Abstract
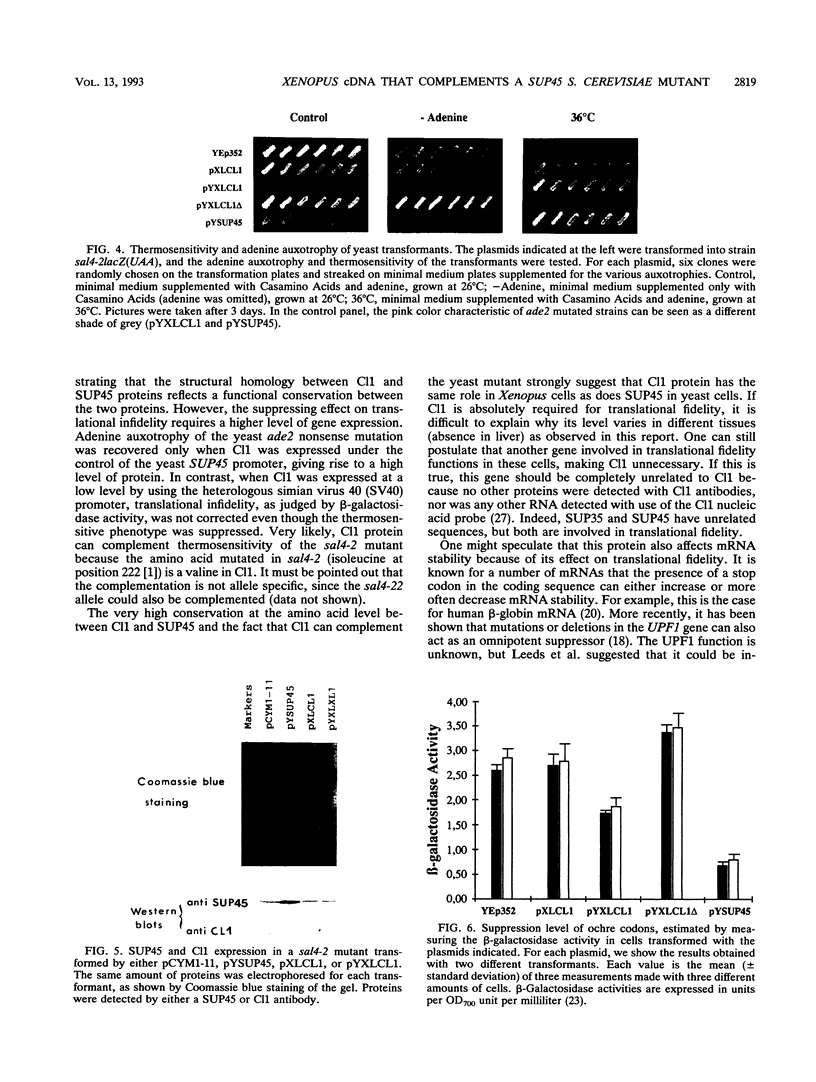
We have performed a differential screen of a Xenopus egg cDNA library and selected two clones (Cl1 and Cl2) corresponding to mRNA which are specifically adenylated and recruited into polysomes after fertilization. Sequence analysis of Cl1 reveals that the corresponding protein is 67.5% identical (83% similar) to the product of the Saccharomyces cerevisiae SUP45 (also called SUP1 or SAL4) gene. This gene, when mutated, is an omnipotent suppressor of nonsense codons. When expressed in a sup45 mutant, the Xenopus Cl1 cDNA was able to suppress sup45-related phenotypes, showing that the structural homology reflects a functional homology. Our discovery of a structural and functional homolog in Xenopus cells implies that the function of SUP45 is not restricted to lower eukaryotes and that the SUP45 protein may perform a crucial cellular function in higher eukaryotes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bravo R., Knowland J. Classes of proteins synthesized in oocytes, eggs, embryos, and differentiated tissues of Xenopus laevis. Differentiation. 1979;13(2):101–108. doi: 10.1111/j.1432-0436.1979.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Camonis J. H., Cassan M., Rousset J. P. Of mice and yeast: versatile vectors which permit gene expression in both budding yeast and higher eukaryotic cells. Gene. 1990 Feb 14;86(2):263–268. doi: 10.1016/0378-1119(90)90288-3. [DOI] [PubMed] [Google Scholar]
- Crouzet M., Izgu F., Grant C. M., Tuite M. F. The allosuppressor gene SAL4 encodes a protein important for maintaining translational fidelity in Saccharomyces cerevisiae. Curr Genet. 1988 Dec;14(6):537–543. doi: 10.1007/BF00434078. [DOI] [PubMed] [Google Scholar]
- Crouzet M., Tuite M. F. Genetic control of translational fidelity in yeast: molecular cloning and analysis of the allosuppressor gene SAL3. Mol Gen Genet. 1987 Dec;210(3):581–583. doi: 10.1007/BF00327216. [DOI] [PubMed] [Google Scholar]
- DeMarini D. J., Winey M., Ursic D., Webb F., Culbertson M. R. SEN1, a positive effector of tRNA-splicing endonuclease in Saccharomyces cerevisiae. Mol Cell Biol. 1992 May;12(5):2154–2164. doi: 10.1128/mcb.12.5.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessen P., Fondrat C., Valencien C., Mugnier C. BISANCE: a French service for access to biomolecular sequence databases. Comput Appl Biosci. 1990 Oct;6(4):355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- Firoozan M., Grant C. M., Duarte J. A., Tuite M. F. Quantitation of readthrough of termination codons in yeast using a novel gene fusion assay. Yeast. 1991 Feb;7(2):173–183. doi: 10.1002/yea.320070211. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L. Genetic properties of some amber-ochre supersuppressors in Saccharomyces cerevisiae. Mol Gen Genet. 1975;138(1):53–63. doi: 10.1007/BF00268827. [DOI] [PubMed] [Google Scholar]
- Grenett H. E., Bounelis P., Fuller G. M. Identification of a human cDNA with high homology to yeast omnipotent suppressor 45. Gene. 1992 Jan 15;110(2):239–243. doi: 10.1016/0378-1119(92)90655-9. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., Leupold U. Suppressors in yeast. Curr Top Microbiol Immunol. 1974;64(0):1–47. doi: 10.1007/978-3-642-65848-8_1. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Le Guellec R., Paris J., Couturier A., Roghi C., Philippe M. Cloning by differential screening of a Xenopus cDNA that encodes a kinesin-related protein. Mol Cell Biol. 1991 Jun;11(6):3395–3398. doi: 10.1128/mcb.11.6.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds P., Peltz S. W., Jacobson A., Culbertson M. R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991 Dec;5(12A):2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- Leeds P., Wood J. M., Lee B. S., Culbertson M. R. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol. 1992 May;12(5):2165–2177. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Mullins J. J., Chen C. M., Gross K. W., Maquat L. E. Novel metabolism of several beta zero-thalassemic beta-globin mRNAs in the erythroid tissues of transgenic mice. EMBO J. 1989 Sep;8(9):2613–2619. doi: 10.1002/j.1460-2075.1989.tb08401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L. Regulation of amphibian oocyte maturation. Cell Differ. 1985 Jun;16(4):211–221. doi: 10.1016/0045-6039(85)90570-6. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Palmer E., Wilhelm J. M., Sherman F. Phenotypic suppression of nonsense mutants in yeast by aminoglycoside antibiotics. Nature. 1979 Jan 11;277(5692):148–150. doi: 10.1038/277148a0. [DOI] [PubMed] [Google Scholar]
- Paris J., Le Guellec R., Couturier A., Le Guellec K., Omilli F., Camonis J., MacNeill S., Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Osborne H. B., Couturier A., Le Guellec R., Philippe M. Changes in the polyadenylation of specific stable RNA during the early development of Xenopus laevis. Gene. 1988 Dec 10;72(1-2):169–176. doi: 10.1016/0378-1119(88)90139-4. [DOI] [PubMed] [Google Scholar]
- Paris J., Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990 Jul;140(1):221–224. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K. E., LeGuellec K., Philippe M., Mitchison T. J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992 Oct 8;359(6395):540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Singh A., Ursic D., Davies J. Phenotypic suppression and misreading Saccharomyces cerevisiae. Nature. 1979 Jan 11;277(5692):146–148. doi: 10.1038/277146a0. [DOI] [PubMed] [Google Scholar]
- Smith R. C. Protein synthesis and messenger RNA levels along the animal-vegetal axis during early Xenopus development. J Embryol Exp Morphol. 1986 Jun;95:15–35. [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Surguchov A. P. 'Omnipotent' nonsense suppressors: new clues to an old puzzle. Trends Biochem Sci. 1988 Apr;13(4):120–123. doi: 10.1016/0968-0004(88)90062-x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Avanesyan M. D., Zimmermann J., Inge-Vechtomov S. G., Sudarikov A. B., Smirnov V. N., Surguchov A. P. Ribosomal recessive suppressors cause a respiratory deficiency in yeast Saccharomyces cerevisiae. Mol Gen Genet. 1982;185(2):319–323. doi: 10.1007/BF00330805. [DOI] [PubMed] [Google Scholar]