Abstract
Dendritic cells (DCs) are major antigen presenting cells (APCs) that can initiate and control host immune responses toward either immunity or tolerance. These features of DCs, as immune orchestrators, are well characterized by their tissue localizations as well as by their subset-dependent functional specialties and plasticity. Thus, the level of protective immunity to invading microbial pathogens can be dependent on the subsets of DCs taking up microbial antigens and their functional plasticity in response to microbial products, host cellular components and the cytokine milieu in the microenvironment.
Vaccines are the most efficient and cost-effective preventive medicine against infectious diseases. However, major challenges still remain for the diseases caused by sexually-transmitted pathogens, including HIV, HPV, HSV and Chlamydia. We surmise that the establishment of protective immunity in the female genital mucosa, the major entry and transfer site of these pathogens, will bring significant benefit for the protection against sexually-transmitted diseases. Recent progresses made in DC biology suggest that vaccines designed to target proper DC subsets may permit us to establish protective immunity in the female genital mucosa against sexually-transmitted pathogens.
Keywords: Dendritic cells, Vaccines, Genital, Mucosa, Sexually-Transmitted Diseases
1. Critical features of DCs for host immunity
Dendritic cells are the major antigen presenting cells (APCs) that can induce and control host immune responses toward either immunity or tolerance [1–3]. DCs are also key immune mediators that link innate to adaptive immunity [4–7]. The roles of DCs in orchestrating host immune responses are not just limited to T cell-mediated cellular responses; DCs also play crucial roles in the induction and activation of antibody-mediated humoral responses in both healthy and diseases [8–12].
DCs localize throughout our bodies and, more importantly, position themselves where microbial pathogens invade. This feature of DCs defines them as the primary APCs. They capture the invading microbial pathogens at the frontline of infections, migrate to lymph nodes (LNs), and present antigens to lymphocytes to initiate antigen-specific adaptive immune responses [13, 14]. In the immune induction phase, DCs display two key features, functional specialty and plasticity, that can ultimately determine the outcomes of the host immune responses during and after microbial infections. The presence of phenotypically and functionally distinct subsets of DCs in local tissues, including skin [15–19] and different mucosae (reviewed in [20]), indicate that the pathways by which DCs orchestrate host immune responses are much more intricate than what we currently understand. Clearly, these pathways must be tightly regulated. Nonetheless, it seems that the functional specialization of different subsets of DCs is an important component for hosts to successfully defend against a variety of microbial pathogens, which invade through varying mechanisms.
The complexity of the mechanisms by which DCs orchestrate host immune responses is further extended by their ability to display functional plasticity. Microbial products and even certain cellular components are able to stimulate DCs through multiple receptors, particularly via pattern-recognition receptors (PRRs), such as toll-like receptors (TLRs) and C-type lectins [21–25]. Depending on the DC receptors that are ligated followed by the types and strength of intracellular signals delivered, DCs can be activated differently and thus can result in different outcomes of immune response. The plasticity of DCs also relies on elaborate signal codes that are generated by multiple stimuli simultaneously. Thus, the DC activation and maturation process is much more sophisticated than simply licensing DCs for the activation of lymphocytes. This particular process must also enable DCs to sense their environment and assume an activated phenotype that carefully instructs the qualitative nature of the immune responses. Taken together, DC subset-driven functional specialty and plasticity may allow DCs to cope with the challenges of their environment. These two features can eventually control and even dictate the magnitude and quality of the responses induced by vaccines and adjuvants. It is therefore fundamental to harness the functional specialties and plasticity of human DCs for the design of improved vaccines against microbial pathogens, including sexually-transmitted pathogens.
DCs also have unique and reciprocal interactions with innate immune cells. The interactions of DCs with natural killer (NK), NKT, and γδ T cells occur in the periphery and in the secondary lymphoid organs [6, 7, 26]. In the secondary lymphoid organs, the activation of NK cells is largely dependent on the interaction with DCs [27]. Activated NK cells display enhanced cytotoxicity and IFNγ secretion, which can further render DCs to induce type 1 immune responses [6]. Mature DCs also activate NKT cells to induce IFNγ and IL-4 secretion [28] and γδ T cells to induce IFNγ and TNFα secretion [29]. The roles of DCs in linking between innate and adaptive immune responses are further highlighted by the findings that activated NKT cells can kill tumor cells [26, 30], while CD40L expressed on the activated NKT cells induces the activation of DCs via CD40 [6].
2. DCs and immune tolerance
The ability of DCs to orchestrate host immune responses reaches beyond the elicitation and establishment of protective immunity against microbial pathogens. It also fulfills a key function for the induction as well as the maintenance of immune tolerance, which is crucial to protect the host from immune attacks driven by autoreactive lymphocytes (reviewed in [31, 32]). The first evidence that DCs were responsible for immune tolerance was provided by Kurts et al. [33] in a mouse model. They found that in the steady state, DCs present ovalbumin (OVA) peptides on major histocompatibility complex (MHC) class I molecules. OT I cells transferred to these mice proliferated in the draining LNs of kidney and pancreas but were eventually deleted. Other studies, using direct DC targeting of antigen, suggested that DCs are the APCs that induce immune tolerance for self-antigens in vivo [31, 32]. Targeting non-self antigens through DEC-205 also resulted in tolerance by the deletion of antigen-specific CD4+ T cells, whereas combined administration of DC-targeted antigen with an agonistic anti-CD40 antibody led to prolonged T cell activation [2, 34]. Furthermore, injection of mice with dying syngeneic TAP−/− splenocytes loaded with OVA led to presentation of cell-associated OVA by the CD8α+ DCs, followed by deletion of OVA-specific CD8+ T cells [35]. In humans, Dhodapkar et al. [36, 37] first demonstrated that the injection of immature DCs pulsed with influenza matrix peptide and keyhole limpet hemocyanin (KLH) in healthy individuals resulted in decreased matrix-peptide-specific IFN-γ-producing CD8+ T cells. Interestingly, they also found that those healthy subjects had increased numbers of the same antigen-specific IL-10-producing T cells. Taken together, these observations suggest that the outcome of antigen presentation by DCs in the steady state may be systemic antigen-specific tolerance. Therefore, immature DCs residing in peripheral tissues can act as tolerogenic APCs. They can capture a broad spectrum of antigens, including autoantigens, through different mechanisms [14]. Unless there is a proper activation signal, they become tolerogenic [34, 38, 39] .
However, the nature of tolerogenic DCs needs to be further investigated. For example, a constitutive presentation of H+/K+ ATPase by DCs does not induce autoimmunity nor ATPase-specific T cell tolerance [40]. Other immunoregulatory mechanisms might also be involved in the induction and maintenance of antigen-specific immune tolerance. Recent studies have similarly shown that immune tolerance can be induced by DCs matured with endogenous non-inflammatory signals [41, 42]. We have additionally reported that antigens targeted to DCs via one of the PRRs, DC-asialoglycoprotein receptor (DC-ASGPR) [43], can induce antigen-specific IL-10-producing regulatory T cells both in human in vitro and non-human primate in vivo. More importantly, this was applied to both foreign and self antigens as well as to both naïve and memory T cell responses. DCs express a variety of PRRs (reviewed in [21–25]) that can specifically recognize different types of microbial as well as host-cell-driven endogenous stimuli. Therefore, DCs must be able to discriminate between self and non-self, and they must enable the immune system to mount immunity to pathogens while silencing auto-reactive lymphocytes [38, 44].
3. Critical features of DC subsets for the design of vaccines against STDs
After Ralph Steinman and his colleagues discovered DCs in the early 1970s [45, 46], our understanding of the role of DCs as immune orchestrator and their application to human medicines has been well progressed. DCs are now divided into two major subsets, myeloid DCs (mDCs) discovered by Ralph Steinman’s group and plasmacytoid DCs (pDCs) discovered by Yong-Jun Liu’s group in 1997 [47]. Both mDCs and pDCs are found in blood as well as in tissues. One of the major characteristics of pDCs is the secretion of high levels of type 1 IFN in response to viral infection [48]. pDCs also play an important role in innate immunity [49]. Although the contribution of pDCs in the direction of T cell priming in vivo remains to be further characterized, pDCs are also able to present antigens via MHC I and II [50–53]. pDCs have also been implicated in the induction of tolerance [54, 55].
The presence of phenotypically and functionally distinct mDC subsets in different tissues (reviewed in [56]) and their plasticity recapitulate the value of DCs as the primary targets for the design of effective vaccines. DC subsets in blood, lymphoid organs, and other mucosae have been extensively reviewed [57–68]. Below, we review the subsets of DCs and their critical features that need to be considered for the design of effective vaccines against sexually-transmitted pathogens. Although we propose to design vaccines to target DCs in the female genital tract, particularly in the vaginal mucosa, such vaccines might also be administered via non-mucosal routes or through a combination of mucosal (intravaginal: IVAG) (reviewed in [69]) and non-mucosal routes. In this review, therefore, we discuss the subsets of skin DCs and the DCs in the female genital tract, especially in the vaginal mucosa. The DC network in the human vagina has not been well understood. However, data from recent studies in animals [70] and humans [71, 72] have shown that DCs in the vaginal mucosa can be the immune initiators and controllers in the vaginal mucosa and female genital tracts.
3.1. Skin and skin DC subsets
3.1.1. Mammalian skin
Skin and mucosae are the outermost anatomical barriers against pathogens as well as against damages from internal and external stresses. Mammalian skin is composed of the epidermis and dermis. The outermost layer, the epidermis, is mainly composed of proliferating basal cells and differentiated supra keratinocytes. It also contains Merkel cells and melanocytes as well as Langerhans cells (LCs). The proliferating basement membrane between the epidermis and dermis can control the traffic of cells as well as biomolecules, including cytokines and growth factors, between the two major compartments. The dermis is composed of connective tissues that cushion the body from stress and strain. It also contains the hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels and blood vessels as well as dermal DCs, lymphocytes, and other immune cells.
3.1.2. Skin DC subsets
In human skin, there are three major subsets of mDCs: LCs are in the epidermis while CD14+ and CD1a+ DCs are in the dermis [15]. In vitro studies show that subsets of skin-resident mDCs can display specialized functions to induce and activate T and B cell responses. LCs are more efficient than CD14+ dermal DCs at cross-presenting antigens to CD8+ T cells. Skin LCs can also induce CD4+ T cells to secrete Th2-type cytokines. Similar observations were made in a mouse in vivo study. Upon delivery of antigens to LC-rich epidermis, Th2-type T cell responses were preferentially induced [16]. Interestingly, transcutaneous immunization of antigens to mice resulted in potent CD4+ and CD8+ T cell-mediated protective immunity to viruses [73, 74]. In contrast to LCs, CD14+ dermal DCs can polarize naïve CD4+ T cells into follicular helper T cells (Tfh) and can thus prime humoral immunity [17]. Skin LCs are also known to induce Th17 responses [18, 75]. In addition, skin LCs can efficiently activate regulatory T cells [76], as well as Th22-type CD4+ T cell responses [19]. Nonetheless, these immunological functions of human skin DC subsets for the induction and activation of T and B cell responses are yet to be established in vivo.
In mice, LCs can present exogenous antigens on MHC I and MHC II both in vitro and in vivo [77–79]. In contrast, a study using herpes simplex virus (HSV) has questioned the contribution of LCs to the induction of antigen-specific responses in vivo. In this study [80], HSV-specific T cell responses were not due to the LCs, but to CD8α+ DCs. Furthermore, another study also showed that dermal CD103+ DCs, but not dermal CD11b+ DCs or LCs, present antigens to naïve CD8+ T cells [81]. The roles of CD103+ DCs in cross-priming antigen-specific CD8+ T cells have been further confirmed by other studies [82–84]. It still remains to be determined whether these differences with regard to the function of LCs between mice and humans derive from the differences in their immune systems. All DCs are capable of presenting viral antigens to CD4+ T cells [85].
3.2. Vaginal mucosa and DC subsets in the vagina
3.2.1. Female genital tract and vaginal mucosa
The female genital tract is comprised of the upper (oviducts, ovaries, uterus, and endocervix) and lower (ectocervix and vagina) reproductive tract [20]. The upper tract is covered by a simple columnar epithelium (type I mucosa) [86] that is hormonally regulated for fertilization and fetal development. The columnar epithelium is characterized by the presence of tight junctions between cells, making it impermeable to entry of large molecules, including microbial pathogens. In contrast, the lower reproductive tract is lined with more than 25 layers of stratified squamous epithelial cells (type II mucosa) [69, 87–89]. The epithelium in the lower tract, although not impermeable, is robust and provides a substantial physical barrier. Only type I mucosal epithelium expresses polymeric immunoglobulin (Ig) receptors, which can transfer dimeric IgA to the lumen [69, 90, 91], with IgA being the abundant isotype in the type I mucosa. In contrast, antigen-specific antibodies in the vaginal mucosa are dominated by IgG [88]. The epithelium as a physical barrier and the interaction between the epithelium and sexually transmitted pathogens have been recently reviewed [92, 93].
The lower female genital tract, particularly the vaginal mucosa, is constantly exposed to foreign antigens and is a unique microenvironment that must control unwanted types of immune responses [69, 94–96]. Meanwhile, the vaginal immune system is also capable of eliciting mucosal immune responses in the vagina [20, 71, 97–105]. Furthermore, intravaginal (IVAG) administration of vaccines can elicit certain levels of immune responses in the vagina in animal models [97–100] as well as in humans [71, 101–105].
Nonetheless, the protective immunity induced in the vaginal mucosa by the current mucosal vaccine models needs to be enhanced [69]. As part of the need for new approaches, a new clinical trial of HIV trimeric gp140 protein CN54 (Infectious Disease Research Institute: IDRI) is currently underway and data from this trial will be available in 2013. In particular, the establishment of cellular immunity, which is a critical immune arm against many sexually-transmitted viruses, has been one of the major challenges for the design of vaccines against sexually-transmitted diseases. In this regard, we envision that safe vaccines that can mount potent cellular as well as humoral immunity in the female genital tract, including the vagina, can be developed in the near future. However, such vaccines may need to be designed to target the right antigens to the right subsets of DCs in the vaginal mucosa. Selection of optimal adjuvants that can further promote the vaccine-targeted DC subset-induced immune responses may also determine the efficacy of new vaccines. Thus, understanding the immunology of the human vagina, especially for the subsets of DCs and their functional specialties and plasticity, will be fundamental for the design of such vaccines.
3.2.2. Vaginal DC subsets
Early studies in animals showed that the type II vaginal mucosa, which is covered with stratified squamous epithelium [69, 87, 88], shares several common features with the skin. LCs are found in the epithelium and CD11c+ DCs in the lamina propria (LP) [88, 94]. There are at least four populations of LCs in the epithelium that have been identified: I-A+/F4/80+, I-A+/F4/80–, I-A−/CD205+, and I-A+/CD205− populations by immunohistochemistry [106] and CD11b+ F4/80hi, CD11b+ F4/80int, and CD11b− F4/80− populations by flow cytometry [107]. However, it is not known whether these populations have specific functions in the immune responses of the vaginal mucosa. DC-SIGN+ CCR5+ LP-DCs were also reported in animals [108, 109]. In mice, both lymphoid DCs and tissue-resident DCs are known to prime T cell responses in mice infected with HSV-1 [110]. Zhao et al. [70] also reported that vaginal LP-DCs, but not LCs, are capable of inducing protective Th1-type responses to HSV-2 infection in mice. However, during infection or inflammation, both monocyte-derived DCs [107] and pDCs [111] are recruited from peripheral blood to the vaginal mucosa.
In humans, the presence of LCs in the vaginal epithelium is known [112, 113], but no further information is yet available. Moreover, the immunological functions of DCs localized in the human vagina remain unknown. Below, we review the subsets of DCs known to localize in the human vagina, expression of PRRs, and DC functional plasticity, based upon our recent study [114].
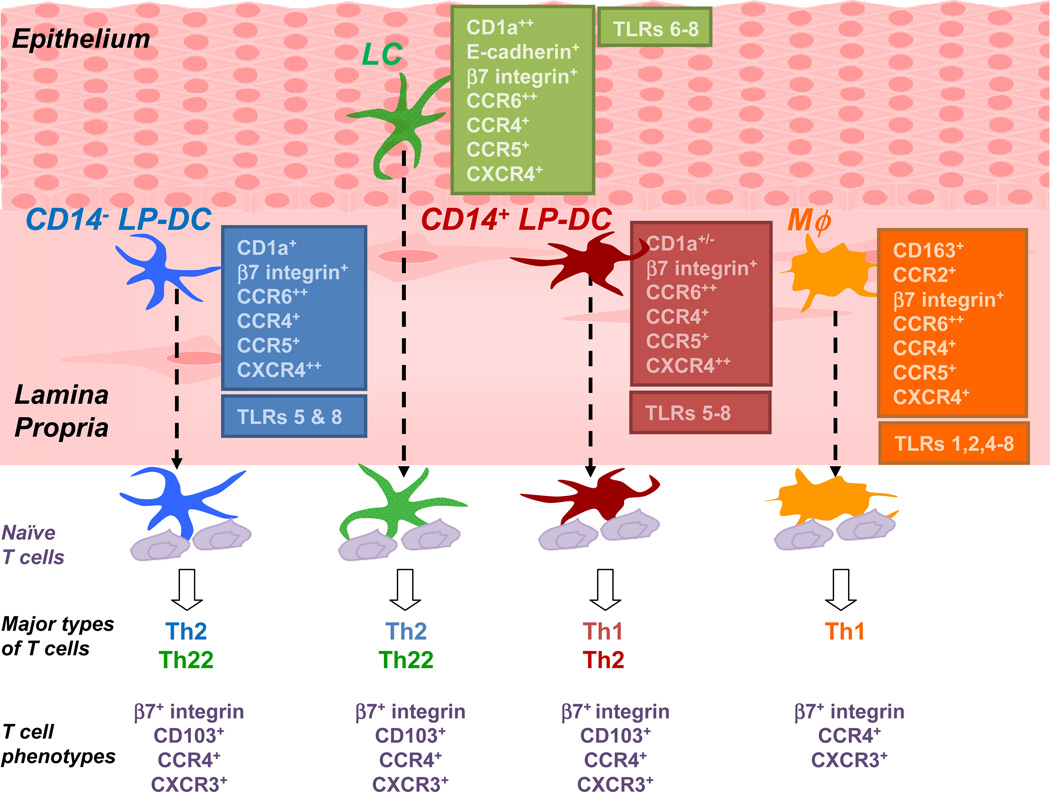
Human vaginal mucosa contains four major subsets of myeloid-originated APCs: CD207+ LCs in the epithelium, CD1c+CD14− DCs (CD14− LP-DCs), CD1c+CD14+ (CD14+ LP-DCs), and CD1c−CD14+ macrophages (Mϕ) in the LP (Fig. 1). In a steady state, the frequencies of pDCs, B cells, and BDCA3+ cells in the human vagina are low. These four major APC subsets display shared and distinct surface phenotypes. LCs express both CD1a and E-cadherin. LCs, as well as the CD14− LP-DCs and CD14+ LP-DCs, express both CD86 and CD83. CD1c−CD14+ cells express CD86, but not CD83. Mϕ express CD163 and CCR2, a homing receptor for monocytes and Mϕ [115]. CCR5 and CXCR4, co-receptors for HIV, are expressed on all four subsets of APCs [116]. Interestingly, the vaginal LP-DCs and Mϕ express increased levels of both CCR5 and CXCR4 compared to LCs. Both LCs and LP-DCs exhibit similar levels of CCR6, which is known to be expressed on intestinal DCs [117]. β7-integrin is detected on LCs and LP-DCs, but not on Mϕ. Both CCR4 and CX3CR1 are similarly expressed on the four subsets of APCs in the vagina, while CCR7 and CD103 are not detected on the surface of the vaginal APC subsets. Taken together, these characteristics of the four major subsets of vaginal APCs suggest that each subset of the APCs might possess both common and distinct functions in directing the immune responses in the vagina.
Figure 1. Human vaginal mucosa harbors four major populations of antigen-presenting cells that display shared and distinct functions at inducing T cell responses.
Four subsets of myeloid-derived APCs are found in human vaginal mucosa: LCs in the epithelium and CD14− LP-DCs, CD14+ LP-DCs and macrophages (MØ) in the submucosa. Each population displays shared and distinct phenotype and functions at inducing naive T cells responses.
In support of the expression levels of co-stimulatory molecules, the vaginal DCs, including LCs, are able to induce greater allogeneic CD4+ and CD8+ T cell proliferation than Mϕ. All four subsets of the vaginal APCs induce similar percentages of IFNγ+CD4+ T cells, but LCs and CD1c+ LP-DCs are more potent than CD14+ LP-DCs and Mϕ at inducing Th2-type CD4+ and CD8+ T cell responses. However, the vaginal APC subsets are not able to induce significant levels of Th17 responses, although Th17 cells contribute to the protective immunity against mucocutaneous candidiasis [118–120] and skin DCs are known to be potent Th17 inducers [18]. Furthermore, none of the vaginal APC subsets display functional specialty to polarizing T cell differentiation into Tfh. In contrast, CD14+ skin dermal-DCs are potent inducers of IL-21+CD4+ T cells [17]. Furthermore, vaginal LCs and CD14− LP-DCs induce similar levels of naïve CD4+ T cell proliferation, whereas skin LCs are superior to CD1a+ dermal DCs at inducing naïve CD4+ T cell proliferation [17, 121]. These features of the vaginal APC subsets clearly distinguish them from the subsets of APCs in the skin. The subset-dependent functional plasticity of the human vaginal APCs can be further promoted by activating DCs through different PRRs. Vaginal DCs express increased levels of PRRs, including MDA-5 [122] and TLR7, which play important roles in anti-viral immune responses. Mϕ express increased levels of TLRs 1, 2, 4, and 6, which allow them to recognize bacterial products. The roles of IL-22 in adaptive immune responses have not been well understood. However, the vaginal LCs and CD14− LP-DCs are able to induce Th22 responses that are further promoted by R848, a ligand for TLRs 7 and 8. Taken together, data from our recent study [114] illustrate that each subset of the DCs and Mϕ in the human vagina can display common as well as distinct functional specialties and plasticity. These features are critical for the design of effective vaccines against sexually-transmitted diseases, although a series of studies to further understand the immunology of the human vagina needs to be done.
4. DC-targeting vaccine for STDs
The pivotal roles of DCs in immunity make them an attractive target for the manipulation of host immune responses [57, 123]. To date, attempts to leverage the use of DCs in vaccination have largely involved cell-based approaches, particularly against cancers. For instance, the in vitro generation of DCs, loading DCs with antigens and the injection of the antigen-loaded DCs to patients, have been explored by several groups (reviewed in [124]). However, this approach has limitations in several aspects, including the preparation of vaccines and antigens as well as costs. In addition, clinical efficacy of such vaccines still needs to be improved. Therefore, the rationale to the design of in vivo DC-targeting vaccines has been well accepted in the fields of immunology and vaccines. A study from a decade ago showed that a minute amount of protein antigens targeted to in vivo DCs via the C-type lectin DEC-205 together with anti-CD40 agonistic antibody results in antigen-specific CD8+ T cell responses in mice [125]. In the absence of adjuvant, however, targeting protein antigens to in vivo DCs via DEC205 resulted in antigen-specific immune tolerance [34, 35]. In the last decade, studies have shown that the DC-targeting vaccine strategy is an efficient way to mount both cellular and humoral immunity [125–127]. The efficiency of antigen presentation on MHC class I and II molecules was increased approximately 100-fold by targeting protein antigens to the DC surface receptors compared to protein antigen without targeting [125, 128, 129]. In addition to DEC205, studies have shown that protein antigens targeted to DCs via a number of other cell surface receptors, such as DC-SIGN [130], CD1b [131], LOX-1 [132], and the mannose receptor (MR) [133–136] are able to elicit antigen-specific immune responses in vitro and in vivo. Table 1 summarizes receptors that are currently being investigated for the design of antibody-based in vivo DC-targeting vaccines. Human trials with prototypes of DC-targeting vaccines composed of anti-DEC205 and anti-MR antibodies are currently ongoing.
Table 1.
Current studies on the antibody-based in vivo DC targeting vaccines.
| Targeting receptors |
Experimental models |
Antigens | Adjuvants | Immune responses |
References |
|---|---|---|---|---|---|
| Mannose Receptor (MR) | Mouse: Human MR transgenic mouse | OVA | CpG | Abs, CD4+ and CD8+ T cells | [153, 154] |
| Human in vitro | Chorionic gonadotropin beta subunit | CD40 ligand | CD4+ and CD8+ T cells | [155] | |
| NY-ESO-1 | CD4+ and CD8+ T cells | [156] | |||
| pmel17 | CD4+ and CD8+ T cells | [136] | |||
| DEC205 | Mouse | OVA | Agonistic anti-CD40 mAb | CD4+ and CD8+ T cells | [34, 35, 125] |
| HIVgag | Poly IC and agonistic anti-CD40 mAb | CD4+ T cells | [128] | ||
| Human in vitro | NY-ESO-1 | CD4+ and CD8+ T cells | [156] | ||
| HIVgag-p24 | CD4+ and CD8+ T cells | [157] | |||
| DC-SIGN | Mouse | OVA | LPS | CD4+ and CD8 T cells | [158] |
| Human in vitro | KLH | CD4+ and CD8+ T cells | [159] | ||
| LOX-1 | Mouse cancer model | OVA | CD8+ T cells | [132] | |
| Human in vitro | Influenza HA1 and PSA | CD4+ T cells | [43] | ||
| DCIR | Human in vitro | Influenza M1, MART-1, and HIVgag | TLR7/8 | CD8+ T cells | [138] |
| Langerin | Mouse | HIV Gag-p24 | Agonistic anti-CD40 | CD8+ T cells | [160] |
| Dectin-1 | Mouse | OVA | TLR3 ligand | CD4+ and CD8+ T cells | [161] |
| Human in vitro | MART-1 peptide and Influenza M1 | CD8+ T cells | [137] | ||
| Tumor cells and CMV | TLR2 and TLR7 ligands | CD4+ and CD8+ T cells | [162] | ||
| Clec9A | Mouse | OVA | CpG, Poly IC, and LPS | Abs, Follicular CD4+, CD8+ T cells | [126] |
| CD40 | Human in vitro | NY-ESO peptide Influenza M1 and CMV peptides | CD8+ T cells | [139] [139] |
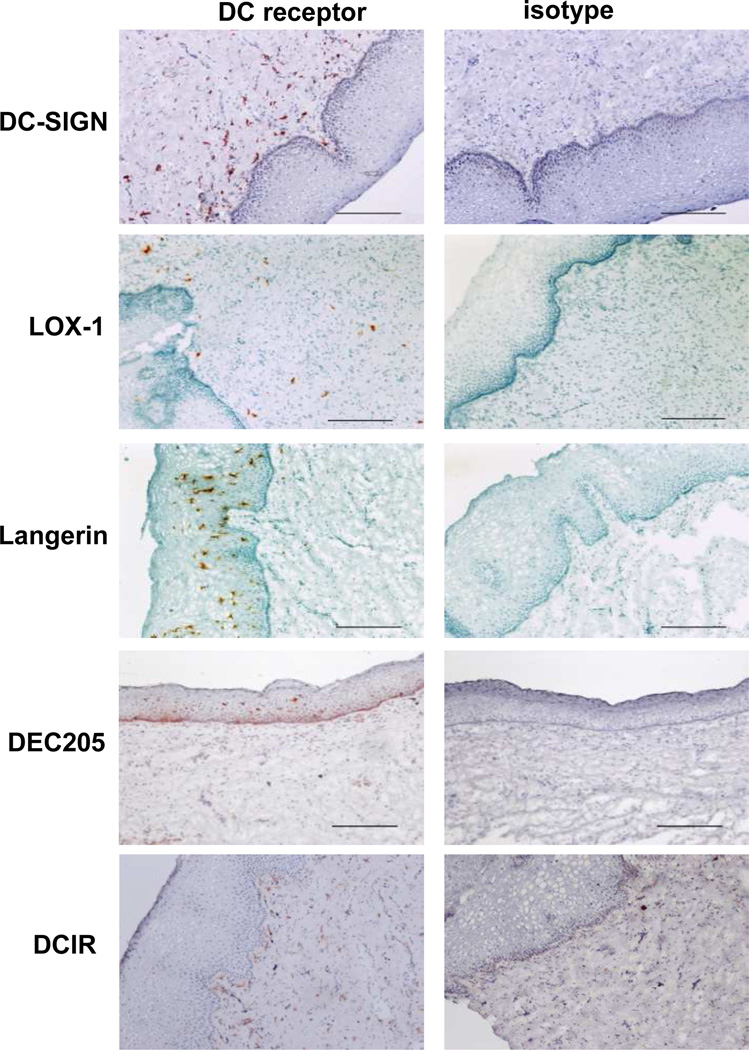
In principle, we can apply the current concept that has been developed and tested by others and by us for the development of DC targeting vaccines against sexually-transmitted pathogens and diseases, such as cervical cancer. To design optimal DC-targeting vaccines that can elicit potent and protective cellular and humoral immunity in the female genital tract, however, there are still a number of questions that need to be addressed. First, the receptor targeted by vaccines should be preferentially expressed on DCs, particularly on the surface of the selected DC subsets. This allows for further induction of the desired types of immune responses without triggering unwanted types of responses. Receptors targeted by the current experimental vaccine models are summarized in Table 1. Data from our studies show that C-type lectins such as LOX-1 [43], Dectin-1 [137], DC-ASGPR [43], and DC-SIGN (not shown) are mainly expressed on dermal DCs in the human skin, while DCIR is expressed on both LCs and dermal DCs [138]. However, the expression of such candidate receptors on the surface of human vaginal APCs are yet to be fully investigated. Our recent data show that cells expressing DC-SIGN, LOX-1, and DCIR are mainly localized in the LP of the human vaginal mucosa (Fig. 2). Both DEC205 and Langerin are mainly expressed on the cells in the epithelium. Data in Fig. 2 also suggests that cells expressing DC-SIGN, LOX-1, and DCIR are not necessarily the same. It is assumed that subsets of DCs and macrophages in the human vaginal LP express shared as well as distinct C-type lectins. Taken together, these data suggest that vaccines targeting selected subsets of the vaginal APCs could be designed. However, the expression of DC surface receptors on the subsets of the vaginal APCs is yet to be more extensively investigated.
Figure 2. DC receptors expressed in human vagina mucosa.
Immunohistochemistry staining of frozen tissue sections with anti-DC-SIGN (clone 15C4, in house), anti-LOX-1 (clone 15C4, in house), anti-Langerin (clone 15E2, in house), anti-DEC205 (clone MG38, eBioscience), anti-DCIR (clone 9E8, in house) antibodies or isotype controls. Digital images were taken using an Olympus BX60 with a UPlanFl 10×/0.30 Ph1 objective, a Nikon Digital Camera DXM 1200C camera and Nikon Elements software (Nikon). (×10, bar is 100µm)
Second, antigens targeted to the receptor should be properly internalized, processed and presented in both MHC classes I and II. For instance, CD40 and MR targeted antibody conjugates to early endosomes, whereas DEC205 targeted antigen primarily to late compartments [139]. The receptor least efficient at internalization, CD40, was the most efficient at cross presentation. This did not reflect DC activation by CD40, but rather its relatively poor uptake or intra-endosomal degradation compared with MR or DEC205. These features of individual candidate receptors are crucial elements that certainly need to be considered for the development of optimal DC-targeting vaccines.
Third, selection of optimal adjuvants that can further promote the functional specialties of the targeted DC subsets will be another critical factor for the design of DC-targeting vaccines. Thus, strategies aimed at inducing immunity require more than just targeting of antigen to the selected subsets of APCs. In addition to the types of adjuvants, timing of antigen delivery and maturation stimulus are also crucial for eliciting strong immune responses that lead to the establishment of protective immunity. Data from the first phase 1 trials assessing in vivo DC-targeting vaccines composed of anti-MR antibody show that stronger immune responses and longer durations of immune responses are observed when the vaccines are co-administered locally with TLR agonists [140]. For safety reasons, adjuvants will not be given systemically. Blander and Medzhitov have also reported that the efficacy of antigen presentation from phagocytosed cargo largely rely on the presence of TLR ligands within the same cargo [141]. This could be due to the governing of MHC class II-restricted antigen presentation by TLR-induced signals occurring in the phagosomes as antigens are delivered. Another study also reported that conjugating antigens with TLR agonists enhanced the magnitude and quality of Th1-type CD4+ T cells and CD8+ T cell responses in nonhuman primates [142]. However, if engagement of several DC surface receptors by anti-DC surface receptors alone triggers immunostimulatory signaling pathways, this could preclude the need of adjuvant.
Fourth, vaccines need to contain proper antigenic components that can bring protective immune responses. Thus, appropriate antigens for targeted microbial pathogens must be carefully chosen. Antigens of sexually-transmitted pathogens and the types of immune responses, humoral and cellular, to the pathogens are summarized in Table 2. These protein antigens can be incorporated into the vaccine prototype, but need to be further tested.
Table 2.
Protein antigens of sexually-transmitted microorganisms
| Pathogen | Protein Ags | Type of immune responses | References | |
|---|---|---|---|---|
| T cell responses |
B cell responses |
|||
| HIV | gp41, gp120, gp160, gag, pol, nef | yes | yes | [163–165] |
| HPV | E6, E7, L1 | yes | yes | [166–170] |
| HSV-2 | gpB, gpD and gpG | yes | yes | [171–174] |
| Chlamydia trachomatis | CprA, OMP2, MOMP, CT144, CT823, PorB, PmpD, pgp3 | yes | yes | [175–179] |
| Candida albicans | Als1/Als3, MP65, Fba, Met6, Hwp1, | yes | [180–182] | |
| Neisseria gonorrhoeae | Opa | yes | [183, 184] | |
| Treponema pallidum | Trp, Tp92 | yes | yes | [185–187] |
| HCV | HCV-core, NS3, NS4, NS5, lipopeptides | yes | yes | [188, 189] |
Finally, the efficiency with which the antigen is delivered to the DCs is largely dependent on the choice of immunization routes. In general, DCs originating from a specific tissue have the ability to instruct lymphocytes to home back to that tissue [143]. Different DC subsets in the same tissues might provide even more detailed instructions. Thus, the rationale for the IVAG administration of DC targeting vaccines can be well accepted. Furthermore, such mucosal vaccines are expected to elicit strong mucosal immune responses followed by the establishment of protective immunity in the female genital tract. However, it is important to keep in mind that the microenvironment in the lower female genital tract is unique in terms of the types of biological factors that could influence the outcomes of vaccine-induced immune responses. Female sex hormones [92, 144, 145], use of hormonal contraception [146, 147] and depo-medroxyprogesterone as well as pregnancy [148], could certainly alter the functions of APCs in the vagina. Epithelial cells in the vaginal mucosa express a variety of TLRs [149–151], and so the immune responses elicited by IVAG administration of vaccines with free TLR ligands could be influenced by the epithelial cells [92, 93, 145, 152]. To avoid or minimize such effects, adjuvants may need to be conjugated to the vaccines. However, it is still important to ensure that the adjuvants do not dampen the types of immune responses elicited by the subsets of DCs targeted by the vaccines. In addition, the presence of soluble factors (reviewed in [87]), including interleukin (IL)-1α, IL-6, and transforming growth factor-β, secreted from epithelial cells also need to be considered.
In conclusion, the DC targeting protein vaccines described in this review are a potential new vaccine platform. Both antigens and adjuvants can be carried by antibodies specific for the selected vaginal DC subsets. There are still several scientific and practical questions, such as vaccine formulation and delivery, that need to be better understood. However, it is certainly possible that vaccines designed based on our current knowledge on the DCs could establish protective immunity in the female genital tract that could prevent sexually-transmitted microbial infections. These vaccines can also be considered in males, too.
ACKNOWLEDGMENTS
We thank Dr. Carson Harrod (BIIR) for reading and editing this manuscript. This study was funded by 1RC1AI087379-01 (NIH), 1RC2A148460-01 (NIH), and U19 AI057234 (NIH), and by the Baylor Health Care System Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting interest
REFERENCES
- 1.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, et al. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–1516. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munz C, Dao T, Ferlazzo G, de Cos MA, Goodman K, Young JW. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105:266–273. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 8.Joo H, Coquery C, Xue Y, Gayet I, Dillon SR, Punaro M, et al. Serum from patients with SLE instructs monocytes to promote IgG and IgA plasmablast differentiation. J Exp Med. 2012;209:1335–1348. doi: 10.1084/jem.20111644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 11.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 12.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 13.Delamarre L, Mellman I. Harnessing dendritic cells for immunotherapy. Semin Immunol. 2011;23:2–11. doi: 10.1016/j.smim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 15.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez D, Harder G, Fattouh R, Sun J, Goncharova S, Stampfli MR, et al. Cutaneous antigen priming via gene gun leads to skin-selective Th2 immune-inflammatory responses. J Immunol. 2005;174:1664–1674. doi: 10.4049/jimmunol.174.3.1664. [DOI] [PubMed] [Google Scholar]
- 17.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, et al. Functional Specializations of Human Epidermal Langerhans Cells and CD14(+) Dermal Dendritic Cells. Immunity. 2008 doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathers AR, Janelsins BM, Rubin JP, Tkacheva OA, Shufesky WJ, Watkins SC, et al. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J Immunol. 2009;182:921–933. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- 19.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci U S A. 2009;106:21795–21800. doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 23.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 24.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 25.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 27.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 29.Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, et al. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 31.Moser M. Dendritic cells in immunity and tolerance-do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/s1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 32.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 33.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 37.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 40.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med. 2002;196:1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 49.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med. 2007;204:1923–1933. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 52.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class. I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 54.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 55.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 57.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 59.Allam JP, Bieber T, Novak N. Dendritic cells as potential targets for mucosal immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9:554–557. doi: 10.1097/ACI.0b013e32833239a9. [DOI] [PubMed] [Google Scholar]
- 60.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 62.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 65.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johansson C, Kelsall BL. Phenotype and function of intestinal dendritic cells. Semin Immunol. 2005;17:284–294. doi: 10.1016/j.smim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 69.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 72.Duluc D, Gannevat J, Anguiano E, Zurawski S, Carley M, Boreham M, et al. Functional diversity of human vaginal APC subsets in directing T cell responses Mucosal. Immunology. 2012 doi: 10.1038/mi.2012.104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004;113:998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dell K, Koesters R, Gissmann L. Transcutaneous immunization in mice: induction of T-helper and cytotoxic T lymphocyte responses and protection against human papillomavirus-induced tumors. Int J Cancer. 2006;118:364–372. doi: 10.1002/ijc.21360. [DOI] [PubMed] [Google Scholar]
- 75.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flacher V, Tripp CH, Stoitzner P, Haid B, Ebner S, Del Frari B, et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol. 2010;130:755–762. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 81.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 82.Ritter U, Meissner A, Scheidig C, Korner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 83.Brewig N, Kissenpfennig A, Malissen B, Veit A, Bickert T, Fleischer B, et al. Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J Immunol. 2009;182:774–783. doi: 10.4049/jimmunol.182.2.774. [DOI] [PubMed] [Google Scholar]
- 84.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bedoui S, Davey GM, Lew AM, Heath WR. Equivalent stimulation of naive and memory CD8 T cells by DNA vaccination: a dendritic cell-dependent process. Immunol Cell Biol. 2009;87:255–259. doi: 10.1038/icb.2008.105. [DOI] [PubMed] [Google Scholar]
- 86.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–480. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 87.Iijima N, Thompson JM, Iwasaki A. Dendritic cells and macrophages in the genitourinary tract. Mucosal Immunol. 2008;1:451–459. doi: 10.1038/mi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96:431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 90.Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997;36:23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 91.Johansson M, Lycke NY. Immunology of the human genital tract. Curr Opin Infect Dis. 2003;16:43–49. doi: 10.1097/00001432-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Kaushic C. The role of the local microenvironment in regulating susceptibility and immune responses to sexually transmitted viruses in the female genital tract. J Reprod Immunol. 2009;83:168–172. doi: 10.1016/j.jri.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 93.Kumamoto Y, Iwasaki A. Unique features of antiviral immune system of the vaginal mucosa. Curr Opin Immunol. 2012;24:411–416. doi: 10.1016/j.coi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mestecky J, Moldoveanu Z, Smith PD, Hel Z, Alexander RC. Mucosal immunology of the genital and gastrointestinal tracts and HIV-1 infection. J Reprod Immunol. 2009;83:196–200. doi: 10.1016/j.jri.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Russell MW, Mestecky J. Tolerance and protection against infection in the genital tract. Immunol Invest. 2010;39:500–525. doi: 10.3109/08820131003674834. [DOI] [PubMed] [Google Scholar]
- 96.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 97.Parr EL, Parr MB. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology. 1999;98:639–645. doi: 10.1046/j.1365-2567.1999.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gillgrass AE, Tang VA, Towarnicki KM, Rosenthal KL, Kaushic C. Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue. J Virol. 2005;79:3117–3126. doi: 10.1128/JVI.79.5.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu HY, Abdu S, Stinson D, Russell MW. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect Immun. 2000;68:5539–5545. doi: 10.1128/iai.68.10.5539-5545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iwasaki A. The role of dendritic cells in immune responses against vaginal infection by herpes simplex virus type 2. Microbes Infect. 2003;5:1221–1230. doi: 10.1016/j.micinf.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 101.Hopkins WJ, Elkahwaji J, Beierle LM, Leverson GE, Uehling DT. Vaginal mucosal vaccine for recurrent urinary tract infections in women: results of a phase 2 clinical trial. J Urol. 2007;177:1349–1353. doi: 10.1016/j.juro.2006.11.093. quiz 591. [DOI] [PubMed] [Google Scholar]
- 102.Lewis DJ, Fraser CA, Mahmoud AN, Wiggins RC, Woodrow M, Cope A, et al. Phase I randomised clinical trial of an HIV-1(CN54), clade C, trimeric envelope vaccine candidate delivered vaginally. PLoS ONE. 2011;6:e25165. doi: 10.1371/journal.pone.0025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uehling DT, Hopkins WJ, Balish E, Xing Y, Heisey DM. Vaginal mucosal immunization for recurrent urinary tract infection: phase II clinical trial. J Urol. 1997;157:2049–2052. [PubMed] [Google Scholar]
- 104.Ogra PL, Ogra SS. Local antibody response to poliovaccine in the human female genital tract. J Immunol. 1973;110:1307–1311. [PubMed] [Google Scholar]
- 105.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parr MB, Parr EL. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491–498. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- 107.Iijima N, Linehan MM, Saeland S, Iwasaki A. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc Natl Acad Sci U S A. 2007;104:19061–19066. doi: 10.1073/pnas.0707179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 112.Hussain LA, Kelly CG, Fellowes R, Hecht EM, Wilson J, Chapman M, et al. Expression and gene transcript of Fc receptors for IgG, HLA class II antigens and Langerhans cells in human cervico-vaginal epithelium. Clin Exp Immunol. 1992;90:530–538. doi: 10.1111/j.1365-2249.1992.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, Munoz-Molina R, Balderas-Carrillo O, de la Luz Diaz-Soberanes M, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans' cells in the human female genital tract. Immunology. 2006;117:220–228. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duluc D, Gannevat J, Anguiano E, Zurawski S, Carley M, Boreham M, et al. Functional diversity of human vaginal APC subsets in directing T-cell responses. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Williams IR. Chemokine receptors and leukocyte trafficking in the mucosal immune system. Immunol Res. 2004;29:283–292. doi: 10.1385/IR:29:1-3:283. [DOI] [PubMed] [Google Scholar]
- 118.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 119.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furio L, Briotet I, Journeaux A, Billard H, Peguet-Navarro J. Human langerhans cells are more efficient than CD14(−)CD1c(+) dermal dendritic cells at priming naive CD4(+) T cells. J Invest Dermatol. 2010;130:1345–1354. doi: 10.1038/jid.2009.424. [DOI] [PubMed] [Google Scholar]
- 122.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 123.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 124.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 125.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol. 2011;187:842–850. doi: 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- 127.Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bozzacco L, Trumpfheller C, Huang Y, Longhi MP, Shimeliovich I, Schauer JD, et al. HIV gag protein is efficiently cross-presented when targeted with an antibody towards the DEC-205 receptor in Flt3 ligand-mobilized murine DC. Eur J Immunol. 2010;40:36–46. doi: 10.1002/eji.200939748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 131.Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 132.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 133.Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit EC, Lanzavecchia A, et al. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 134.Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ, Verwoerd D, et al. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 135.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 136.Ramakrishna V, Treml JF, Vitale L, Connolly JE, O'Neill T, Smith PA, et al. Mannose receptor targeting of tumor antigen pmel17 to human dendritic cells directs anti-melanoma T cell responses via multiple HLA molecules. J Immunol. 2004;172:2845–2852. doi: 10.4049/jimmunol.172.5.2845. [DOI] [PubMed] [Google Scholar]
- 137.Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, et al. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185:3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar A, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, et al. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 140.Morse MA, Chapman R, Powderly J, Blackwell K, Keler T, Green J, et al. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res. 2011;17:4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 142.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 144.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaushic C, Roth KL, Anipindi V, Xiu F. Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J Reprod Immunol. 2011;88:204–209. doi: 10.1016/j.jri.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 146.Lavreys L, Baeten JM, Martin HL, Jr, Overbaugh J, Mandaliya K, Ndinya-Achola J, et al. Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS. 2004;18:695–697. doi: 10.1097/00002030-200403050-00017. [DOI] [PubMed] [Google Scholar]
- 147.Wang CC, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K, et al. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–209. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- 148.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 149.Andersen JM, Al-Khairy D, Ingalls RR. Innate immunity at the mucosal surface: role of toll-like receptor 3 and toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol Reprod. 2006;74:824–831. doi: 10.1095/biolreprod.105.048629. [DOI] [PubMed] [Google Scholar]
- 150.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–2432. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 151.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 152.Ferreira VH, Nazli A, Khan G, Mian MF, Ashkar AA, Gray-Owen S, et al. Endometrial epithelial cell responses to coinfecting viral and bacterial pathogens in the genital tract can activate the HIV-1 LTR in an NF{kappa}B-and AP-1-dependent manner. J Infect Dis. 2011;204:299–308. doi: 10.1093/infdis/jir260. [DOI] [PubMed] [Google Scholar]
- 153.Dasgupta S, Bayry J, Lacroix-Desmazes S, Kaveri SV. Human mannose receptor (CD206) in immune response: novel insights into vaccination strategies using a humanized mouse model. Expert Rev Clin Immunol. 2007;3:677–681. doi: 10.1586/1744666X.3.5.677. [DOI] [PubMed] [Google Scholar]
- 154.He LZ, Crocker A, Lee J, Mendoza-Ramirez J, Wang XT, Vitale LA, et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J Immunol. 2007;178:6259–6267. doi: 10.4049/jimmunol.178.10.6259. [DOI] [PubMed] [Google Scholar]
- 155.He LZ, Ramakrishna V, Connolly JE, Wang XT, Smith PA, Jones CL, et al. A novel human cancer vaccine elicits cellular responses to the tumor-associated antigen, human chorionic gonadotropin beta. Clin Cancer Res. 2004;10:1920–1927. doi: 10.1158/1078-0432.ccr-03-0264. [DOI] [PubMed] [Google Scholar]
- 156.Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011;186:1218–1227. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- 157.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tacken PJ, Ginter W, Berod L, Cruz LJ, Joosten B, Sparwasser T, et al. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood. 2011;118:4111–4119. doi: 10.1182/blood-2011-04-346957. [DOI] [PubMed] [Google Scholar]
- 159.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 160.Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci U S A. 2011;108:2384–2389. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 162.Weck MM, Appel S, Werth D, Sinzger C, Bringmann A, Grunebach F, et al. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 2008;111:4264–4272. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- 163.Zolla-Pazner S. Identifying epitopes of HIV-1 that induce protective antibodies. Nat Rev Immunol. 2004;4:199–210. doi: 10.1038/nri1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Salmon-Ceron D, Durier C, Desaint C, Cuzin L, Surenaud M, Hamouda NB, et al. Immunogenicity and safety of an HIV-1 lipopeptide vaccine in healthy adults: a phase 2 placebo-controlled ANRS trial. AIDS. 2010;24:2211–2223. doi: 10.1097/QAD.0b013e32833ce566. [DOI] [PubMed] [Google Scholar]
- 165.Gorse GJ, Rogers JH, Perry JE, Newman FK, Frey SE, Patel GB, et al. HIV-1 recombinant gp160 vaccine induced antibodies in serum and saliva. The NIAID AIDS Vaccine Clinical Trials Network. Vaccine. 1995;13:209–214. doi: 10.1016/0264-410x(95)93138-y. [DOI] [PubMed] [Google Scholar]
- 166.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 167.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 168.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 169.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93:284–292. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 170.Trimble CL, Frazer IH. Development of therapeutic HPV vaccines. Lancet Oncol. 2009;10:975–980. doi: 10.1016/S1470-2045(09)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gorander S, Harandi AM, Lindqvist M, Bergstrom T, Liljeqvist JA. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J Virol. 2012;86:7544–7553. doi: 10.1128/JVI.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011;121:4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim M, Taylor J, Sidney J, Mikloska Z, Bodsworth N, Lagios K, et al. Immunodominant epitopes in herpes simplex virus type 2 glycoprotein D are recognized by CD4 lymphocytes from both HSV-1 and HSV-2 seropositive subjects. J Immunol. 2008;181:6604–6615. doi: 10.4049/jimmunol.181.9.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu K, Jiang D, Zhang L, Yao Z, Chen Z, Yu S, et al. Identification of B- and T-cell epitopes from glycoprotein B of herpes simplex virus 2 and evaluation of their immunogenicity and protection efficacy. Vaccine. 2012;30:3034–3041. doi: 10.1016/j.vaccine.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 175.Picard MD, Cohane KP, Gierahn TM, Higgins DE, Flechtner JB. High-throughput proteomic screening identifies Chlamydia trachomatis antigens that are capable of eliciting T cell and antibody responses that provide protection against vaginal challenge. Vaccine. 2012;30:4387–4393. doi: 10.1016/j.vaccine.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 176.Starnbach MN, Loomis WP, Ovendale P, Regan D, Hess B, Alderson MR, et al. An inclusion membrane protein from Chlamydia trachomatis enters the MHC class I pathway and stimulates a CD8+ T cell response. J Immunol. 2003;171:4742–4749. doi: 10.4049/jimmunol.171.9.4742. [DOI] [PubMed] [Google Scholar]
- 177.Eko FO, He Q, Brown T, McMillan L, Ifere GO, Ananaba GA, et al. A novel recombinant multisubunit vaccine against Chlamydia. J Immunol. 2004;173:3375–3382. doi: 10.4049/jimmunol.173.5.3375. [DOI] [PubMed] [Google Scholar]
- 178.Eko FO, Okenu DN, Singh UP, He Q, Black C, Igietseme JU. Evaluation of a broadly protective Chlamydia-cholera combination vaccine candidate. Vaccine. 2011;29:3802–3810. doi: 10.1016/j.vaccine.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Donati M, Sambri V, Comanducci M, Di Leo K, Storni E, Giacani L, et al. DNA immunization with pgp3 gene of Chlamydia trachomatis inhibits the spread of chlamydial infection from the lower to the upper genital tract in C3H/HeN mice. Vaccine. 2003;21:1089–1093. doi: 10.1016/s0264-410x(02)00631-x. [DOI] [PubMed] [Google Scholar]
- 180.Bar E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, et al. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol. 2012;188:5636–5643. doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- 181.Spellberg B, Ibrahim AS, Lin L, Avanesian V, Fu Y, Lipke P, et al. Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J Infect Dis. 2008;197:967–971. doi: 10.1086/529204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xin H, Dziadek S, Bundle DR, Cutler JE. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc Natl Acad Sci U S A. 2008;105:13526–13531. doi: 10.1073/pnas.0803195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Plummer FA, Chubb H, Simonsen JN, Bosire M, Slaney L, Nagelkerke NJ, et al. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J Clin Invest. 1994;93:1748–1755. doi: 10.1172/JCI117159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu Y, Feinen B, Russell MW. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol. 2011;2:52. doi: 10.3389/fmicb.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morgan CA, Molini BJ, Lukehart SA, Van Voorhis WC. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J Immunol. 2002;169:952–957. doi: 10.4049/jimmunol.169.2.952. [DOI] [PubMed] [Google Scholar]
- 186.Giacani L, Molini B, Godornes C, Barrett L, Van Voorhis W, Centurion-Lara A, et al. Quantitative analysis of tpr gene expression in Treponema pallidum isolates: Differences among isolates and correlation with T-cell responsiveness in experimental syphilis. Infect Immun. 2007;75:104–112. doi: 10.1128/IAI.01124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis. 2000;181:1401–1413. doi: 10.1086/315399. [DOI] [PubMed] [Google Scholar]
- 188.Beauvillain C, Meloni F, Sirard JC, Blanchard S, Jarry U, Scotet M, et al. The scavenger receptors SRA-1 and SREC-I cooperate with TLR2 in the recognition of the hepatitis C virus non-structural protein 3 by dendritic cells. J Hepatol. 2010;52:644–651. doi: 10.1016/j.jhep.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 189.Zhou Y, Zhang Y, Yao Z, Moorman JP, Jia Z. Dendritic cell-based immunity and vaccination against hepatitis C virus infection. Immunology. 2012;136:385–396. doi: 10.1111/j.1365-2567.2012.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]