for immunoblot analyses, while the other half brain was fresh frozen, cut into 15 μm thick coronal sections on cryostat, and the sections were mounted on charged slides for immunohistochemical analysis. Human control and AD patient brain tissue was provided by the Department of Pathology of Johns Hopkins School of Medicine. Tissues were prepared as formalin-fixed and paraffin-embedded sections. The sections were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) and endogenous peroxidases were quenched by incubation with 0.3% hydrogen peroxide in methanol for 30 minutes. Citrate buffer (pH 6.0) was applied to the tissue sections for antigen retrieval as needed prior to primary antibody incubations. The slides were incubated with primary antibodies raised against human Aβ (Cell Signaling), human PHF-Tau (AT180; Pierce Endogen), LC3B (Sigma) overnight at 4°C. A standard streptavidin-biotin-peroxidase labeling method (Vector ABC kit; USA) was used to visualize Aβ and p-Tau immunoreactivities. Tissues were stained for 5 minutes with the chromogen 3,3’-diaminobenzidine (Vector, USA) and counterstained with hematoxylin, dehydrated, and cover-slipped. Primary antibodies were omitted in negative controls.
2.10. Statistical analysis
One or two way ANOVA were used for comparisons among the different treatment groups at different time points, and Scheffe post-hoc tests were performed for pairwise comparisons among treatment groups. For comparisons involving two groups paired two-tailed t -test was performed.
3. Results
3.1 Oxidative stress adversely affects mitochondria and triggers autophagosome accumulation in neurons
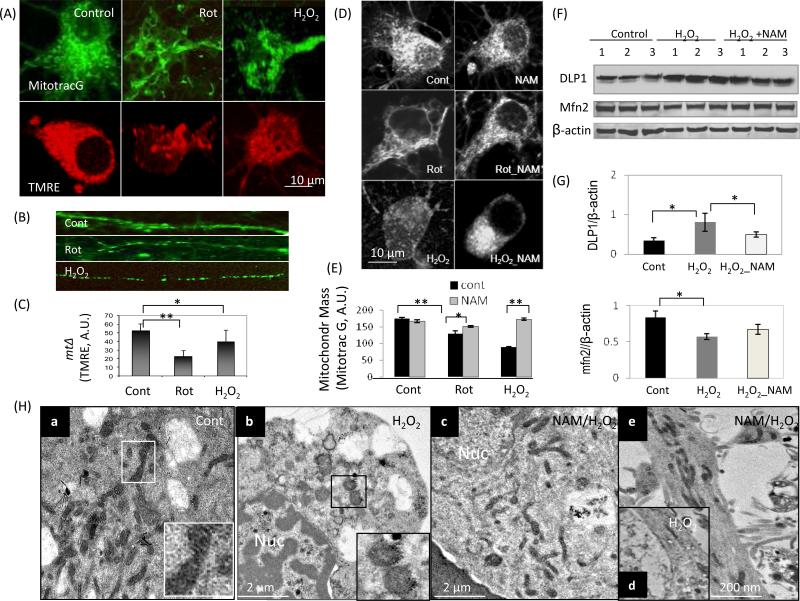
Cultured cortical neurons (7 days in culture) were loaded with the fluorescent probe MitoTracker@ Green which incorporates into mitochondria in a membrane potential-independent manner, or TMRE (a MMP indicator) after exposure to either H2O2 or the mitochondrial toxin rotenone for 6 h (Fig. 1A). Significant mitochondrial morphological changes appeared in neurons exposed to both of the oxidative insults. In H2O2- and rotentone-treated neurons mitochondrial density was reduced in the neuronal cell body (Fig. 1A). In the neurites the size of the mitochondria was reduced with many of the mitochondria appearing rounded rather than exhibiting the normal elongated morphology, character of mitochondrial fissing (Fig. 1B). The MMP was reduced in cortical neurons exposed to oxidative insults compared to vehicle-treated control neurons (Fig. 1C). Mitochondrial mass was preserved in NAM-treated neurons exposed to oxidative/metabolic insults (Fig. 1D and E). The amount of DLP1, a GTPase which plays a pivotal role in mitochondrial fission associated with neuronal injury (Smirnova et al., 2001; Cho et al., 2000), was increased in cortical cells after exposure to oxidative insults (H2O2 and rotenone ), but was attenuated in NAM-treated cells exposed to the same insults (Fig. 1F) indicating increased mitochondrial resistance to oxidative stress. The amount of mitofusin2 (Mfn2) was slightly reduced in cells exposed to oxidative stress. NAM also protected neurons from rotenone-induced loss of the mitochondrial proteins COX IV, the complex 1 subunit NDUFB8 and cytochrome c (D. Liu, unpublished data). The effects of oxidative insults on the ultrastructure of neurons were evaluated by electron microscopy (EM). Neurons in control cultures exhibited a normal appearance of organelles with abundant healthy mitochondria and a few small autophagosomes (Fig. 1Ha). After exposure to H2O2 for 6 h, loss of mitochondrial mass was evident (Fig. 1Hb). Mitochondrial preserved was observed in NAM-treated cells (Fig. 1Hc) and neuronal processes (Fig. 1He) compared to untreated cells exposed to H2O2 (Fig. 1Hd).
Figure 1.
NAM preserves mitochondrial mass, morphology and functionality under conditions of oxidative stress in neurons. Cortical neurons were loaded with the fluorescent probe MitoTracker Green (MitoTracG) and the mitochondrial membrane potential (MMP) indicator TMRE following exposure to vehicle (Control), H2O2 (20 μM) or rotenone (1 μM) for 6 h. (A) Representative images showing mitochondrial morphological changes with reduced mitochondrial mass (MitoTrac Green) and MMP (TMRE) in neurons exposed to oxidative stress. Images are representative of more than 40 neurons examined in each group. (B) Reduced mitochondrial size and loss of the normal elongated shape of the mitochondria occurred in neuronal processes (MitoTracker Green) after exposure of neurons to the indicated oxidative insults. (C) MMP is reduced in neurons exposed to oxidative insults. Values are the mean ± SD of measurements made on 3-4 separate cultures (10-15 neurons evaluated/culture). *p<0.05. **p<0.01. (D and E) NAM treatment preserves mitochondrial mass in neurons exposed to oxidative stress. Values are the mean ± SD of measurements made on 3-4 separate cultures (10-15 neurons evaluated/culture). (F) Levels of DLP1 (a mitochondrial fission-related protein) were elevated in cells exposed to oxidative insults, and NAM treatment attenuated the DLP1 response to oxidative stress. Levels of the mitochondrial fusion protein mfn2 were reduced in cells exposed to oxidative stress. (G) Densitometric values of protein bands (normalized to the β-actin band). Values are the mean ± SD of determinations made on samples from 3 different experiments (samples from 6-7 cultures/experiment were pooled). *p<0.05. (H) Electron microscopy showing ultrastructural alterations in neurons caused by exposure to H2O2 (20 μM) for 6 h. Panel a shows a neuron in a control culture with the inset showing an enlarged view of the boxed area in the lower magnification image. The images illustrate the ‘healthy’ appearance of the mitochondria which are elongated with cristae evident. Panel b shows a neuron in a culture that had been exposed to hydrogen peroxide for 6 h, with the inset showing an enlarged view of the boxed area in the lower magnification image. The latter images illustrate abnormal mitochondria which are round and swollen, with cristae damaged. Panels c and e show neurons that were treated with NAM and then exposed to hydrogen peroxide; mitochondria in the cell body (panel c) and neurites (panel e) appear healthy. Panel d shows the loss of mitochondria in a neurite of a neuron exposed to hydrogen peroxide. Nuc, nucleus.
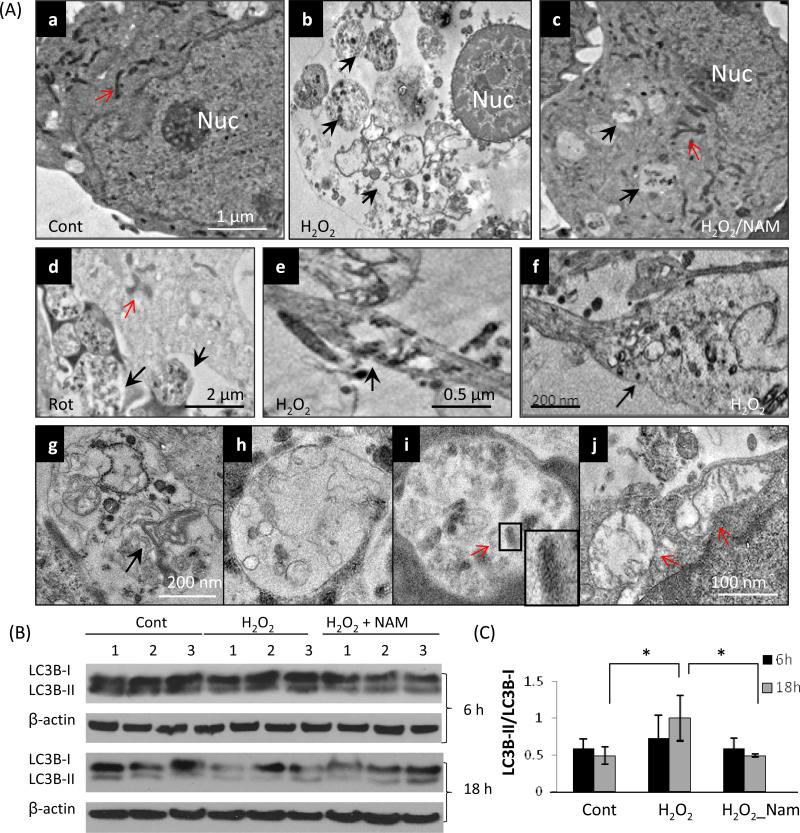
Since autophagy/mitophagy is the major process for turnover of damaged and dysfunctional organelles including mitochondria, autophagy/mitophagy was examined by electron microscopy (EM) ultrastructural analysis on neurons subjected to oxidative/metabolic insults. Autophagsomes are membrane-bound structures containing cellular organelles that exhibit intact membranes, whereas autolysosomes are membrane-bound structures with an amorphous electron-dense content reflecting acidification and degradation of organellar contents. EM images showed that after exposure to oxidative insults (6 h), numerous autophagosomes appeared in the cell body (Fig. 2Ab, d), neuronal processes (Fig. 2Ae) and process terminals (Fig. 2Af). A variety of structures were observed inside the autophagosomes including membrane stacks and dense aggregates (Fig. 2Ag), fused autolysosomes (Fig. 2Ah), and degenerated mitochondria (mitophagy, Fig. 2Ai). Some remaining mitochondria had degenerative features including swelling, enlarged matrix and disrupted cristae (Fig. 2Aj, arrows). Autophagosome accumulation was reduced in NAM-treated cells exposed to H2O2 (Fig. 2Ac).
Figure 2.
Oxidative stress induces autophagy/mitophagy in neurons. Cortical neurons were exposed to H2O2 for 6 h. (A) Electron microscopy revealed numerous autophagosomes in the cell body (b, d), neurites (e) and neurite terminals (f, arrow) in H2O2-treated neurons, compared to a relative paucity of autophagosomes in control neurons (a). Black arrows indicate autophagosomes and red arrows indicate mitochondria. Large autophagosomes contained membranous structures and dense aggregates (g), autolysosomes (h), and degenerated mitochondria (mitophagy) (i, arrows). The remaining mitochondria in H2O2-treated neurons had degenerative features including swelling, enlarged matrix and disrupted cristae (j, arrows). These electron micrographs are representative of ultrastructural features observed in more than 60 neurons examined from at least 3 separate cultures for each treatment condition. (B) Immunoblot revealed slightly increased levels of the LC3-II/LC3-I ratio in cells exposed to oxidative insults at 6 h, while at 18 h the levels of LC3-I were reduced resulting in an elevated LC3-II/LC3-I ratio. The LC3-I level and the LC3-II/LC3-I ratio were maintained in NAM-treated neurons exposed to H2O2. (C) Results of densitometric analysis of immunoblots. Values are the mean ± SD of determinations made on samples from 3 different experiments (samples from 6-7 cultures/experiment were pooled); values were normalized to the β-actin band.
A major autophagy marker is LC3 (microtubule-associated protein 1, light chain 3) (Nakatogawa et al., 2007), a mammalian ortholog of yeast Atg8; LC3 is a ubiquitin-like protein that is covalently attached to phosphatidylethanolamine during autophagosome biogenesis (Nakatogawa et al., 2007, Kabeya et al., 2000). LC3 exists in a cytosolic form (LC3-I) and an autophagosome membrane-associated form (LC3-II). Previous studies have shown that the LC3-II/LC3-I ratio provides a biochemical measure of the autophagic processing status of cells, including neurons (Kadowaki and Karim, 2009; Sadasivan et al., 2010; Wong and Cuervo, 2010; Solano et al., 2012). Immunoblots revealed a moderate increase in the LC3-II/LC3-I ratio in cells exposed to oxidative insults at 6 h, while at 18 h, levels of LC3-I were reduced considerably and the LC3-II/LC3-I ratio was increased. The LC3-I level and the LC3-II/LC3-I ratio were maintained unchanged in NAM-treated cells exposed to H2O2 (Fig. 2B and C), suggesting NAM can protect neurons from oxidative damage and preserve autophagy-lysosome function.
3.2 Nicotinamide protects neurons from oxidative damage and Aβ toxicity involving NAD+ biosynthesis, PI3K signaling and autophagy
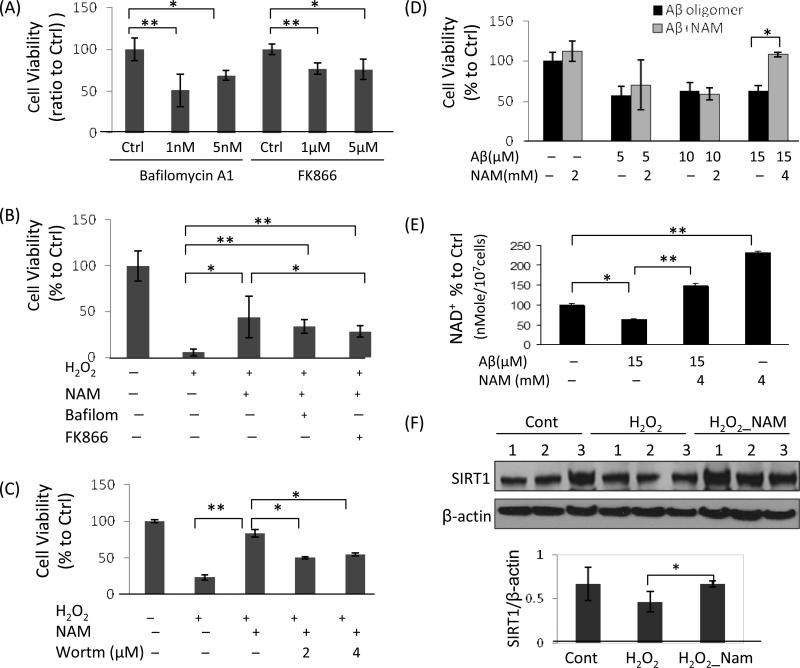
The neuroprotective actions of NAM were examined in cultured neurons exposed to oxidative stress. To clarify the roles of NAD+ biosynthesis and autophagy in the neuroprotective actions of NAM, cortical neurons were treated with FK866, an inhibitor of nicotinamide phosphoribosyltransferase (NAMPT), the key enzyme that catalyzes NAD+ biosynthesis from NAM (Hasmann et al., 2003; Sasaki et al., 2006), or the autophagy inhibitor Bafilomycin A1 (Yoshimori et al., 1991). Both FK866 and Bafilomycin A1 reduced cortical neuron viability in basal culture conditions (Fig. 3A). NAM enhanced cell viability in H2O2-treated neurons, and the neuroprotective effects were significantly attenuated by treatment with FK866 and Bafilomycin A1 (Fig. 3B) indicating that both NAD+ biosynthesis and autophagy are essential for neuron survival. Interestingly, wortmannin, an inhibitor of phosphatidylinositol-3-kinase (PI3K), also attenuated the neuroprotective effects of NAM in neurons subjected to oxidative stress, suggesting the involvement of PI3K-related signaling pathways (Fig. 3C). We also found that aggregated Aβ reduced neuronal survival and cellular NAD+ levels, and that NAM increased cellular NAD+ levels and cell viability in the presence of Aβ (Fig. 3D and E). Since SIRT1 is an NAD+-dependent deacetylase, and SIRT1 levels are closely related to cellular NAD+ availability (Liu et al., 2009; Revollo et al., 2004), we measured SIRT1 protein levels in neurons exposed to oxidative insults in the presence or absence of NAM. SIRT1 protein levels were maintained at a significantly higher level in neurons treated with NAM and then exposed to H2O2 compared to neurons exposed to H2O2 alone (Fig. 3F).
Figure 3.
NAM enhances the survival of neurons exposed to oxidative stress and Aβ oligomers. (A) Cortical neurons were treated for 20 h with the autophagy inhibitor Bafilomycin A1 (1 nM) or with FK866 (1 μM), an inhibitor of nicotinamide phosphoribosyltransferase (NAMPT). Values are the mean and SD of measurements made in 4 separate cultures. (B) NAM protected neurons against oxidative insults, and the neuroprotective effects were significantly attenuated by co-treatment with Bafilomycin A1 (1 nM) or FK866 (1 μM). Values are the mean and SD of measurements made in 4 separate cultures. (C) Wortmannin, an inhibitor of phosphatidylinositol-3-kinase (PI3K), also attenuated the neuroprotective effects of NAM. Values are the mean and SD of measurements made in 4 separate cultures. (D) NAM protected cortical neurons against the toxicity of Aβ oligomers (mean and SD of 4 separate cultures). (E) Cellular NAD+ levels were reduced after exposure to Aβ oligomers, but were maintained in NAM-treated neurons exposed to Aβ oligomers. Values are the mean and SD of measurements made in 3 separate cultures. (F) Immunoblot analysis revealed that levels of the NAD+-dependent deacetylase SIRT1 were preserved in NAM-treated cortical neurons exposed to oxidative stress. Values are the mean ± SD of determinations made on samples from 3 different experiments (samples from 8-10 cultures/experiment were pooled). *p<0.05; **p<0.01.
3.3 NAM treatment ameliorates cognitive decline and neuropathology in 3xTgAD mice
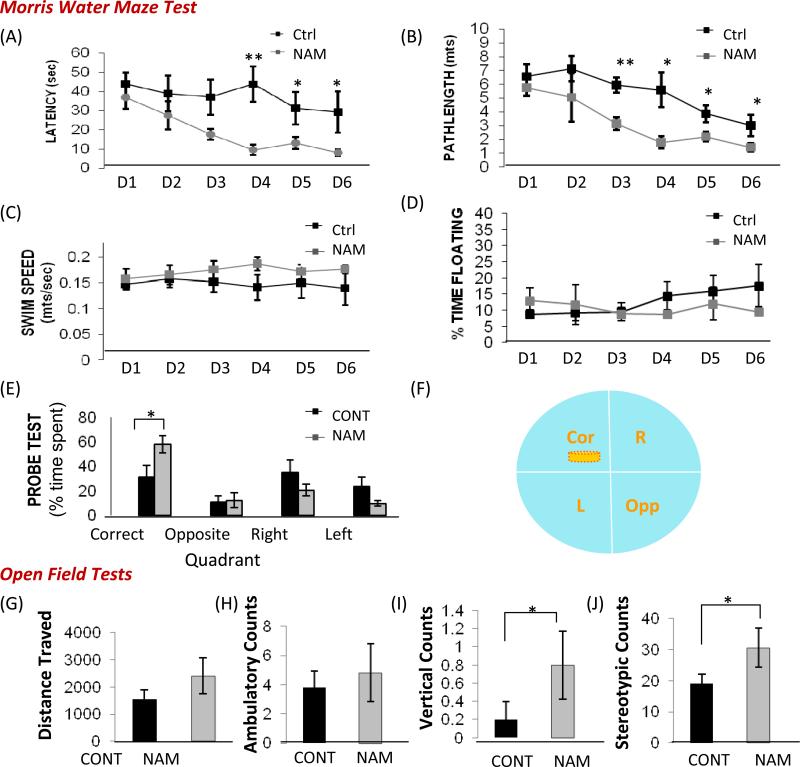
3xTgAD mice develop progressive accumulation of Aβ oligomers and hyper-phosphorylated tau in the hippocampus and cerebral cortex, and learning and memory deficits as they age (Oddo et al., 2003; Liu et al., 2010). Since compromised brain energy metabolism, oxidative stress and mitochondrial dysfunction are involved in the progression of AD, we determined whether NAM might improve brain bioenergetics, and thus modify the disease process and cognitive decline in a mouse model of AD. NAM was provided in the drinking water beginning at 4 months of age in 3xTgAD mice. After 8 months of treatment, water maze and open field tests were performed to evaluate learning and memory capacity. Mice were tested in the hidden platform version of the water maze on 6 consecutive days, and goal latency, path length, percentage of time floating and swim speed were evaluated. Compared to age-matched untreated 3xTgAD mice, NAM-treated 3xTgAD mice exhibited reduced goal latencies (Fig. 4A) and reduced path lengths (Fig. 4B), suggesting improved learning and memory ability. The swimming speeds and floating times did not differ significantly in vehicle- and NAM-treated 3xTgAD mice (Fig. 4C and D). A probe trial test was performed at the end of the 6 consecutive days of training. NAM-treated 3xTgAD mice spent significantly more time in the target quadrant compared to control 3xTgAD mice (Fig. 4E and F). In the open field test, distance traveled and ambulatory counts were similar in control and NAM-treated 3xTgAD mice (Fig. 4G and H). NAM-treated 3xTgAD mice exhibited significantly more vertical counts and stereotyped movements (Fig. 4I and J), suggesting an anxiolytic effect of NAM in 3xTgAD mice.
Figure 4.
NAM ameliorates spatial memory deficits and an anxiety-like phenotype in 3xTgAD mice. 3xTgAD mice were treated with NAM or vehicle (control) for 8 months beginning at 4 months of age (see Methods) and were then tested in the water maze (A-F) and the open field (G-J). Mice were tested in the hidden platform version of the water maze on 6 consecutive days. Goal latencies (A), path length (B), swim speed (C) and percentage of time floating (D) were measured. Values are the mean ± SD (n = 10 mice per group). *p<0.05, **p<0.01 compared to corresponding value for NAM-treated 3xTgAD mice. Results of probe trial test after 6 consecutive training days showed that NAM-treated 3xTgAD spent significantly more time in the target quadrant compared to control 3xTgAD mice (E and F). There were no significant differences in distance traveled (G) and ambulatory counts (H) in the open field between control and NAM-treated 3xTgAD mice. However, NAM-treated 3xTgAD mice exhibited a greater number of vertical counts (I) and stereotypical behavior counts (J) compared to vehicle-treated 3xTgAD mice. *p<0.05.
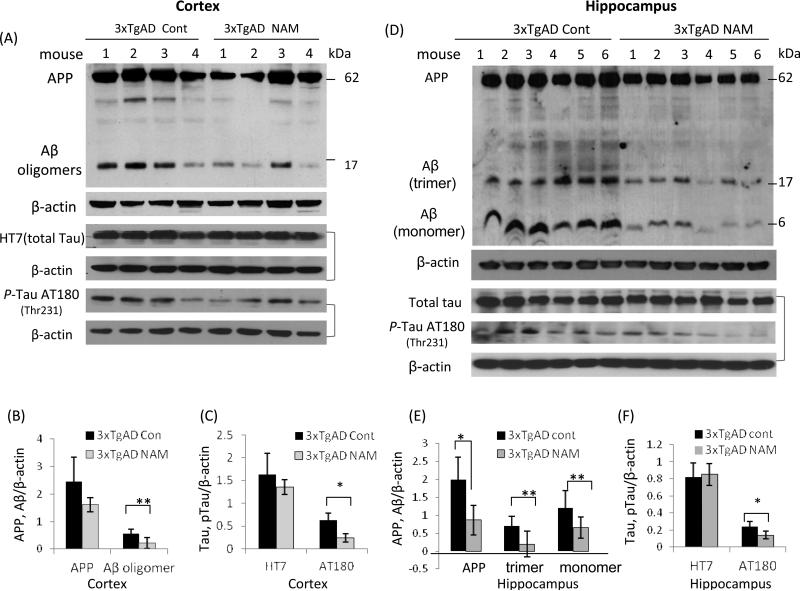
To determine whether the beneficial effect of NAM on cognitive function is associated with changes of Aβ and Tau pathologies, immunohistochemical and immunoblot studies were performed in brain tissues from 3xTgAD mice with or without NAM treatment one month after behavioral testing (13 months of age). Immunoblots with a commonly used Aβ antibody (6E10), which recognizes both APP and Aβ, showed that the levels of Aβ oligomers (the major Aβ oligomer was a trimer of approximately 17 kDa) were lower in both the cerebral cortex and hippocampus samples of NAM-treated 3xTgAD mice compared to untreated 3xTgAD mice (Fig. 5). Levels of p-Tau, determined using an antibody that binds Tau when it is phosphorylated at Thr231 (AT180), were lower in cortical tissue and hippocampus samples from NAM-treated 3xTgAD mice compared to vehicle-treated 3xTgAD mice (Fig. 5), consistent with the results of Green et al. (Green et al., 2008). Levels of total Tau (HT7) were not significantly different between NAM-treated and vehicle-treated mice (Fig. 5).
Figure 5.
NAM treatment ameliorates Aβ and tau pathologies in the brains of 3xTgAD mice. (A) Immunoblots showing levels of full-length APP, Aβ oligomers, Tau and p-Tau in samples of cortical tissue from NAM-treated 13 month-old 3xTgAD mice compared to vehicle-treated 3xTgAD mice. Each lane represents a sample from an individual mouse. (B and C) Results of densitometric analysis of immunoblots of full-length APP, Aβ oligomers (trimers, about 17 kDa), total Tau (T46), and hyper-phosphorylated tau (AT180) from cortical tissue samples of 13 month-old untreated and NAM-treated 3xTgAD mice (4 mice/group). (D - F) Immunoblots and densitometric analysis of APP, Aβ, total Tau and p-Tau (AT180) in protein samples from the hippocampus of untreated and NAM-treated 3xTgAD mice. Density of protein bands were normalized to β-actin bands; values are the mean ± SD (6 mice/group).
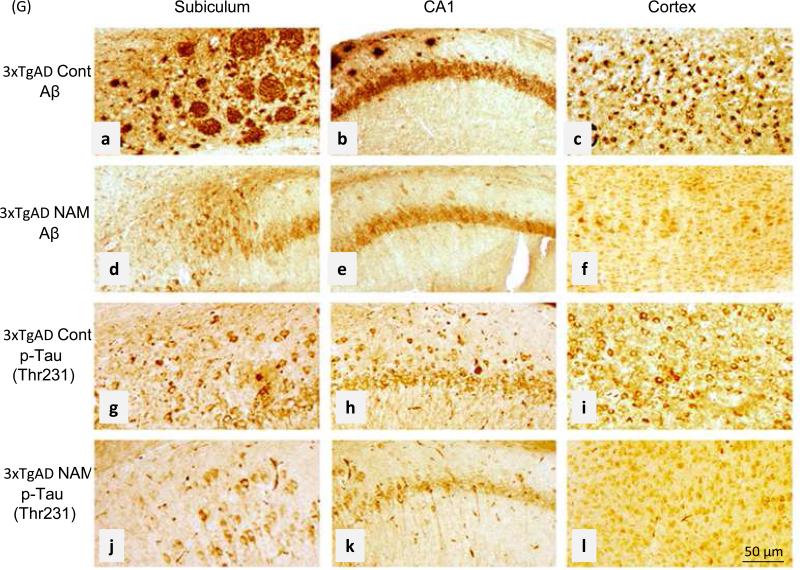
Immunohistochemical staining of brains of untreated 3xTgAD mice revealed dense extracellular Aβ aggregations with plaques of variable sizes that were particularly prominent in the subiculum (Fig. 6). Intracellular Aβ deposition also detected high in pyramidal neurons located at neocortex layer IV-V using an antibody specific for Aβ only (Supplemental Figure 2B and C). The extracellular accumulations of Aβ and intracellular accumulations of hyper-phosphorylated tau (Thr231) in neurons in the subiculum, CA1 region of hippocampus and cerebral cortex were greatly reduced in NAM-treated 3xTgAD mice compared to untreated 3xTgAD mice (Fig. 6). In NAM-treated mice, levels of intracellular Aβ immunoreactivity also appeared reduced compared to vehicle-treated mice (Fig. 6).
Figure 6.
NAM treatment reduces Aβ accumulation and neuronal p-Tau immunoreactivity in 3xTgAD mice. Representative brain sections showing the indicated brain regions from control 3xTgAD mice and NAM-treated 3xTgAD mice immunostained with either Aβ or p-Tau (AT180 ) antibodies as indicated. Note that levels of extracellular and cell-associated Aβ immunoreactivity and intracellular p-Tau (Thr 231) immunoreactivity are reduced in the NAM-treated mice. These images are representative of results observed in 6 vehicle-treated and 6 NAM-treated 3xTgAD mice. Scale bars = 50 μm.
3.4 NAM treatment improves mitochondrial dynamics and autophagy-lysosome procession
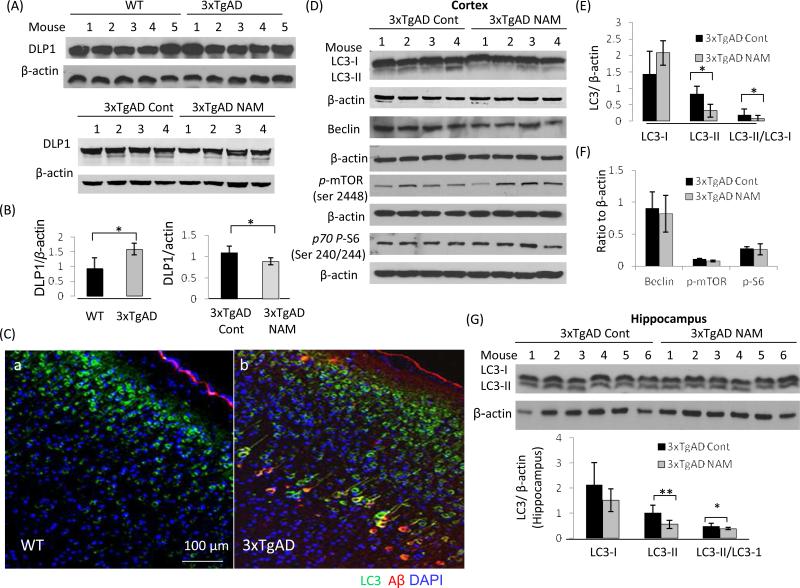
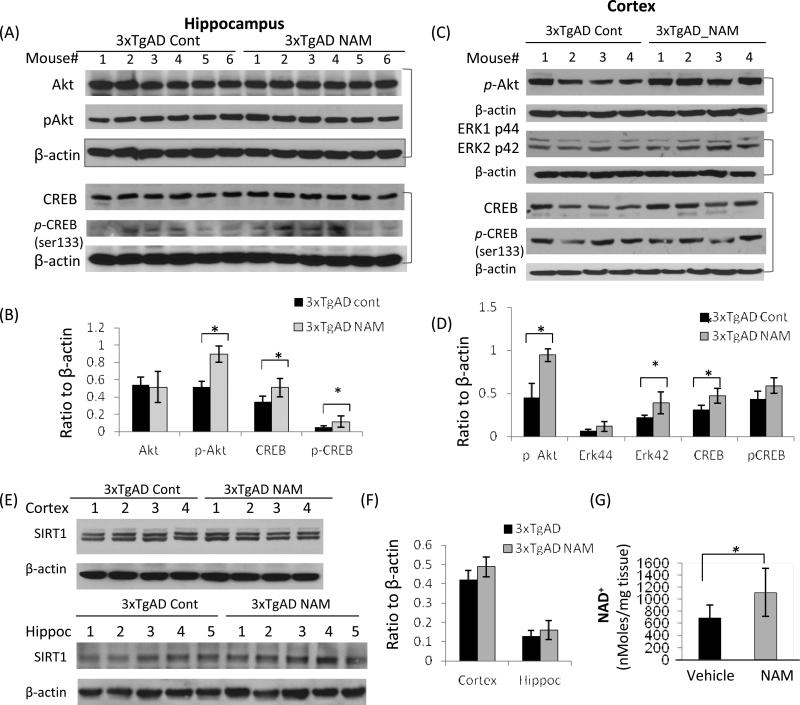
Based on the neuroprotective effects of NAM on cultured neurons, we examined markers of mitochondrial dynamics and autophagy-lysosome function in the brains of control and NAM-treated 3xTgAD mice. Immunoblots showed that the levels of the mitochondrial fission protein DLP1were increased in cerebral cortex tissue samples of 13 month-old 3xTgAD mice compared to samples collected from age-matched wild type mice (Fig. 7A and B), suggesting the existence of mitochondrial stress in 3xTgAD mice. DLP1 levels were lower in cortical tissue samples collected from NAM-treated 3xTgAD mice compared to samples from vehicle-treated 3xTgAD mice (Fig. 7A and B), indicating that NAM could reduce metabolic and oxidative stress on mitochondria, thereby attenuating aberrant mitochondrial fragmentation.
Figure 7.
NAM normalizes mitochondrial dynamics and improves autolysosomal processing in 3xTgAD mouse brains. (A- C) Immunoblots and densitometric analysis showing relative levels of DLP1 and OPA1in cortex samples from age-matched wild type mice (WT), vehicle-treated 3xTgAD mice and NAM-treated 3xTgAD mice. (C- E) Immunoblot and densitometric analysis of LC3-I, LC3-II, beclin, pmTOR and p70p-S6 protein levels in cerebral cortex samples from vehicle- and NAM-treated 3xTgAD mice (4 mice/group). (F and G) Immunoblot and densitometric analysis of LC3-I, LC3-II levels and LC3-II/LC3-I ratio in hippocampal tissue samples of NAM-treated and control 3xTgAD mice. Values are the mean ±SD (6 mice/group).
Immunoblots revealed that autophagy-related proteins LC3-I and LC3-II levels were higher in 3xTgAD mouse brains compared to wild type mice (Supplemental Figure 2F). The higher LC3 staining was also detected in association with neurons and cerebral blood vessels in brain tissue sections from AD patients where its levels were greater than in brain tissue sections from age-matched control subjects (Supplemental Figure 3). Immunofluorescence double-labeling revealed regional variation of Aβ, p-Tau and LC3 distributions, with the presence of relatively high levels of LC3 and Aβ in layer IV-V pyramidal neurons in the cerebral cortex of 3xTgAD mice (Supplemental Figure 2A - C). LC3 was co-localized with Aβ immunoreactivity in some but not all pyramidal neurons. Some neurons exhibited robust LC3 immunoreactivity with little Aβ immunoreactivity, and vice-versa, suggesting diversity of pathologic stages of individual neurons. Immunoblots showed that in NAM-treated 3xTgAD mice, the LC3-II/LC3-I ratio was reduced compared to vehicle-treated 3xTgAD mice in the cortex and hippocampus (Fig. 7D and G). The latter result suggests that NAM treatment may ameliorate neuropathology related build-up of autophagosomes and/or enhance autophagy-lysosome procession. The levels of beclin, a protein involved in the early stages of the autophagic complex formation, were not significantly different between NAM-treated and vehicle-treated 3xTgAD mice (Fig. 7D and F).
The rapamycin target mTOR has been recognized as an important regulator of autophagy (Yu et al., 2010). By sensing and monitoring nutrient levels, mTOR can stimulate protein translation by phosphorylating the p70 ribosomal protein S6 kinase (pS6K) (Shah et al., 2004). Levels of phosphomTOR and phospho-S6 kinase were not significantly different between control and NAM-treated 3xTgAD mice (Fig. 7D - F), suggesting the actions of NAM are exerted by an mTOR-independent mechanism, which may involve signaling pathways regulating autophagy under normal nutritional conditions (Lipinski et al., 2010).
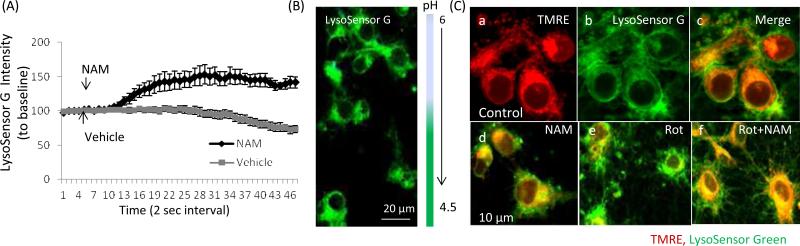
Studies of cardiac myocytes have suggested that NAM stimulates NAD+ biosynthesis, which promotes autophagy and lysosomal acidification and protease activation (Hsu et al., 2009). To examine whether NAM could affect autophagy-lysosome process, rat primary cortical neuronal cultures were loaded with acidic organelle pH indicator LysoSensor™ Green, which selectively accumulates in acidic organelles (including lysosomes, autolysosomes and mitophagosomes). The fluorescence intensity was monitored by live cell time-lapse imaging, with images collected at 2 sec intervals. LysoSensor G fluorescence intensity was quantified from multiple cells and represented as an average percent change from the baseline levels. Adding NAM to cells induced an increased cytosol fluorescence intensity indicating enhanced organelle acidification (Fig. 8A-C), which could enhance autolysosome proteolysis and subsequent clearance and recycling of autophagosome contents.
Figure 8.
NAM enhances organellar acidification and autolysosomal processing in neurons. (A) Rat primary cortical neuronal cultures were loaded with the acidic organelle pH indicator LysoSensor™ Green, which selectively accumulates in acidic organelles including lysosomes and autolysosomes. The fluorescence intensity was monitored by time-lapse confocal imaging images (images were acquired every 2 seconds). Adding NAM to cells induced a transient increase of LysoSensor G fluorescence intensity, indicating enhanced acidification of the organelles. Values are the mean and SD of measurements made in 12 neurons from 3-4 separate cultures. (B) Representative image of cortical neurons loaded with LysoSensor™ Green. Flourescence intensity increases as the pH decreases. (C) Cells were loaded with mtΔψ indicator TMRE (red) and LysoSensor™ G. Under basal culture conditions there was little or no co-localization of the TMRE and LysoSensor signals (Ca-c). Acidic organelle clustering with increased intensity (green) and reduced mitochondrial Δψm (red) were detected in neurons exposed to rotenone for 6 h (Ce). In NAM-treated cells, MMP was partially preserved with co-localization of TMRE and LysoSensor G labeling (yellow), suggesting occurrence of mitophagy (Cf).
3.5 NAM activates p-Akt, MAPK/ERK1/2, and CREB signaling pathways
We next asked whether NAM affected signaling pathways known to play important roles in learning and memory and neuroprotection. Greater amounts of activated (phosphorylated) Akt kinase were detected in the hippocampus and cerebral cortex of NAM treated 3xTgAD mouse brains (6 individual animal/group), compared to vehicle-treated control mice (Fig. 9A-D). The results are consistent with our studies of cultured neurons where the PI3K inhibitor wortmannin abolished the neuroprotective effect of NAM against oxidative stress (Fig. 3C). Signaling pathways involving PI3K, MAPK/ERK42/44and Akt/Foxo3 have been reported to be involved in neuronal survival (Dudek et al., 1997) and autophagy regulation under normal nutritional conditions (Lipinski et al., 2010). We found that the levels of ERK42 were elevated in cerebral cortex of NAM-treated 3xTgAD mice compared to control 3xTgAD mice (Fig. 9C and D). Levels of CREB (cAMP-responsive element binding protein), a transcription factor critical for synaptic plasticity and long-term memory (Sakamoto et al., 2011) were also elevated in hippocampus and cortex tissues from NAM-treated 3xTgAD mice compared to vehicle-treated 3xTgAD mice (Fig. 9A-D).
Figure 9.
Evidence that NAM enhances Akt, ERK, and CREB signaling pathways. (A and C) Immunoblots of hippocampal and cerebral cortex tissue samples from NAM-treated and age-matched (13 month-old) control 3xTgAD mice showing relative levels of Akt, p-Akt, CREB, p-CREB, p-ERK1, p-ERK2 and β-actin. (B and D) Results of densitometric analysis of relative levels of the indicated proteins (normalized to β-actin levels in the same samples). Values are the mean ± SD (n = 4-6 mice/group). (E) Immunoblots performed on tissue samples from the cerebral cortex and hippocampus of NAM-treated and age-matched control 3xTgAD mice showing relative levels of SIRT1, SIRT3, NAMPT and β-actin. (F) Results of densitometric analysis of relative levels of the indicated proteins (normalized to β-actin levels in the same samples). Values are the mean and SD (n = 5 mice). *p<0.05. (G) NAD+ levels in cerebral cortex tissue samples from vehicle-treated and NAM-treated (250 mg/kg) mice 6 h after i.p. injection. Values are the mean ± SD (n = 5 mice). *p<0.05.
NAM is an essential precursor in the mammalian NAD+ biosynthesis salvage pathway, and a previous study showed that NAM administration increases NAD+ levels in cultured neurons and thereby maintain the NAD+-dependent histone deacetylase (HDAC) sirtuin 1 (SIRT1) level (Liu et al., 2009), which may play roles in aging and cell survival (Haigis and Guarente, 2006). Although NAM is also an inhibitor of SIRT1, immunoblots showed that SIRT1 protein levels were slightly elevated in NAM-treated 3xTgAD mouse cortex tissue (Fig. 9E and F). NAM can be converted to NAD+ in vivo through the NAMPT-mediated salvage biosynthesis pathway which supports SIRT1 expression and activity. Brain NAD+ levels were increased in NAM-treated mouse brains compared to age matched vehicle-treated mice after NAM administration (Fig. 9 G). The protein level of NAMPT, the rate-limiting enzyme in the NAD+ salvage biosynthesis (Imai et al.,2009), was not significantly affected by NAM treatment (D. Liu, unpublished data), suggesting its enzyme activity may be sufficient to support the exogenously-derived NAM in the NAD+ salvage biosynthesis process. SIRT1 protein levels could be an indicator of NAD+ levels in the brain which mediates the stress resistance signaling in NAM-treated 3xTgAD mice.
4. Discussion
Long-term (8 months) administration of NAM ameliorated cognitive decline in 3xTgAD mice, which was associated with reduced Aβ and Tau pathologies, preservation of mitochondrial integrity and enhanced autophagy-lysosome function, as well as activation of neuroprotective signaling pathways.
We observed significant mitochondrial loss and aberrant mitochondrial dynamics in cultured neurons exposed to oxidative/metabolic stress (hydrogen peroxide and rotenone), as revealed by observation of mitochondrial morphology indicated by increased mitochondrial fragmentation and increased levels of DLP1, a key mediator of mitochondrial fission. A reduction of mitochondrial mass in neurons subjected to oxidative stress was also evident in EM images, and was associated with increased autophagosomes and mitophagy in neurons. The early changes of mitochondrial dynamics and autophagosome accumulation under metabolic and oxidative stress conditions may represent an adaptive stress response to remove damaged organelles including mitochondria. Oxidative stress-induced mitochondrial fission has been noted as an early event in other models of neurodegenerative diseases (Barsoum et al., 2006). It has been reported that increased autophagy may occur early in the disease process in AD (Cataldo et al., 1996). In addition, selective inhibition of autophagy and lysosomal proteolysis results in AD-like abnormalities in neurons (Lee et al., 2011), whereas enhancement of autophagy by genetic deletion of the lysosomal protease inhibitor cystatin B reduced Aβ pathology in a mouse model of AD (Yang et al., 2011).
NAM counteracted the neurotoxic actions of oxidative/metabolic insults and maintained mitochondrial integrity and NAD+-dependent SIRT1 levels in cultured neurons. NAM also protected cultured neurons against the neurotoxic actions of Aβ by a mechanism involving elevation of cellular NAD+ levels. It has been proposed that NADH:NAD+ redox state modulates ROS production in neurons (Starkov et al., 2003). When the NADH:NAD+ redox potential is high, NADH acts as an electron donor to generate H2O2. Thus, elevation of cellular NAD+ levels may not be the only mechanism by which dietary supplementation with NAM improves cognitive function and reduces Aβ and Tau pathologies in the 3xTgAD mice. Indeed, Ghosh et al. (2012) recently reported that cultured neurons from 3xTgAD mice exhibit lower NAD(P)H redox states compared to wild type mice at a young age, and that the ability of 3xTgAD neurons to regenerate NADH declines progressively with age. When 3xTgAD neurons were treated with NAM, they exhibited elevated levels of the antioxidant glutathione. Thus, in addition to maintaining cellular NAD+ levels, NAM may protect neurons against AD-related proteotoxicity by elevating endogenous antioxidant defenses (Figure 10). Consistent with the latter mechanism, we found that levels of the mitochondrial fission protein DLP1 were greater in neuronal cultures exposed to oxidative stress and in brain cortex samples from untreated 3xTgAD mice compared to NAM-treated 3xTgAD mice, indicating that NAM can reduce mitochondrial oxidative stress and/or increase resistance of mitochondria to metabolic and oxidative stress.
Figure 10.
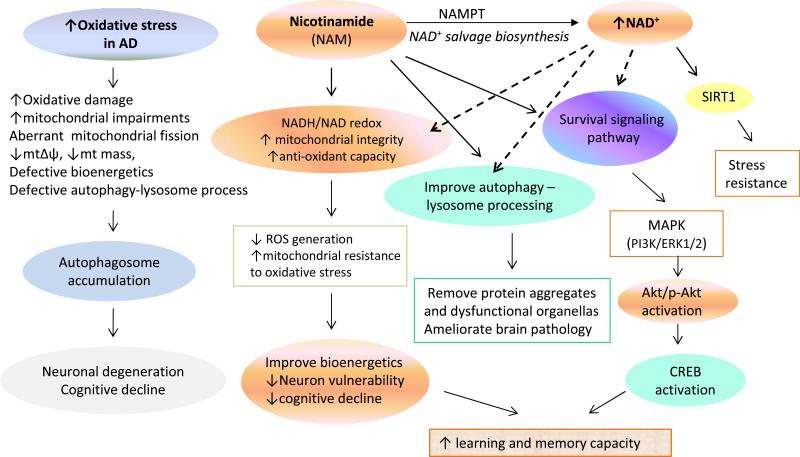
Model for the mechanisms by which NAM counteracts AD-like disease processes and maintains cognitive function in 3xTgAD mice. Compromised brain energy metabolism, and age-related accumulation of oxidative stress may contribute to the adverse effects of APP, PS1 and tau mutations on neuronal plasticity and cognitive function. Important aspect of the pathogenic process are perturbed mitochondrial bioenergetics and aberrant mitochondrial dynamics, oxidative stress and impaired autophagy. NAM, the precursor in NAD+ salvage biosynthesis increases mitochondrial resistance to oxidative stress by modulating the NAD+: NADH redox state to reduce ROS and enhances autophagylysosomal processing of damaged organelles. NAM may also preserve mitochondrial function by acting as an antioxidant to suppress ROS production. NAM increases activation of PI3K-Akt, MAPK/ERK1/2 and SIRT1 cell survival and stress response signaling pathways, as well as transcription factor CREB, a protein critical for synaptic plasticity and long-term memory, thereby ameliorating cognitive decline in AD mice.
We found that NAM treatment resulted in improved memory acquisition and retention in water maze tests in 3xTgAD mice. However, in contrast to the study of Green et al. (Green et al., 2008), we found that NAM-treated 3xTgAD mice had lower levels of Aβ oligomers in the hippocampus and cerebral cortex. One difference between the two studies that may account for the differential effect of NAM on Aβ levels is that we treated 3xTgAD mice with NAM for a longer time period (8 months), whereas treatment was for 4 months in the Green et al. study (Green et al., 2008). In addition, we also found that levels of AT180 immunoreactive hyperphosphorylated Tau (Thr231) were lower in NAM-treated 3xTgAD mice compared to control 3xTgAD mice. Our findings are similar to those reported by Green et al. (2008), who also found that NAM treatment reduced AT180 p-Tau levels. 3xTgAD express a mutant form of Tau that causes frontotemporal lobe dementia in humans (P301L mutation). Deters et al. (2008) investigated the phosphorylation state of Tau in transgenic mice that express P301L mutant tau, and found that hyperphosphorylated Tau continued to accumulate with age.
Our findings suggest that the mechanism by which NAM mitigates Aβ and tau pathologies and enhances cognitive function in 3xTgAD mice also involves elevation of cellular NAD+ levels and activation of signaling pathways involved in cell survival and plasticity (Akt, ERKs and CREB). CREB plays major roles in synaptic plasticity and long-term memory (Sakamoto et al., 2011), and impaired CREB function has been associated with the pathogenesis of AD (Vitolo et al., 2002; Espana et al., 2010). The higher levels of phosphorylated Akt in the brains of NAM-treated 3xTgAD mice may play roles in the enhancement of cognitive function because Akt and ERKs and are key kinases in the metabolic signaling pathways of neurotrophic factors such as BDNF that are involved in synaptic plasticity (Segal et al., 2003; Wu et al., 2008; Cheng et al., 2010). Indeed, NAM treatment elevates BDNF levels in a mouse model of Huntington's disease (Hathorn et al., 2011). It was previously reported that 3xTgAD mice exhibit an anxiety-like phenotype when tested in the open field (Nelson et al., 2007), and we found that NAM lessened this anxiety-like behavior. This anxiolytic effect of NAM might also result from increased CREB activity and consequent BDNF production (Breuillaud et al., 2012).
It has been reported that NAM can inhibit class III NAD+-dependent histone deacetylases (HDACs) including SIRT1 (Liu et al., 2009; Green et al., 2008). However, NAM is readily converted to NAD+ by salvage biosynthesis, consistent with our finding of elevated brain NAD+ levels measured 6 h after NAM administration. In addition, we previously showed that that NAM sustains cellular NAD+ levels and maintained SIRT1 protein levels in cultured neurons subjected to excitotoxins, and that NAM treatment was neuroprotective in a stroke model (Liu et al., 2009). We found that NAM treatment resulted in a small elevation of SIRT1 protein levels in the brains of 3xTgAD mice, which could be an indicator of elevated cellular NAD+ levels due to the dependency of SIRT1 activity on NAD+, and/or increased CREB activity which has been reported to induce SIRT1 expression (Noriega et al., 2011). SIRT1 might also contribute to the oxidative stress resistance in NAM-treated neurons, and better cognitive function in NAM-treated 3xTgAD mice. Other studies have suggested that Class III HDAC inhibitors can suppress the activity of multiple HDACs in addition to SIRT1, and account for their neuroprotective effects (Green et al., 2008; Langley et al., 2005; Roth et al., 2001).
Aβ may play a role in the mitochondrial abnormalities in 3xTgAD mice because previous studies have shown that Aβ can cause mitochondrial fragmentation in cultured neurons (Wang et al., 2008), and we observed similar adverse effects of Aβ on cultured neurons. We found that levels of the mitochondrial fission protein DLP1 were elevated in the cerebral cortex of 3xTgAD mice compared to nontransgenic mice, and levels of DLP1 were also elevated in brain tissue samples from AD patients compared to age-matched control subjects. The latter findings are consistent with mitochondrial fragmentation occurring in neurons in AD and, indeed, it has been suggested that Aβ interacts directly with DLP1 to promote mitochondrial fission in AD (Manczak et al., 2011). Treatment of 3xTgAD mice with NAM resulted in reduced Aβ and DLP1 levels, suggesting reduced mitochondrial fragmentation.
Autophagy is involved in several physiological processes including protein and organelle turnover and responses to changes in energy states (Singh et al., 2011). Perturbed autophagy may contribute to various pathological conditions (Komatsu et al., 2006; Rubinsztein et al., 2007; Hidvegi et al., 2010). Although basal levels of autophagy ensure the turnover of old and damaged organelles, excessive accumulation of autophagosomes is often a harbinger of cellular dysfunction and death (Banerjee et al., 2010). We observed an accumulation of autophagosomes in neurons exposed to oxidative stress, and the presence of degenerated mitochondria in autophagic vacuoles (mitophagy). Immunoblots revealed an elevated LC3-II/LC3-I ratio in neurons exposed to H2O2, which was reduced in NAM-treated cells, presumably due to reduced cellular oxidative damage and/or enhanced autolysosomal processing and clearance. Recent studies have shown that both the APP and tau are associated with the autophagic pathway (Li et al., 2010), and that autophagic degradation of mitochondria increases in AD (Moreira et al., 2007). However, abnormal aggregation and post-translational modifications (phosphorylation and oxidative modification) of these proteins may impair or disturb the autophagy-lysosome system, resulting in the accumulation of autophagosomes in neurons. We found that the autophagy-related protein LC3 was abundant in the brain of 3xTgAD mice and in the brain tissue of patients with AD, mainly distributed in neuronal type cells and cerebral blood vessels. The accumulation of autophagosome components could be an adaptive response to the pathological conditions in AD brains, or may signify defective autophagylysosomal processing. During late stages of macroautophagy, lysosomes fuse with LC3-II-associated autophagosomes, and the contents are then degraded and recycled. Inhibition of autolysosomal degradation will result in the buildup of late-stage autophagosomes.
It has been reported that presenilin 1 mutations and presenilin 1 deficiency impair the acidification of lysosomes, thus disrupting lysosomal proteolysis and clearance of autophagic vacuoles (Lee et al., 2010). 3xTgAD mice were generated from a presenilin-1 mutant PS1M146V knock-in mouse embryo (Oddo et al., 2003). Therefore, together with Aβ, the mutant PS1 may promote the accumulation of LC3-II-associated autophagosomes in neurons due to disrupted lysosomal proteolysis. Our study showed that NAM could enhance acidification of intracellular acidic organelles. In NAM-treated 3xTgAD mice the LC3-II/LC3-I ratio was reduced, suggesting improved autophagy-lysosome processing. This effect of NAM on autophagy may result from the elevation of cellular NAD+ levels in neurons because multiple steps in autophagy process including lysosomal acidification for the optimal function of lysosomal proteases and autophagosome-lysosome fusion requires NAD+ (Hsu et al., 2009).
Our findings do not definitively establish that elevation of NAD+ levels in neurons is the only mediator of neuroprotection afforded by NAM treatment. However, while not precluding additional mechanisms, our data are consistent with a significant contribution of elevated NAD+ levels to the neuroprotective actions of NAM (Figure 10). Our data show that NAM treatment increases cellular NAD+ levels in cultured hippocampal neurons and in brain tissues samples from 3xTgAD mice receiving NAM. We further show that the NAMPT inhibitor FK866 significantly attenuates the neuroprotective action of NAM on neurons. We previously reported that treatment of cultured hippocampal neurons with that NAD+ or NADH is neuroprotective (Liu et al., 2008; 2009). Others have provided evidence that maintenance of cellular NAD+ levels is critically involved in the enhanced autophagy and neuroprotection afforded by Nampt activity in neurons (Wang et al., 2012). Moreover, previous studies of non-neuronal cells have shown that NAM enhances autophagy by a mechanism involving elevation of cellular NAD+ levels (Hsu et al., 2009; Jang et al., 2012). It was also reported that NAD+ induces autophagy in cultured neuroblastoma cells (Han et al., 2011). Other mechanisms of action that may also contribute to the neuroprotective effects of NAM documented in the present study include effects on oxidative stress responses, cell survival signaling and SIRT1 activity (Figure 10).
Collectively, our findings suggest that NAM counteracts the adverse effects of PS1, APP and tau mutations by improving brain energy metabolism and facilitating the function of multiple molecular pathways involved in protein and organellar quality control and synaptic plasticity (Figure 10). 3xTgAD mice treated with NAM exhibited improved memory acquisition and memory retention compared to control 3xTgAD mice, and this cognitive enhancement was associated with reduced Aβ and tau pathologies, and evidence of preserved mitochondrial quality and improved autophagic efficiency. In addition, the elevated levels of activated Akt and CREB in cortical cells of NAM-treated 3xTgAD mice suggests that NAM can enhance neurotrophic signal transduction pathways. NAM has been administered to humans in high doses in numerous studies and has an excellent safety profile (Knip et al., 2000). Together with previous findings (Green et al., 2008), our data therefore suggest a potential therapeutic benefit of nicotinamide in patients with or at risk for AD. Other approaches for elevating cellular NAD+ levels are also possible. For example, nicotinamide riboside can be phosphorylated and converted into NAM by nicotinamide riboside kinases. It was recently reported that nicotinamide riboside protects mice against diet-induced obesity by a mechanism involving elevation of NAD+ levels and activation of sirtuins 1 and 3 in skeletal muscle of mice (Canto et al., 2012). However, the latter study suggested that peripheral administration of nicotinamide riboside does not result in an elevation of NAD+ levels in the brain. Given the present findings and those of Green et al. (2008), together with the well-established safety of long-term dietary supplementation with NAM, clinical trials of NAM in human subjects with or at risk for AD are warranted.
Supplementary Material
Acknowledgements
We thank M. Mughal for assistance with management of the 3xTgAD mouse colony, R. Leapman for supporting our EM studies, and P. Ghosh and S.L. Chan for helpful discussion and providing antibodies. This research was supported by the Intramural Research Program of the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has a conflict of interest to disclose.
Supplemental Information
Supplemental information for this article includes three figures and figure legends.
References
- Alano CC, Garmier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Montiel T, Quiroz-Báez R, Massieu L. β-Amyloid neurotoxicity is exacerbated during glycolysis inhibition and mitochondrial impairment in the rat hippocampus in Vivo and in isolated nerve terminals: Implications for Alzheimer's disease. Exp. Neurol. 2002;176:163–174. doi: 10.1006/exnr.2002.7912. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Beal MF, Thomas B. Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci. 2010;33:541–549. doi: 10.1016/j.tins.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlevi Y, Spada AR. Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol. Dis. 2011;43:46–51. doi: 10.1016/j.nbd.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends. Biochem. Sci. 2006;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer's disease. J. Alzheimers Dis. 2002;4:225–32. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- Breuillaud L, Rossetti C, Meylan EM, Mérinat C, Halfon O, Magistretti PJ, Cardinaux JR. Deletion of CREB-regulated transcription coactivator 1 induces pathological aggression, depression-related behaviors, and neuroplasticity genes dysregulation in mice. Biol Psychiatry. 2012;72:528–536. doi: 10.1016/j.biopsych.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim Biophys Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Manczak M, Mao P, Shirendeb U. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects againsts high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer's disease. J. Neurosci. 1996;16:186–199. doi: 10.1523/JNEUROSCI.16-01-00186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trends. Endocrinol. Metab. 2010;21:589–98. doi: 10.1016/j.tem.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherra SJ, III, Dagda RK, Chu CT. Review: Autophagy and neurodegeneration: survival at a cost? Neuropathol. Appl. Neurobiol. 2010;36:125–132. doi: 10.1111/j.1365-2990.2010.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007;282:11590–601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Deters N, Ittner LM, Götz J. Divergent phosphorylation pattern of tau in P301L tau transgenic mice. Eur J Neurosci. 28:137–147. doi: 10.1111/j.1460-9568.2008.06318.x. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Brinbaum M, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinas Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic. Biol. Med. 2011;51:1014–1026. doi: 10.1016/j.freeradbiomed.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana J, Valero J, Miñano-Molina AJ, Masgrau R, Martín E, Guardia-Laguarta C, Lle A, Giménez-Llort LG, Rodríguez-Alvarez J, Saura CA. β-Amyloid disrupts activity-dependent gene transcription required for memory through the CREB coactivator CRTC1. J. Neurosci. 2010;30:9402–9410. doi: 10.1523/JNEUROSCI.2154-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J. Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Starkov A, Blass JP, Ratan RR, Beal MF. Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim. Biophys. Acta. 2010;1802:122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes. Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Han J, Shi S, Min L, Wu T, Xia W, Ying W. NAD+ treatment induces delayed autophagy in Neuro2a cells partially by increasing oxidative stress. Neurochem. Res. 2011;36:2270–2277. doi: 10.1007/s11064-011-0551-x. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hasmann M, Schemainda I. FK866, a Highly Specific Noncompetitive Inhibitor of Nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- Hathorn T, Snyder-Keller A, Messer A. Nicotinamide improves motor deficits and upregulates PGC-1α and BDNF gene expression in a mouse model of Huntington's disease. Neurobiol. Dis. 2011;41:43–50. doi: 10.1016/j.nbd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, Michalopoulos G, Perlmutter DH. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;9:229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- Hipkiss AR. NAD+ availability and proteotoxicity. Neuromolecular. Med. 2009;11:97–100. doi: 10.1007/s12017-009-8069-y. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Hariharan N, Alcendor RR, Oka S, Sadoshim AJ. Nicotinamide phosphoribosyltransferase regulates cell survival through autophagy in cardiomyocytes. Autophagy. 2009;5:1229–1231. doi: 10.4161/auto.5.8.10275. [DOI] [PubMed] [Google Scholar]
- Hung SY, Huan WP, Liou HC, Fu WM. Autophagy protects neuron from Aβ-induced cytotoxicity. Autophagy. 2009;5:502–510. doi: 10.4161/auto.5.4.8096. [DOI] [PubMed] [Google Scholar]
- Imai S. Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr. Pharm. Des. 2009;15:20–8. doi: 10.2174/138161209787185814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SY, Kang HT, Hwang ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem. 2012;287:19304–19314. doi: 10.1074/jbc.M112.363747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Karim MR. Cytosolic LC3 ratio as a quantitative index of macroautophagy. Methods Enzymol. 2009;452:199–213. doi: 10.1016/S0076-6879(08)03613-6. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karna P, Zughaier S, Pannu V, Simmons R, Narayan S, Aneja R. Induction of reactive oxygen species-mediated autophagy by a novel microtubule-modulating agent. J. Biol. Chem. 2010;285:18737–18748. doi: 10.1074/jbc.M109.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto EM, Scavone C, Mattson MP, Camandola S. Curcumin requires tumor necrosis factor alpha signaling to alleviate cognitive impairment elicited by lipopolysaccharide. Neurosignals. 2012;9:1–14. doi: 10.1159/000336074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AM, Frackowiak RS, Newman SK, Bloomfield PM, Seaward J, Roques P, Lewington G, Cunningham VJ, Rossor MN. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer's disease. Neurosci. Lett. 1995;186:17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA. European Nicotinamide Diabetes Intervention Trial Group. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr. Drug. Targets CNS Neurol. Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy. J. Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang X, Le W. Autophagy dysfunction in Alzheimer's disease. Neurodegener. Dis. 2010;7:265–271. doi: 10.1159/000276710. [DOI] [PubMed] [Google Scholar]
- Lipinski MM, Hoffman G, Ng A, Zhou W, Py BF, Hsu E, Liu X, Eisenberg J, Liu J, Blenis J, Xavier RJ, Yuan J. A genome-wide siRNA screen reveals multiple mTORC1 independent signaling pathways regulating autophagy under normal nutritional conditions. Dev. Cell. 2010;18:1041–1052. doi: 10.1016/j.devcel.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Croteau DL, Souza-Pinto N, Pitta M, Tian J, Wu C, Jiang H, Mustafa K, Keijzers G, Bohr VA, Mattson MP. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. J. Cereb. Blood Flow Metab. 2011;31:680–692. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Pitta M, Lee JH, Ray B, Lahiri DK, Furukawa K, Mughal M, Jiang H, Villarreal J, Cutler RG, Greig NH, Mattson MP. The KATP channel activator diazoxide ameliorates amyloid-β and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer's disease. J. Alzheimers Dis. 2010;22:443–457. doi: 10.3233/JAD-2010-101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protect neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Pitta M, Mattson MP. Preventing NAD+ depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann. N. Y. Acad. Sci. 2008;1147:275–282. doi: 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J. Biol. Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- Miwa S, Lawless C, von Zglinicki T. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell. 2008;7:920–923. doi: 10.1111/j.1474-9726.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer's Disease. J. Alzheimers Dis. 2006;9:101–110. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos RX, Zhu X, Lee HG, Smith MA, Casadesus G, Perry G. Autophagy in Alzheimer's disease. Expert Rev. Neurother. 2010;10:1209–1218. doi: 10.1586/ern.10.84. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Nelson RL, Guo Z, Halagappa VM, Pearson M, Gray AJ, Matsuoka Y, Brown M, Martin B, Iyun T, Maudsley S, Clark RF, Mattson MP. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp. Neurol. 2007;205:166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12:1069–1076. doi: 10.1038/embor.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of the Huntington's disease mutation in drosophila and mouse models. Nat. Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Bio.l Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA. Redox-active iron mediates amyloid-β toxicity. Free Radic Biol Med. 2001;30:447–450. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Sadasivan S, Zhang Z, Larner SF, Liu MC, Zheng W, Kobeissy FH, Hayes RL, Wang KK. Acute NMDA toxicity in cultured rat cerebellar granule neurons is accompanied by autophagy induction and late onset autophagic cell death phenotype. BMC Neurosci. 2010;11:21. doi: 10.1186/1471-2202-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J. Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA. NAD and vitamin B3: from metabolism to therapies. J. Pharmacol. Exp. Ther. 2008;324:883–893. doi: 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell. 2007;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu. Rev. Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J. Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J. Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano RM, Casarejos MJ, Gómez A, Perucho J, de Yébenes JG, Mena MA. Parkin null cortical neuronal/glial cultures are resistant to amyloid-β1-42 toxicity: a role for autophagy? J Alzheimers Dis. 2012;32:57–76. doi: 10.3233/JAD-2012-120406. [DOI] [PubMed] [Google Scholar]
- Spector R, Johanson CE. Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E. J. Neurochem. 2007;103:425–438. doi: 10.1111/j.1471-4159.2007.04773.x. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J. Alzheimers Dis. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid. Redox. Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo OV, Angelo AS, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc. Natl. Acad. Sci. USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- White MJ, Dicaprio MJ, Greenberg DA. Assessment of neuronal viability with Alamar blue in cortical and granule cell cultures. J. Neurosci. Meth. 1996;70:195–200. doi: 10.1016/s0165-0270(96)00118-5. [DOI] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma. 2008;25:130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134:258–277. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Näslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy via a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J. Cell Biol. 2005;10:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.