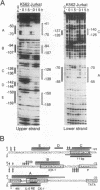
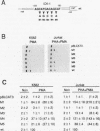
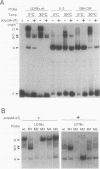
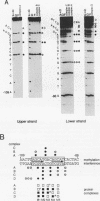
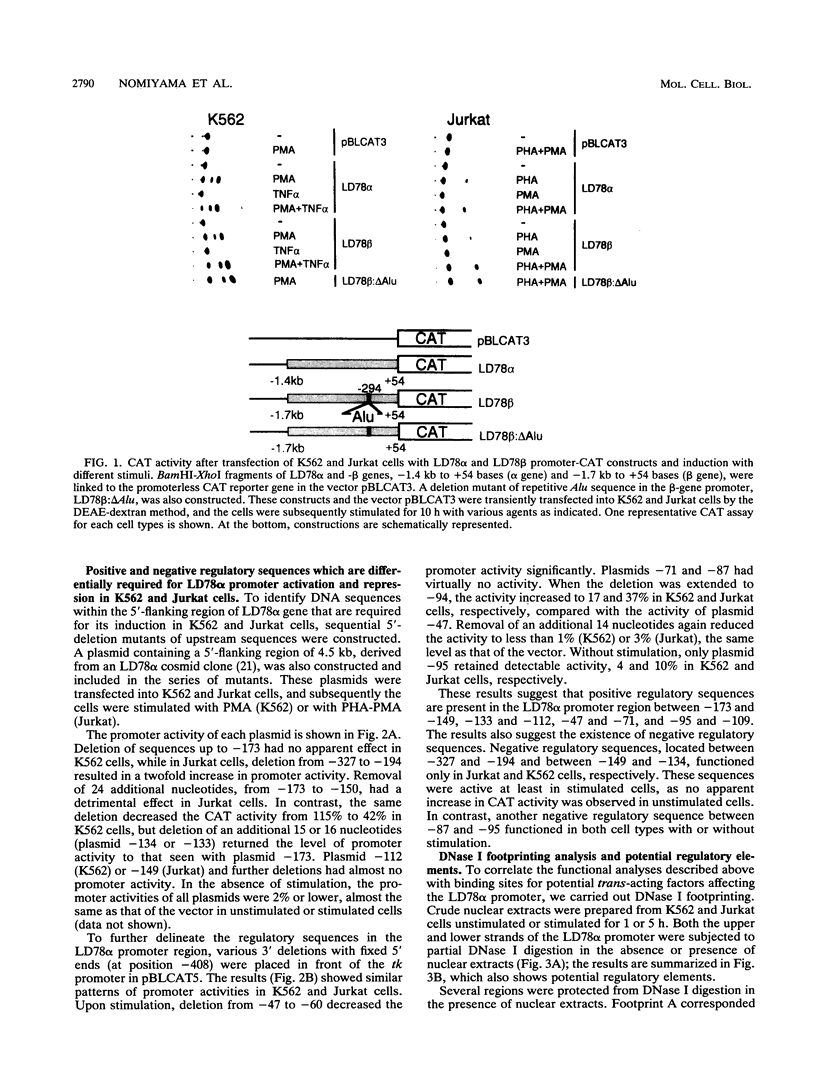
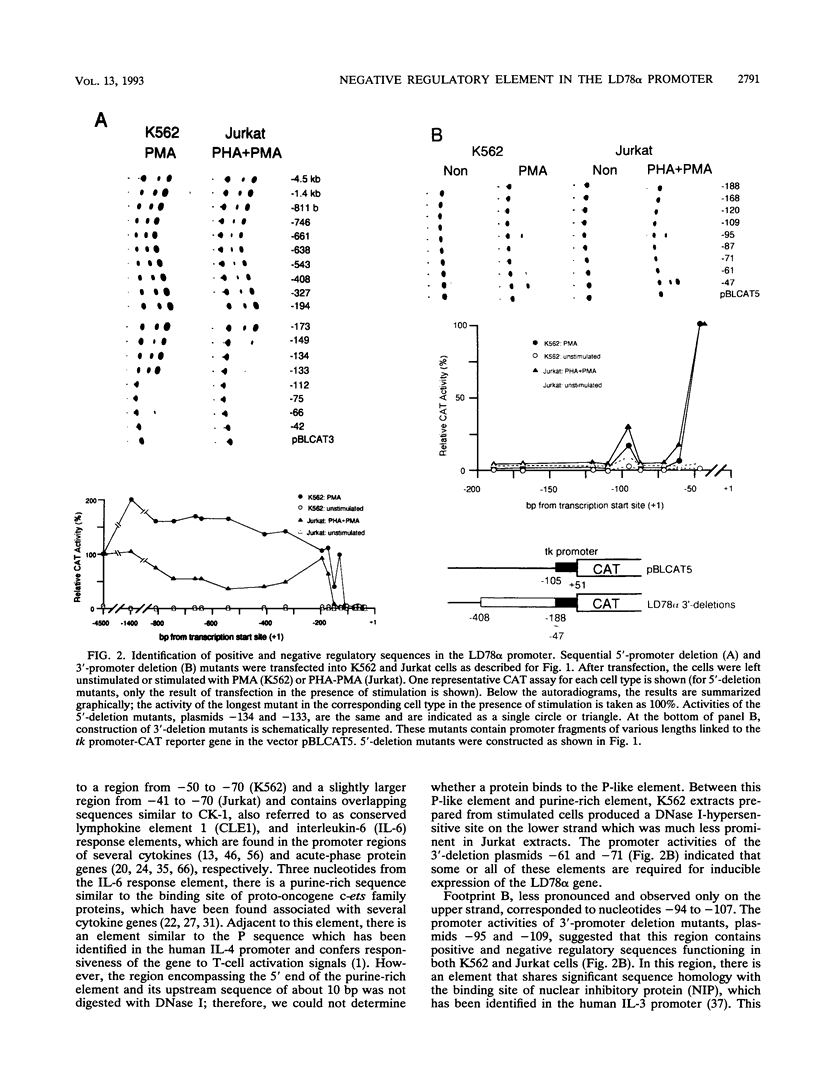
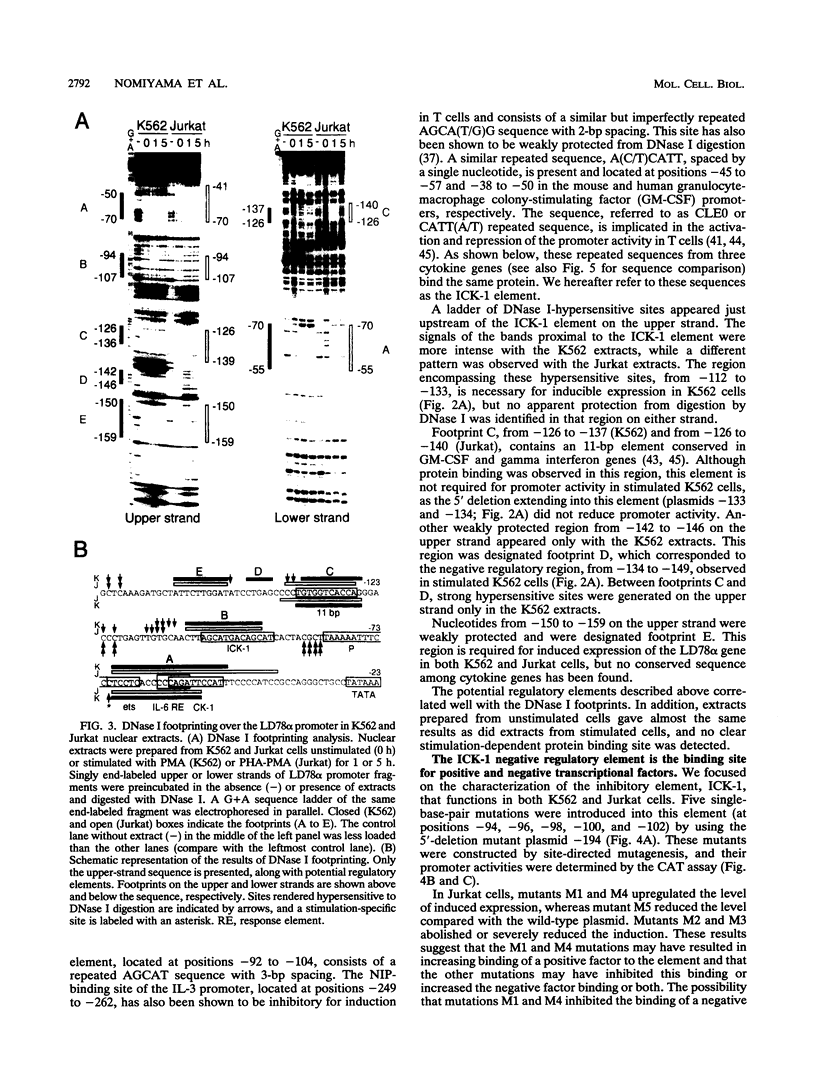
Abstract
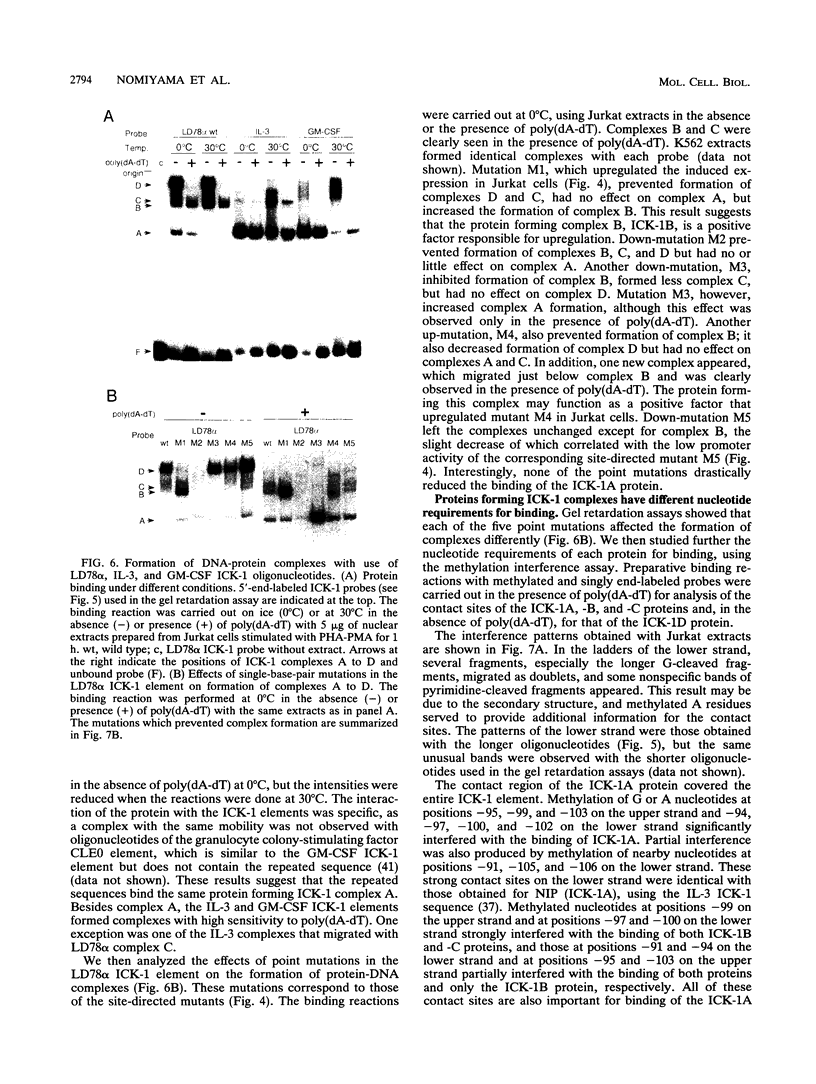
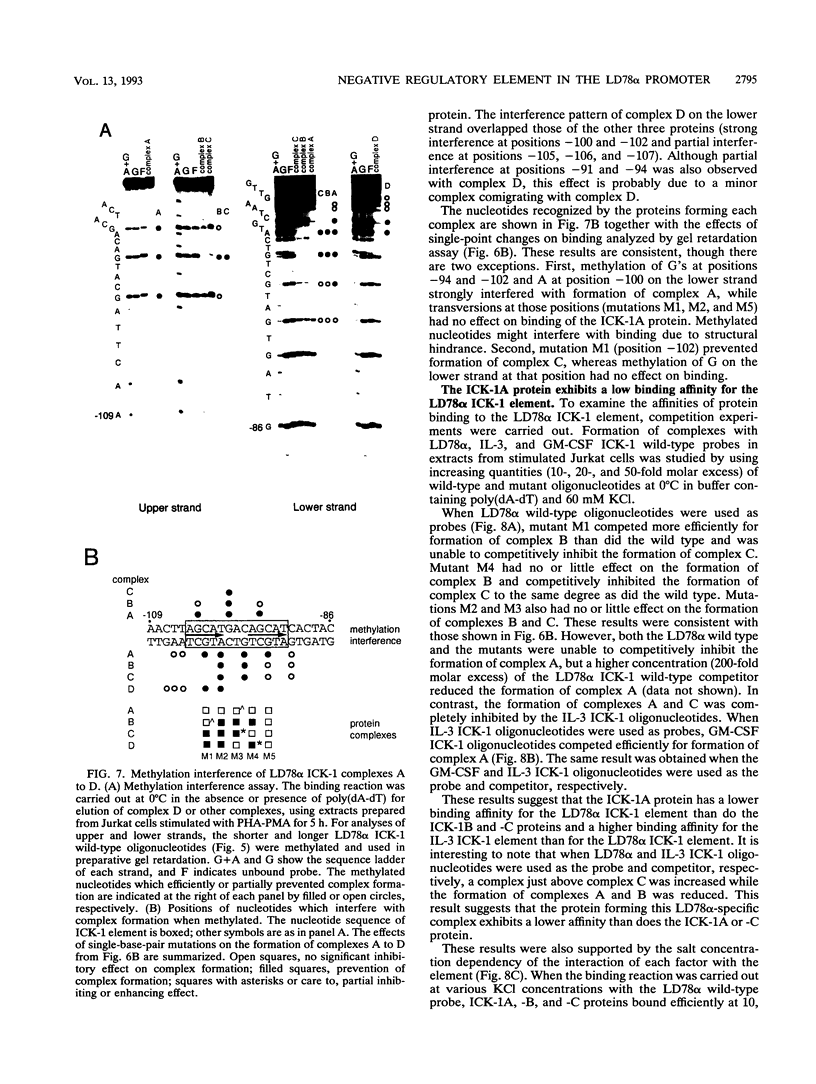
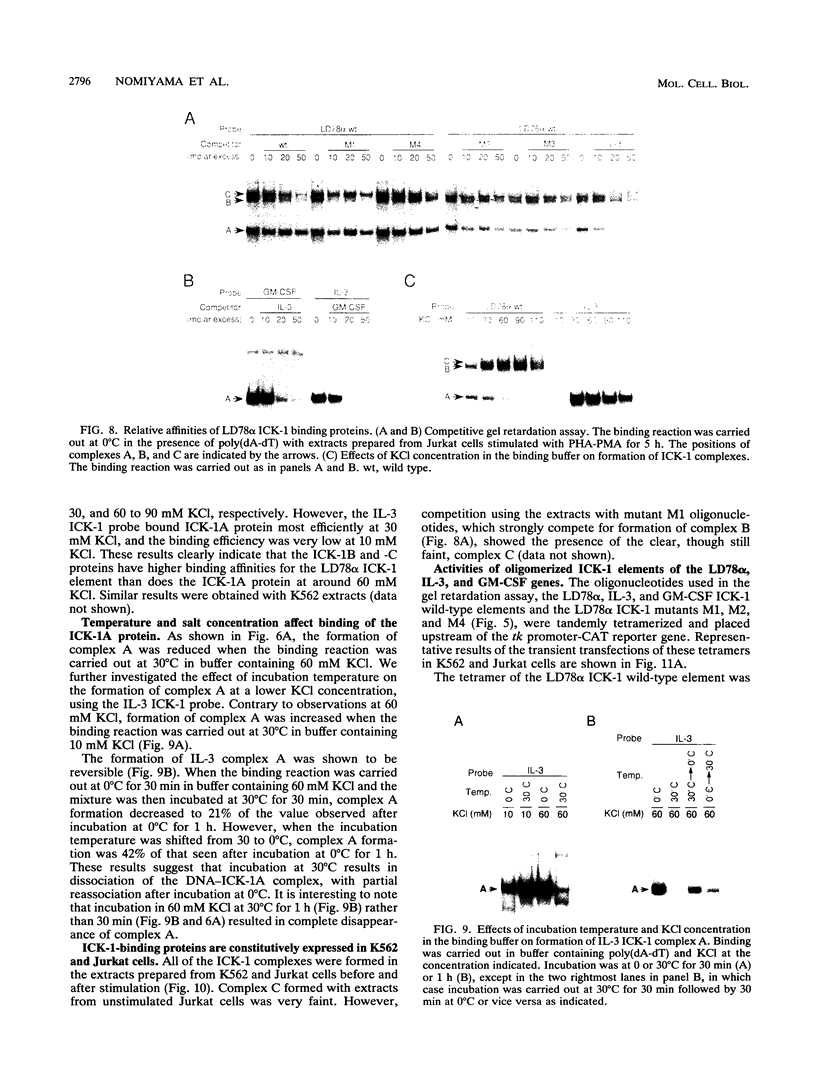
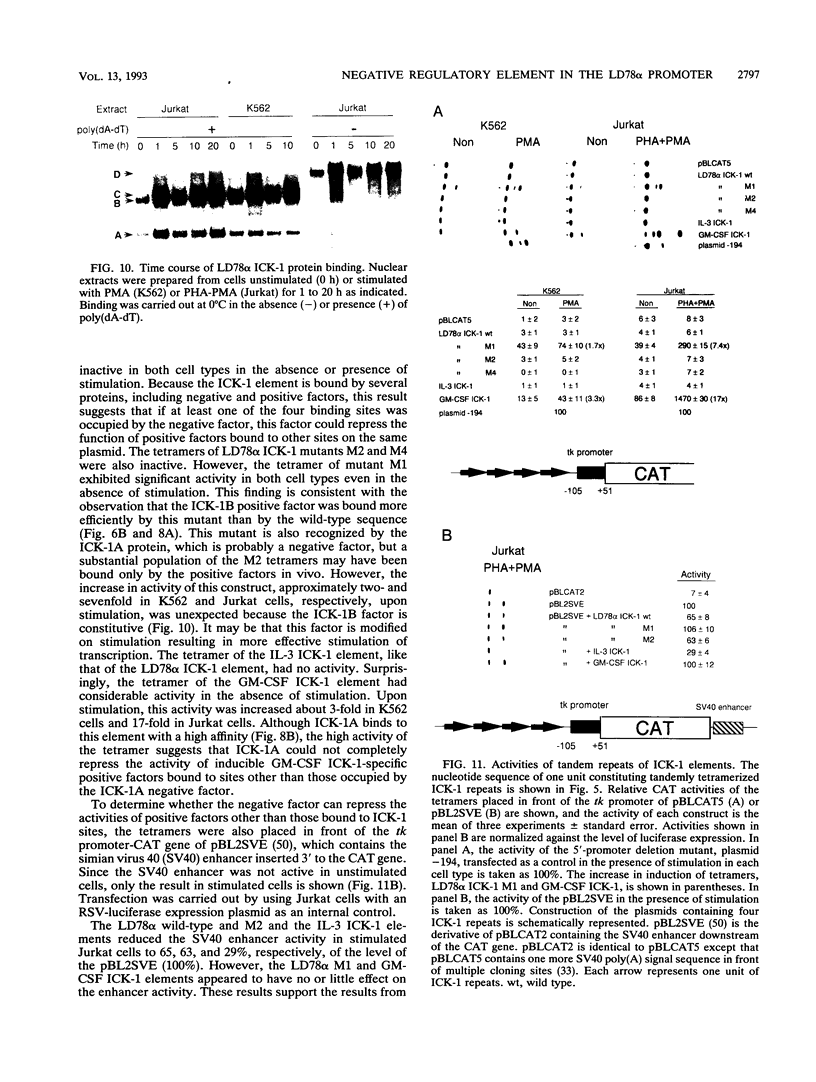
Cytokine LD78 is a human counterpart of the mouse macrophage inflammatory protein 1 alpha/hematopoietic stem cell inhibitor. Promoters of the LD78 alpha and LD78 beta genes showed similar inducible activities in two leukemic cell lines, K562 and Jurkat, but the induction mechanisms differed between the two cell lines. Further characterization of the LD78 alpha promoter indicated that multiple positive and negative regulatory elements are present, some of which are differentially required for induction and repression of the promoter activity in different cells. One of the negative regulatory elements, ICK-1, functioned in both cell lines in the absence and presence of stimulation and was shown to be a recognition site for positive and negative transcriptional factors. This ICK-1 element contained a direct repeat, and similar repeats were also found in the negative regulatory elements of hematopoietic growth factor interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) gene promoters. Nuclear extracts from K562 and Jurkat cells formed several protein-DNA complexes with the LD78 alpha ICK-1 element, one of which was also observed with the IL-3 and GM-CSF ICK-1 elements. Results from in vivo and in vitro analyses suggested that the protein forming this complex functions as a negative factor. The binding affinity of this protein, ICK-1A, to the LD78 alpha ICK-1 element was low and was significantly affected by the incubation temperature and the salt concentration in the binding buffer. ICK-1B, another protein bound specifically by the LD78 alpha ICK-1 element, was shown to be a positive factor important for induction of the promoter. These results suggested that ICK-1A plays an important role in balanced expression of LD78, IL-3, and GM-CSF during hematopoietic cell growth and differentiation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., De Waal Malefyt R., Matsuda I., Arai K., Arai N. An 11-base-pair DNA sequence motif apparently unique to the human interleukin 4 gene confers responsiveness to T-cell activation signals. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2864–2868. doi: 10.1073/pnas.89.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S., Forsdyke R. E., Forsdyke D. R. Three human homologs of a murine gene encoding an inhibitor of stem cell proliferation. DNA Cell Biol. 1990 Oct;9(8):589–602. doi: 10.1089/dna.1990.9.589. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., Crosier P. S., Clark S. C. Effects of hematopoietic growth factors on the survival of primitive stem cells in liquid suspension culture. Blood. 1991 Aug 15;78(4):914–920. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Sherry B., Lu L., Cooper S., Oh K. O., Tekamp-Olson P., Kwon B. S., Cerami A. Enhancing and suppressing effects of recombinant murine macrophage inflammatory proteins on colony formation in vitro by bone marrow myeloid progenitor cells. Blood. 1990 Sep 15;76(6):1110–1116. [PubMed] [Google Scholar]
- Burd P. R., Rogers H. W., Gordon J. R., Martin C. A., Jayaraman S., Wilson S. D., Dvorak A. M., Galli S. J., Dorf M. E. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989 Jul 1;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatelis G., Tekamp-Olson P., Wolpe S. D., Hermsen K., Luedke C., Gallegos C., Coit D., Merryweather J., Cerami A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med. 1988 Jun 1;167(6):1939–1944. doi: 10.1084/jem.167.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop D. J., Wright E. G., Lorimore S., Graham G. J., Holyoake T., Kerr D. J., Wolpe S. D., Pragnell I. B. Demonstration of stem cell inhibition and myeloprotective effects of SCI/rhMIP1 alpha in vivo. Blood. 1992 May 1;79(9):2221–2225. [PubMed] [Google Scholar]
- Fahey T. J., 3rd, Tracey K. J., Tekamp-Olson P., Cousens L. S., Jones W. G., Shires G. T., Cerami A., Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992 May 1;148(9):2764–2769. [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental C., Kanno M., Nomiyama H., Chambon P. Cooperativity and hierarchical levels of functional organization in the SV40 enhancer. Cell. 1988 Sep 23;54(7):943–953. doi: 10.1016/0092-8674(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham G. J., Pragnell I. B. SCI/MIP-1 alpha: a potent stem cell inhibitor with potential roles in development. Dev Biol. 1992 Jun;151(2):377–381. doi: 10.1016/0012-1606(92)90177-i. [DOI] [PubMed] [Google Scholar]
- Graham G. J., Wright E. G., Hewick R., Wolpe S. D., Wilkie N. M., Donaldson D., Lorimore S., Pragnell I. B. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990 Mar 29;344(6265):442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- Hattori M., Abraham L. J., Northemann W., Fey G. H. Acute-phase reaction induces a specific complex between hepatic nuclear proteins and the interleukin 6 response element of the rat alpha 2-macroglobulin gene. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2364–2368. doi: 10.1073/pnas.87.6.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M., Ono T., Nakao M., Nishi H., Kimura A., Nomiyama H., Hamada F., Yoshida M. C., Shimada K. Nucleotide sequence of the third cytokine LD78 gene and mapping of all three LD78 gene loci to human chromosome 17. DNA Seq. 1992;3(4):203–212. doi: 10.3109/10425179209034019. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Bhat N. K., Gottschalk L. R., Lindsten T., Thompson C. B., Papas T. S., Leiden J. M. Sequence-specific binding of human Ets-1 to the T cell receptor alpha gene enhancer. Science. 1990 Nov 9;250(4982):814–818. doi: 10.1126/science.2237431. [DOI] [PubMed] [Google Scholar]
- Irving S. G., Zipfel P. F., Balke J., McBride O. W., Morton C. C., Burd P. R., Siebenlist U., Kelly K. Two inflammatory mediator cytokine genes are closely linked and variably amplified on chromosome 17q. Nucleic Acids Res. 1990 Jun 11;18(11):3261–3270. doi: 10.1093/nar/18.11.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Tanahashi H., Misumi Y., Sakaki Y. Nuclear factors interacting with an interleukin-6 responsive element of rat alpha 2-macroglobulin gene. Nucleic Acids Res. 1989 Nov 25;17(22):9425–9435. doi: 10.1093/nar/17.22.9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kelly K., Davis P., Mitsuya H., Irving S., Wright J., Grassmann R., Fleckenstein B., Wano Y., Greene W., Siebenlist U. A high proportion of early response genes are constitutively activated in T cells by HTLV-I. Oncogene. 1992 Aug;7(8):1463–1470. [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- Kukita T., Nakao J., Hamada F., Kukita A., Inai T., Kurisu K., Nomiyama H. Recombinant LD78 protein, a member of the small cytokine family, enhances osteoclast differentiation in rat bone marrow culture system. Bone Miner. 1992 Dec;19(3):215–223. doi: 10.1016/0169-6009(92)90871-a. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lai C. F., Sigman D. S., Gaynor R. B. Cloning of a cellular factor, interleukin binding factor, that binds to NFAT-like motifs in the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7739–7743. doi: 10.1073/pnas.88.17.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord B. I., Dexter T. M., Clements J. M., Hunter M. A., Gearing A. J. Macrophage-inflammatory protein protects multipotent hematopoietic cells from the cytotoxic effects of hydroxyurea in vivo. Blood. 1992 May 15;79(10):2605–2609. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majello B., Arcone R., Toniatti C., Ciliberto G. Constitutive and IL-6-induced nuclear factors that interact with the human C-reactive protein promoter. EMBO J. 1990 Feb;9(2):457–465. doi: 10.1002/j.1460-2075.1990.tb08131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. A., Dorf M. E. Differential regulation of interleukin-6, macrophage inflammatory protein-1, and JE/MCP-1 cytokine expression in macrophage cell lines. Cell Immunol. 1991 Jun;135(1):245–258. doi: 10.1016/0008-8749(91)90269-h. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Andrews N. C., Murphy H. S., Kreissman S. G., Nathan D. G. Positive and negative elements regulate human interleukin 3 expression. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5046–5050. doi: 10.1073/pnas.87.13.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Otsuka T., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985 Oct;4(10):2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Shlomai J., Arai K., Arai N. Characterization of the mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) gene promoter: nuclear factors that interact with an element shared by three lymphokine genes--those for GM-CSF, interleukin-4 (IL-4), and IL-5. Mol Cell Biol. 1991 Dec;11(12):5894–5901. doi: 10.1128/mcb.11.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Nomiyama H., Shimada K. Structures of human genes coding for cytokine LD78 and their expression. Mol Cell Biol. 1990 Jul;10(7):3646–3658. doi: 10.1128/mcb.10.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer S. D., Morita E. A., Martis M. J., Wachsman W., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor promoter region by genetic analysis: correlation with DNase I footprinting. Mol Cell Biol. 1988 May;8(5):1979–1984. doi: 10.1128/mcb.8.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer S., Fraser J., Richards J., Lynch M., Gasson J. The repeated sequence CATT(A/T) is required for granulocyte-macrophage colony-stimulating factor promoter activity. Mol Cell Biol. 1990 Nov;10(11):6084–6088. doi: 10.1128/mcb.10.11.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Nagata S. Regulatory elements responsible for inducible expression of the granulocyte colony-stimulating factor gene in macrophages. Mol Cell Biol. 1990 May;10(5):2002–2011. doi: 10.1128/mcb.10.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaru K., Fukuda M., Maeda S., Shimada K. A cDNA clone used to study mRNA inducible in human tonsillar lymphocytes by a tumor promoter. J Biochem. 1986 Mar;99(3):885–894. doi: 10.1093/oxfordjournals.jbchem.a135549. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Boshart M., Bosch F. X., Schmid W., Fournier R. E., Schütz G. Two genetically defined trans-acting loci coordinately regulate overlapping sets of liver-specific genes. Cell. 1990 Jun 1;61(5):895–904. doi: 10.1016/0092-8674(90)90200-x. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Thurston S. J. A negative regulatory element with properties similar to those of enhancers is contained within an Alu sequence. Mol Cell Biol. 1989 Feb;9(2):355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991 May;3(3):165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Schall T. J., O'Hehir R. E., Goeddel D. V., Lamb J. R. Uncoupling of cytokine mRNA expression and protein secretion during the induction phase of T cell anergy. J Immunol. 1992 Jan 15;148(2):381–387. [PubMed] [Google Scholar]
- Shannon M. F., Pell L. M., Lenardo M. J., Kuczek E. S., Occhiodoro F. S., Dunn S. M., Vadas M. A. A novel tumor necrosis factor-responsive transcription factor which recognizes a regulatory element in hemopoietic growth factor genes. Mol Cell Biol. 1990 Jun;10(6):2950–2959. doi: 10.1128/mcb.10.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shiozaki H., Ide T., Nakao J., Imamura T., Nakamura M., Shimada K., Miura Y., Suda T. Suppressive effect of LD78 on the proliferation of human hemopoietic progenitors. Jpn J Cancer Res. 1992 May;83(5):499–504. doi: 10.1111/j.1349-7006.1992.tb01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. G., Hromas R., Kaushansky K. Transcriptional regulation of interleukin 3 gene expression in T lymphocytes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9650–9654. doi: 10.1073/pnas.87.24.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Tabilio A., Pelicci P. G., Vinci G., Mannoni P., Civin C. I., Vainchenker W., Testa U., Lipinski M., Rochant H., Breton-Gorius J. Myeloid and megakaryocytic properties of K-562 cell lines. Cancer Res. 1983 Oct;43(10):4569–4574. [PubMed] [Google Scholar]
- Tomilin N. V., Iguchi-Ariga S. M., Ariga H. Transcription and replication silencer element is present within conserved region of human Alu repeats interacting with nuclear protein. FEBS Lett. 1990 Apr 9;263(1):69–72. doi: 10.1016/0014-5793(90)80707-p. [DOI] [PubMed] [Google Scholar]
- Widmer U., Yang Z., van Deventer S., Manogue K. R., Sherry B., Cerami A. Genomic structure of murine macrophage inflammatory protein-1 alpha and conservation of potential regulatory sequences with a human homolog, LD78. J Immunol. 1991 Jun 1;146(11):4031–4040. [PubMed] [Google Scholar]
- Wilson D. R., Juan T. S., Wilde M. D., Fey G. H., Darlington G. J. A 58-base-pair region of the human C3 gene confers synergistic inducibility by interleukin-1 and interleukin-6. Mol Cell Biol. 1990 Dec;10(12):6181–6191. doi: 10.1128/mcb.10.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmann A., Hillen W. DNA contacts probed by modification protection and interference studies. Methods Enzymol. 1991;208:365–379. doi: 10.1016/0076-6879(91)08020-i. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Hattori T., Obaru K., Sakai K., Asou N., Takatsuki K., Ohmoto Y., Nomiyama H., Shimada K. Synthesis of a novel cytokine and its gene (LD78) expressions in hematopoietic fresh tumor cells and cell lines. J Clin Invest. 1989 Dec;84(6):1707–1712. doi: 10.1172/JCI114353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel P. F., Balke J., Irving S. G., Kelly K., Siebenlist U. Mitogenic activation of human T cells induces two closely related genes which share structural similarities with a new family of secreted factors. J Immunol. 1989 Mar 1;142(5):1582–1590. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]