Abstract
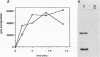
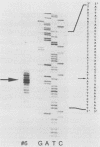
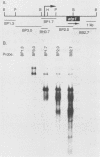
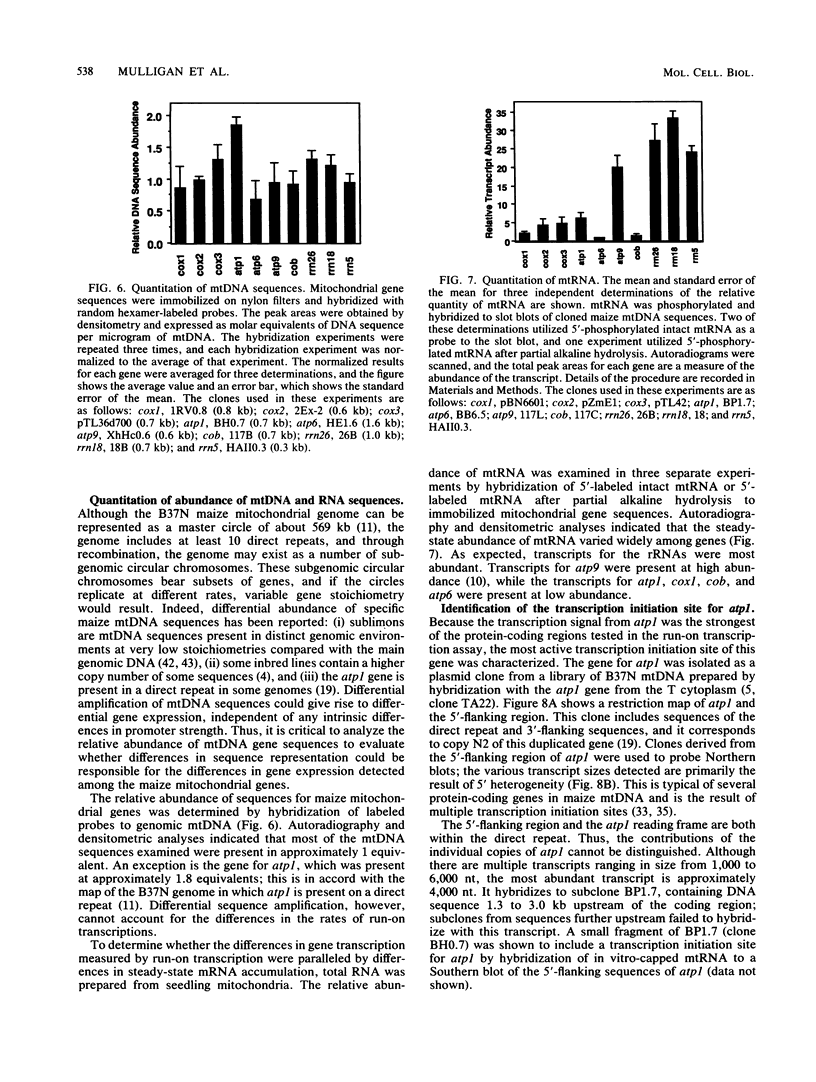
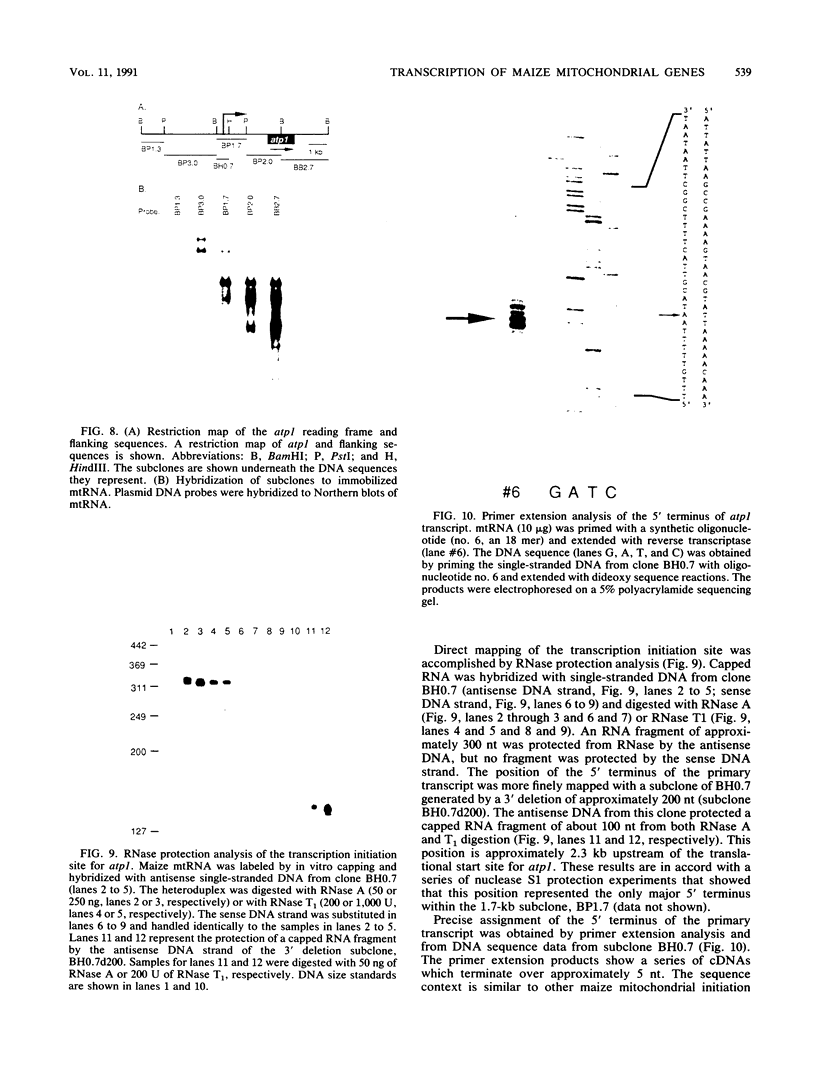
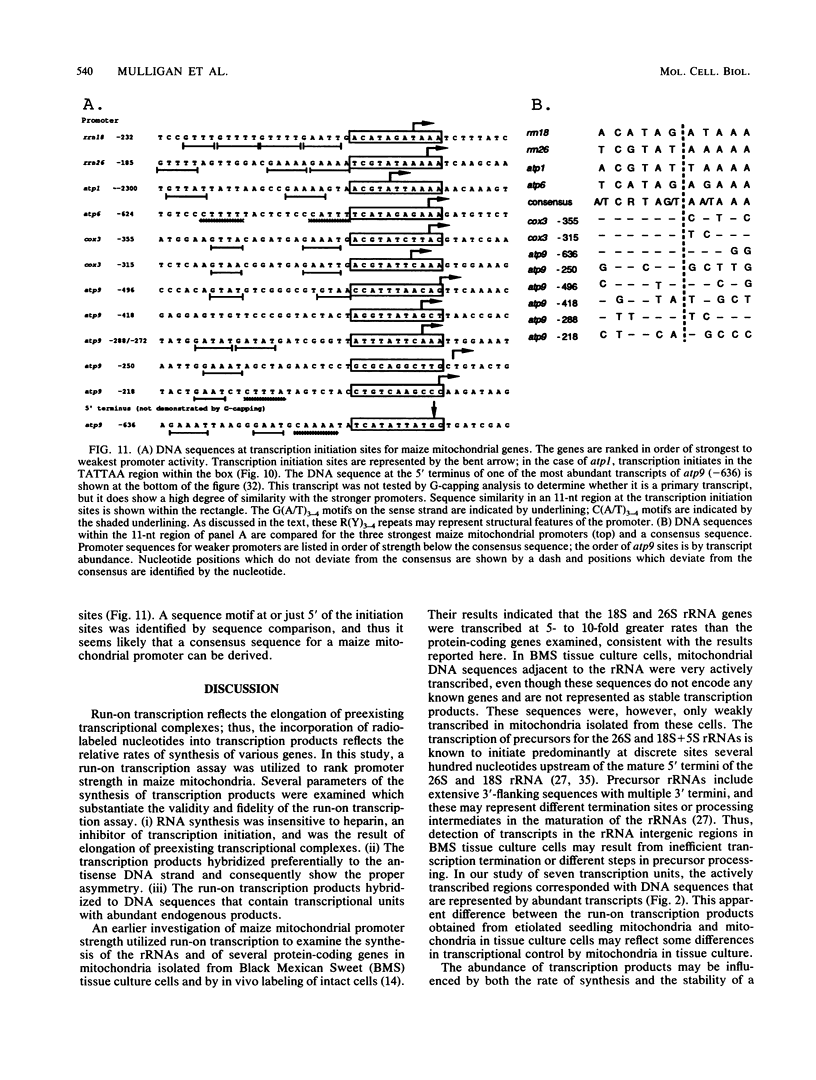
Lysed maize mitochondria synthesize RNA in the presence of radioactive nucleoside triphosphates, and this assay was utilized to compare the rates of transcription of seven genes. The rates of incorporation varied over a 14-fold range, with the following rank order: 18S rRNA greater than 26S rRNA greater than atp1 greater than atp6 greater than atp9 greater than cob greater than cox3. The products of run-on transcription hybridized specifically to known transcribed regions and selectively to the antisense DNA strand; thus, the isolated run-on transcription system appears to be an accurate representation of endogenous transcription. Although there were small differences in gene copy abundance, these differences cannot account for the differences in apparent transcription rates; we conclude that promoter strength is the main determinant. Among the protein coding genes, incorporation was greatest for atp1. The most active transcription initiation site of this gene was characterized by hybridization with in vitro-capped RNA and by primer extension analyses. The DNA sequences at this and other transcription initiation sites that we have previously mapped were analyzed with respect to the apparent promoter strengths. We propose that two short sequence elements just upstream of initiation sites form at least a portion of the sequence requirements for a maize mitochondrial promoter. In addition to modulation at the level of transcription, steady-state abundance of protein-coding mRNAs varied over a 20-fold range and did not correlate with transcriptional activity. These observations suggest that posttranscriptional processes are important in the modulation of mRNA abundance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedinger P., Walbot V. DNA synthesis in purified maize mitochondria. Curr Genet. 1986;10(8):631–637. doi: 10.1007/BF00418131. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas T. K., Getz G. S. Nucleotides flanking the promoter sequence influence the transcription of the yeast mitochondrial gene coding for ATPase subunit 9. Proc Natl Acad Sci U S A. 1986 Jan;83(2):270–274. doi: 10.1073/pnas.83.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K. S., Walbot V. Comparison of the Restriction Endonuclease Digestion Patterns of Mitochondrial DNA from Normal and Male Sterile Cytoplasms of ZEA MAYS L. Genetics. 1982 Sep;102(1):109–128. doi: 10.1093/genetics/102.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Levings C. S. Nucleotide Sequence of the F(1)-ATPase alpha Subunit Gene from Maize Mitochondria. Plant Physiol. 1985 Oct;79(2):571–577. doi: 10.1104/pp.79.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T., Rabinowitz M. Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J Biol Chem. 1983 Nov 25;258(22):14025–14033. [PubMed] [Google Scholar]
- Deng X. W., Gruissem W. Constitutive transcription and regulation of gene expression in non-photosynthetic plastids of higher plants. EMBO J. 1988 Nov;7(11):3301–3308. doi: 10.1002/j.1460-2075.1988.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X. W., Stern D. B., Tonkyn J. C., Gruissem W. Plastid run-on transcription. Application to determine the transcriptional regulation of spinach plastid genes. J Biol Chem. 1987 Jul 15;262(20):9641–9648. [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., Timothy D. H. Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol. 1985 Nov;79(3):914–919. doi: 10.1104/pp.79.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Schuster A. M., Levings C. S., Timothy D. H. Nucleotide sequence of F(0)-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Havlik M. The BamHI, XhoI, SmaI restriction enzyme maps of the normal maize mitochondrial genome genotype B37. Nucleic Acids Res. 1988 Nov 11;16(21):10395–10396. doi: 10.1093/nar/16.21.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Finnegan P. M., Brown G. G. In organello transcription in maize mitochondria and its sensitivity to inhibitors of RNA synthesis. Plant Physiol. 1987 Sep;85(1):304–309. doi: 10.1104/pp.85.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan P. M., Brown G. G. Transcriptional and Post-Transcriptional Regulation of RNA Levels in Maize Mitochondria. Plant Cell. 1990 Jan;2(1):71–83. doi: 10.1105/tpc.2.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Analysis of promoter regions for the spinach chloroplast rbcL, atpB and psbA genes. EMBO J. 1985 Dec 16;4(13A):3375–3383. doi: 10.1002/j.1460-2075.1985.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R. A., Richardson C. C. Enzymatic properties of a proteolytically nicked RNA polymerase of bacteriophage T7. J Biol Chem. 1987 Mar 15;262(8):3790–3799. [PubMed] [Google Scholar]
- Isaac P. G., Brennicke A., Dunbar S. M., Leaver C. J. The mitochondrial genome of fertile maize (Zea mays L.) contains two copies of the gene encoding the alpha-subunit of the F1-ATPase. Curr Genet. 1985;10(4):321–328. doi: 10.1007/BF00365628. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Jones V. P., Leaver C. J. The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J. 1985 Jul;4(7):1617–1623. doi: 10.1002/j.1460-2075.1985.tb03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Hack E., Forde B. G. Protein synthesis by isolated plant mitochondria. Methods Enzymol. 1983;97:476–484. doi: 10.1016/0076-6879(83)97156-2. [DOI] [PubMed] [Google Scholar]
- Leon P., Walbot V., Bedinger P. Molecular analysis of the linear 2.3 kb plasmid of maize mitochondria: apparent capture of tRNA genes. Nucleic Acids Res. 1989 Jun 12;17(11):4089–4099. doi: 10.1093/nar/17.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney A. P., Walbot V. Structural analysis of mature and dicistronic transcripts from the 18 S and 5 S ribosomal RNA genes of maize mitochondria. J Mol Biol. 1990 Jun 20;213(4):633–649. doi: 10.1016/S0022-2836(05)80252-9. [DOI] [PubMed] [Google Scholar]
- Moss B. 5' end labeling of RNA with capping and methylating enzymes. Gene Amplif Anal. 1981;2:253–266. [PubMed] [Google Scholar]
- Mueller D. M., Getz G. S. Steady state analysis of mitochondrial RNA after growth of yeast Saccharomyces cerevisiae under catabolite repression and derepression. J Biol Chem. 1986 Sep 5;261(25):11816–11822. [PubMed] [Google Scholar]
- Mueller D. M., Getz G. S. Transcriptional regulation of the mitochondrial genome of yeast Saccharomyces cerevisiae. J Biol Chem. 1986 Sep 5;261(25):11756–11764. [PubMed] [Google Scholar]
- Mullet J. E., Klein R. R. Transcription and RNA stability are important determinants of higher plant chloroplast RNA levels. EMBO J. 1987 Jun;6(6):1571–1579. doi: 10.1002/j.1460-2075.1987.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. M., Lau G. T., Walbot V. Numerous transcription initiation sites exist for the maize mitochondrial genes for subunit 9 of the ATP synthase and subunit 3 of cytochrome oxidase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7998–8002. doi: 10.1073/pnas.85.21.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. M., Maloney A. P., Walbot V. RNA processing and multiple transcription initiation sites result in transcript size heterogeneity in maize mitochondria. Mol Gen Genet. 1988 Mar;211(3):373–380. doi: 10.1007/BF00425688. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Newton K. J., Walbot V. Maize mitochondria synthesize organ-specific polypeptides. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6879–6883. doi: 10.1073/pnas.82.20.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel A. H., Groot Koerkamp M. J., Stuiver M. H., Van der Horst G. T., Tabak H. F. Effect of point mutations on in vitro transcription from the promoter for the large ribosomal RNA gene of yeast mitochondria. Nucleic Acids Res. 1987 Jul 24;15(14):5597–5612. doi: 10.1093/nar/15.14.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster W., Hiesel R., Isaac P. G., Leaver C. J., Brennicke A. Transcript termini of messenger RNAs in higher plant mitochondria. Nucleic Acids Res. 1986 Aug 11;14(15):5943–5954. doi: 10.1093/nar/14.15.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I. D., Isaac P. G., Leaver C. J. Stoichiometric differences in DNA molecules containing the atpA gene suggest mechanisms for the generation of mitochondrial genome diversity in maize. EMBO J. 1987 Apr;6(4):865–869. doi: 10.1002/j.1460-2075.1987.tb04832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I., Suffolk R., Leaver C. J. Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell. 1989 Jul 14;58(1):69–76. doi: 10.1016/0092-8674(89)90403-0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Gruissem W. Control of plastid gene expression: 3' inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell. 1987 Dec 24;51(6):1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Jones H., Gruissem W. Function of plastid mRNA 3' inverted repeats. RNA stabilization and gene-specific protein binding. J Biol Chem. 1989 Nov 5;264(31):18742–18750. [PubMed] [Google Scholar]
- Travers A. A. DNA conformation and protein binding. Annu Rev Biochem. 1989;58:427–452. doi: 10.1146/annurev.bi.58.070189.002235. [DOI] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R., Dierks P. M. Sequence and transcription analysis of the Petunia mitochondrial gene for the ATP synthase proteolipid subunit. Nucleic Acids Res. 1986 Oct 24;14(20):7995–8006. doi: 10.1093/nar/14.20.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]