Abstract
Increasing evidence suggests that altered immunity and chronic inflammation play a key role in the etiology of many malignancies, but the underlying biological mechanisms involved remain unclear. Systemic markers of immunity may not represent the clinically relevant, site-specific immune response, whereas tissue-based markers may more accurately reflect the local immunologic mechanisms by which precursor lesions develop into cancer. Tissues are often only available in individuals with disease. Previous studies have measured tumor-infiltrating lymphocytes to predict prognosis and survival, but it can be challenging to use tissue-based markers to study the natural history of cancer due to limitations with regard to temporality, the availability of appropriate comparison groups, and other epidemiologic issues. In this commentary, we discuss several epidemiologic study design and study population considerations to address these issues, including the strengths and limitations of using tissue-based markers to study immune response and cancer development. We also discuss how the use of tissue-based immune markers fits into the greater context of molecular epidemiology, which encompasses multiple technologies and techniques, and how implementation of tissue-based immune markers will provide an increased understanding of site-specific biological mechanisms involved in carcinogenesis.
Introduction: Immunity and cancer
Since the introduction of Burnet and Thomas’ cancer immunosurveillance hypothesis (1), the concept that the immune system plays a protective role in tumor development has been vigorously debated. The central principle of the hypothesis that the immune system can indeed prevent tumor formation was supported by clinical observations of higher incidences of cancer in immunodepressed individuals (2). Conversely, accumulated evidence showed that infectious and non-infectious causes of local chronic inflammation increased the risk of developing cancer (3). Schreiber and colleagues proposed that cancer immunosurveillance was a component of a more general process of cancer immunoediting, responsible for both eliminating tumors and sculpting the immunogenic phenotypes of tumors that eventually form in immunocompetent individuals (4).
One element of the immunogenic phenotype of solid tumors is the presence of immune infiltrates, but their role in carcinogenesis is still debated. Since Schreiber’s proposal that immunity plays a dual role by promoting host protection against cancer and facilitating tumor escape from immune destruction, intratumoral lymphocyte infiltrates have been identified as major prognostic factors for several solid cancers (5). By contrast, immune infiltrates have had limited utility in etiologic studies of solid cancers due to tissue inaccessibility and epidemiologic issues such as temporality and the lack of availability of appropriate comparison groups. However, recent developments in molecular pathology promise new opportunities for tissue-based markers of immune infiltrates to enrich molecular epidemiologic investigations aimed at defining the causes of cancer.
Important research questions in tumor immunoepidemiology
Many epidemiologic studies only collect tissue specimens at a single time point, typically the time of diagnosis or selection into the study, which potentially limits the research questions that can be addressed. One of the most explored research questions in individuals with disease has been: What is the association between the presence of certain immune infiltrates and clinical outcomes such as prognosis and survival? Such analyses have been reported in several solid cancers, including breast (6), colorectal (7), ovarian (8), and lung (9) cancers, and melanoma (10).
Rather than exploring the role of immune infiltrates after cancer development, another important question is: How does the immune response, or an inappropriate immune response, contribute to cancer development? The application of molecular epidemiologic techniques using tissue-based immune markers to examine precursor lesions (which we discuss later in this commentary) may provide clues for determining the contribution of inflammation in tumor development.
Yet another distinct question in tumor immunoepidemiology includes: What is the association between immune markers and known risk factors for cancer or tumor subtypes, such as different histologies or anatomical locations within an organ? Such analyses can provide clues about the etiology of these different cancer types. One recent example of this approach is a pooled analysis of breast cancer tumor markers and epidemiological risk factors in the Breast Cancer Association Consortium (11). This study found that estrogen receptor (ER)-positive and progesterone receptor (PR)-positive breast tumors were associated with known risk factors for breast cancer, but ER-negative, PR-negative, and especially triple-negative or core basal phenotype tumors, were not associated, suggesting that the etiology for triple-negative tumors is distinct from other breast cancer subtypes. Following up these findings by carefully examining the immune cells present in tissue from different histologies might help elucidate the biological mehcanisms underlying different tumor subsets. In addition, examining different patterns of immune cells in tumor tissue and their association with known risk factors for cancer might help identify new tumor subsets. Careful evaluation of immune cells in tissues from different breast cancer or other tumor subtypes could also help identify progression-related phenotypes.
All of these questions fall into the emerging field of molecular pathological epidemiology. Molecular pathological epidemiology combines traditional epidemiology and traditional pathology research approaches to understand how exogenous and endogenous factors contribute to cancer development, progression, and response to treatment (12). By identifying links between exposures and biomarkers associated with cancer (e.g., microsatellite instability/stability, immunologic markers), molecular pathological epidemiology can provide clues as to the molecular mechanisms underlying cancer etiology and prognosis (12, 13).
Why tissue-based markers?
Broadly defined, tissue-based immune markers may refer to markers of specific immune cell populations, the expression of specific cytokines in tissues by immune cells that play a role in immune function or signaling, cytokine receptors, or immune presentation molecules (human leukocyte antigen, HLA). Tissue-based markers are likely to become more and more available given the increasing use of biomarkers in clinical care and advances in biomarker discovery and technological innovation (14). Tissue-based markers offer important advantages for molecular epidemiologic evaluations of inflammation and immunity in the development and progression of cancer. First, measuring immune markers in tumor and/or surrounding tissue can provide direct evidence of inflammatory or other immune responses in the specific location where the cancer developed. By contrast, other markers of immune response, such as circulating cytokines, are not specific to the location of tumor development and may reflect immune or inflammatory responses irrelevant to the cancer of interest.
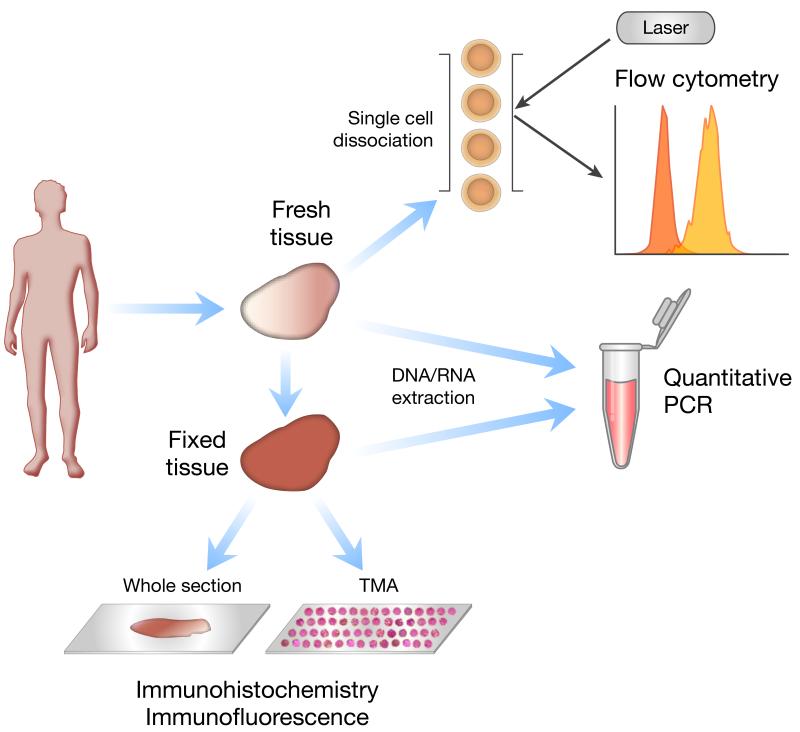
Second, tissue specimens are often collected and archived in epidemiologic studies of cancer. Third, there are several techniques for measuring immune markers in tissue specimens. The utilities of these techniques differ according to specimen type and research question. Figure 1 shows some of the most common molecular assays, although novel strategies for immune marker imaging and detection are rapidly evolving.
Figure 1.
Common techniques for measuring tissue-based immune markers. This schematic depicts example molecular tools that can be used depending on the type of tissue available and assessments desired. Fresh tissue can be dissociated into single cells for flow cytometry, which can be used to assess the type and function of tumor-infiltrating lymphocytes. DNA can be extracted from fresh or fixed tissue for polymerase chain reaction (PCR) or quantitative PCR (qPCR) to detect polymorphisms or mutations in immune-related genes; mRNA can be extracted from tissue for reverse-transcriptase-PCR (RT-PCR) to assess the expression of cytokine genes and other immune markers. Immunohistochemistry (IHC) and immunofluorescence (IF) are commonly used techniques to assess the presence of immune cell populations in fixed tissues; tissue microarray (TMA) technology is a high-throughput method for assessing hundreds of tissues on a single microscope slide.
While there are several strengths for using tissue-based markers, a weakness is that tissue specimens are often not collected from individuals without cancer. Thus, an appropriate comparison, or control, group for epidemiologic studies of tumor-based markers may be unavailable. Some alternatives may include adjacent non-tumor tissue, precursor condition tissue, or tissues from other surgical procedures. While potentially useful, these controls are not “normal” and may not represent the population from which the cancer cases arose. For example, adjacent non-tumor tissue is likely to have been exposed to the same carcinogens as tumor tissue. These carcinogens could have introduced changes such that the non-tumor tissue is no longer representative of truly normal tissue, even though it has not been fully transformed. With respect to immune-response in particular, the tumor itself is likely to influence the immune cells and other immune markers in and around adjacent non-tumor tissue. Similarly, tissue from patients with precursor conditions are already altered in some way and do not represent purely “normal” tissues for comparison, and tissues obtained through other surgical procedures may also have been affected by the disease process that led to the surgery. Tissue processing time and methods are also critical. For example, commercially available “normal” tissues often originate from autopsy subjects (15, 16) and are likely to have remained unprocessed in the corpse for some time. Such delay could lead to changes in immune infiltrate content and degradation of immune markers. Thus, comparisons with autopsy specimens may reflect differences in immune markers due to death rather than differences due to disease.
Another concern with the use of tissue-based immune markers is the lack of clarity in determining if the pattern of immune response contributed to the development of cancer or was a result of or a response to the cancer itself. Oftentimes in tissue-based studies, temporality (exposure preceding outcome) is difficult to establish. However, as we describe later, there are ways to address this concern.
Examples of tissue-based immune markers
Immune cells and the cellular factors produced from them, both immunosuppressive and inflammatory cytokines, play dual roles in promoting or discouraging cancer development. Their role may be determined by the tumor microenvironment and the events that lead to the initial propagation of carcinogenesis. Of the plethora of cells, cytokines, or other soluble factors involved, tumor-associated macrophages (TAMs) or myeloid-derived suppressive cells (MDSCs) and secreted cytokines interleukin (IL)-6, tumor necrosis factor (TNF), and IL-1β (reviewed in (17)) may be the key players in promoting tumor development. Within the microenvironment, TAMs are a heterogeneous cell population, which may include type 1 (M1) and type 2 (M2) macrophages. M1 macrophages infiltrate early in tumor development in response to inflammatory mediators, signals such as those from bacteria, and cellular factors such as granulocyte macrophage colony-stimulating factor and interferon (IFN)-γ. Upon arrival, M1 macrophages upregulate the transcription of IL-12p35, IL-12p40, IL-23, and TNF- α, and they release pro-inflammatory cytokines and chemokines, such as chemokine (C-X-C motif) ligand (CXCL)19 and CXCL10 to attract and encourage Th1, Th17, and natural killer (NK) cell development and differentiation (18-20). M1 macrophages may be pro-inflammatory, promoting cell growth and recruitment through the production of IL-6, TNF-α, IL-23, and IL-12 (21). By contrast, more advanced tumors may have infiltrating M2 macrophages, which express cytokines and chemokines such as chemokine (C-C motif) ligand (CCL)17, CCL22, and CCL24 to encourage the development and recruitment of regulatory T cells (Tregs), and these macrophages may also release factors to encourage Th2 differentiation and recruitment as well as angiogenesis (18, 19). M2 macrophages promote tumor development and immunoregulation by producing transforming growth factor (TGF)-β and IL-10 (18, 22).
MDSCs are a heterogeneous population of immature myeloid cells that exert suppressive functions, regulating T cell responses through nitric oxide, reactive oxygen species, and TGF-β secretion and promote Tregs and an anti-inflammatory response (23, 24). Tregs, often characterized by CD4+ T cells that express CD25 and the master transcription factor forkhead box P3 (Foxp3), play a role in promoting tumor growth by inhibiting the immune response against cancer (25, 26). Similar to Tregs, Th17 cells, which are IL-17-producing CD4+ cells, in the tumor microenvironment may antagonize the differentiation and function of Th1 cells. Th17 cells likely counter the tumor-suppressive immune response and may be involved in the promotion of tumor growth (27). By contrast, IFN-γ-producing CD4+ Th1 cells and CD8+ T cells inhibit and kill tumor cells, impeding tumor growth (8), although the extent to which these cells are able to contribute to anti-cancer immunity is unknown, particularly with the presence of the immunosuppressive condition within the tumor.
Tissue-based immune markers must be interpreted as part of a biological mechanism within the microenvironment. Single markers measured in isolation are not as useful in elucidating that mechanism. Systematic characterization of multiple tissue-based immune markers in epidemiologic studies will help us understand the interactions and mutual regulation of these complex immune networks within the tumor microenvironment.
Tools for studying tissue-based immune markers
Immunity and inflammation at the cancer site can be evaluated using various molecular techniques depending on the type of tissue that is available and the assessments desired (Table 1 lists some examples described here). The type, density, and location of immune cells can be assessed in fresh and ethanol- or formalin-fixed tumor tissues. Previous studies [e.g., (7, 8, 28-33)] have semi-quantitatively evaluated the numbers and proportion of various immune cell subsets, including CD4 T-helper cells, CD8 cytotoxic T cells, dendritic cells, NK cells, macrophages, Tregs, neutrophils, etc. In addition, assessment of immune function through the measurement of cytokines or cytotoxicity mediators such as perforin (30) and granzymes (29) may also be assessed in fresh and fixed tissues.
Table 1.
Common techniques for measuring tissue-based immune markers and some examples of immune markers.
| Technique | Description | Tissue requirements | Research goal | Considerations | Some examples of immune markers* |
|---|---|---|---|---|---|
| Bright-field Immunohistochemistry (IHC) |
Process of detecting antigens (e.g., proteins) in cells of a tissue section by antibodies binding specifically to antigens |
Fixed or frozen tissue |
Semi-quantitative to quantitative assessment of protein expression that distinguishes cell populations |
Preserves tissue and cellular architecture; use of tissue microarrays (TMAs) allow for high- throughput, validated, standardized testing |
CD3, T cells; CD4, T- helper cells; CD8, cytotoxic T cells; CD20, B cells; foxp3, regulatory T cells; MHC I and MHC II, cells that present potential antigens; CD68, macrophages, CD57, natural killer cells |
| Immunofluorescence (IF) |
Similar to IHC, where the antibody can also be tagged to a fluorophore for visualizing the target protein |
Fixed tissue | Quantitative assessment of protein expression that distinguishes cell populations |
Preserves tissue and cellular architecture; use of counterstains and co- localization allow delineation of tissue compartments and cell types |
Simulataneous detection of CD25, CD4, and foxp3 for regulatory T cells; CD8 and granzyme B for cytotoxic T cells |
| Flow cytometry | Technique for counting and examining microscopic particles, such as cells and chromosomes, by suspending them in a stream of fluid and passing them by an electronic detection apparatus |
Fresh or frozen tissue, dissociated cells; with and without stimulation |
Semi-quantitative to quantitative assessment, functional assessment |
Loss of tissue architecture; simultaneous multiparametric analysis of the physical and/or chemical characteristics of hundreds to thousands of cells per second; can be used to sort cell populations for other functional assays (e.g., proliferation and cytotoxicity assays) |
CD11b, CD33, HLA-DR for myeloid-derived suppressor cells; CD44, CD8, and PD1, exhausted T cells |
| Quantitative PCR (qPCR) | Measure the quantity of a PCR product (commonly in real-time) by quantitatively measuring the starting amounts of DNA, cDNA, or RNA. |
Fresh, frozen, or fixed tissues, from which DNA or RNA is extracted |
Determine whether a DNA sequence or cDNA sequence reverse transcribed from extracted RNA is present in a sample and the number of its copies in the sample. |
Loss of tissue architecture; very high degree of precision; can be combined with microdissection; RNA degradation is a possibility depending on tissue handling |
Indoleamine 2,3- dioxygenase (IDO), immunoregulatory enzyme in dendritic cells; foxp3, immunoregulatory protein in regulatory T cells; expression levels of cytokines and chemokines: IL-4, IL-10, IFN-gamma, TGF-beta, etc |
Immunohistochemistry (IHC), one of the most commonly used techniques for studying tissue-based immune markers, is a well-established tool in diagnostic pathology (34) that can be readily applied to assessing the presence of immune cells in tumor tissues. Together with immunofluorescence (IF), IHC allows the visualization of immune cells in structurally-intact tissue, in which architectural compartments such as tumor vs. stroma may be important to distinguish. Several histochemical and immunohistochemical stains, such as myeloperoxidase for neutrophils (35) and CD4 for T-helper cells (36), have been widely used in the clinic. Because markers must be robust to differences in tissue collection and processing procedures, the clinical use of these markers suggests that they may be reliably used in epidemiologic studies. However, because multiple antibodies may be available for the immunohistochemical staining of any particular tissue-based marker, each IHC stain and analysis system must be validated for the particular marker and tissue type of interest (37-39). Some markers of interest may be produced by both immune and non-immune cells (e.g., CD68 is expressed on fibroblasts as well as macrophages (40)). While the staining intensity is likely to differ between cell types, immune infiltrates must be identified in the context of the tissue architecture.
Recent advances in high-throughput methods also increase the appeal of tissue-based studies for molecular epidemiologic investigations. Tissue microarray (TMA) technology can be used to assay hundreds of tissues arrayed on a single microscope slide. Tissue-based immune markers may be assessed by IHC or IF using TMAs of fixed tissues and other biological specimens (41). Moreover, the advent of automated image analysis using quantitative scoring algorithms has reduced the need for manual interpretation by molecular pathologists, which limits inter- and intra-observer variation (38, 42, 43).
Polymerase chain reaction (PCR) or quantitative PCR (qPCR, also real-time qPCR) can be used to amplify the sequences of immune-related genes to detect polymorphisms or mutations in DNA extracted from tissues. Reverse transcriptase-PCR (RT-PCR) can be used to assess immune function by measuring the expression of cytokine genes or other immune markers, such as indoleamine 2,3-dioxygenase (IDO) (44) and foxp3 (45, 46), from mRNA extracted from tissues. Studying the mRNA levels of these as well as other cytokines or immune-related markers such as IL-10 (47), CD28 (48), granzyme B (49), and programmed death (PD)-1 (50) may provide insight into the signals that induce immunity rather than tolerance, or robust cytotoxicity rather than immune exhaustion. Although mRNA cannot fully capture immune function as a whole, it can provide clues that can be used to explore the underlying biological mechanism with additional immune markers/approaches. DNA and RNA can be extracted from fresh, frozen, or fixed tissues, although tissue processing procedures may influence the integrity of the genetic material and also destroy the tumor architecture. Microdissection prior to DNA or RNA extraction can help clarify the location from which the immune signal arose (tumor vs. stroma).
Flow cytometry may be used to assess the function of tumor-infiltrating lymphocytes, particularly cells like MDSCs or Tregs that require multiple markers for accurate identification. In addition, proliferation assays or assays that assess the cytotoxicity of immune subsets provide a more direct assessment of immune function. However, along with flow cytometry, these functional assays require cells isolated from fresh or frozen tissues, which are less commonly available in epidemiologic studies. These assays are also unable to distinguish the tissue compartment source.
For all of these approaches, it is critically important to use reliable, validated methods and careful laboratory technique. For example, IHC staining and analysis require study-by-study validation (37-39), as described above. PCR is notoriously prone to contamination and requires rigorous precautions to avoid spurious results (51, 52). The results from biological assays may differ depending on the platform used, underscoring the importance of thorough biomarker validation (53).
Study populations and study design
One of the first considerations of study design is the choice of the most appropriate population for evaluating the question of interest. Importantly, studies in the general population allow for conclusions regarding interventions that may affect public health, such as cancer screening. However, in-depth studies of biological mechanisms might require studies in special populations at high risk for certain outcomes of interest (e.g., referral clinic patients).
Several study designs can be applied in different populations (54). Tissue-based immune markers may be assessed and correlated with various stages of pre-cancer and cancer using the cross-sectional design, where markers are measured at a single time point, but inference regarding temporality is limited. Depending on the cancer of interest, some case-control studies may have tissue specimens available from control subjects, particularly if precursor conditions are associated with the cancer. However, study participants and their samples need to be selected in such a way as to ensure the representativeness of the participants and their samples and to minimize bias in the selection of the comparison groups. In addition, information needs to be collected on potential confounding variables and used in the analyses, where appropriate. Attention to these issues is needed to address the validity and generalizability of the results.
Randomized clinical trials or cancer prevention trials can be useful resources for studying tissue-based immune markers and the development of cancer, but collection, processing, and storage costs may limit tissue availability in large-scale trials with thousands of participants. Cancer prevention trials, such as screening trials or intervention trials that collect biopsies of precursor lesions, may be useful for assessing tissue-based immune markers in cancer precursors. In addition, cross-sectional studies or randomized trials are sometimes converted to cohort studies after the trial period ends and thus provide longer follow-up that ensures more cancer events. The cohort study design is particularly valuable because exposure can be measured prior to the development of the outcome, allowing the establishment of temporality. For tissue-based studies, this prospective assessment may only be possible for cancers with precursor conditions that are not typically treated but followed over time [e.g., monoclonal gammopathy of undetermined significance as a precursor to multiple myeloma (55)].
Efficiency for studying tissue-base immune markers: Prospective vs. cross-sectional design
Prospective data from cohorts or trials are often preferable because temporality may potentially be evaluated. For tissue-based studies, however, cross-sectional designs in high-risk populations may be more feasible and efficient. A comparison of two National Cancer Institute (NCI) studies of cervical cancer, the Guanacaste Project and the Study to Understand Cervical Cancer Early Endpoints and Determinants (SUCCEED), provides an excellent example. The Guanacaste Project is a cohort study that began following approximately 10,000 women in 1993 (56). After 18 years of follow-up, this study collected biospecimens from only 140 women with histologically confirmed cervical pre-cancer [cervical intraepithelial neoplasia grade 3 (CIN3)] and 45 with cancer (incident or prevalent) (57). In stark comparison, SUCCEED used a cross-sectional design to recruit women referred to one colposcopy clinic over the course of four years (58) and collected tissues from over 300 CIN3 cases and over 100 invasive cervical cancer cases (59). Although prospective data are highly valuable and information from the general population is important for making public health inferences, sometimes it can be efficient to start with a cross-sectional study, particularly for studies of immune markers that require tissue specimens.
Sample size considerations
Four quantities are needed to calculate sample sizes: 1) the expected difference (e.g., mean difference) or association (e.g., odds ratio), 2) an estimate of the variability in the measurements (e.g., standard deviation, standard error, or 95% confidence interval), 3) the desired statistical power (e.g., 0.8 or 0.9), and 4) the number of comparisons and whether the comparisons are agnostic (i.e., no a priori hypotheses, and typically numerous) or based on biologically plausible, a priori hypotheses (typically involving a more limited number of comparisons). These basic considerations are important for all studies of biomarkers, immune or otherwise. As an example for immune markers, most IHC studies of cervical immune infiltrates report results as mean or median cell densities (the number of positively-stained cells per mm2). Table 2 provides example sample size calculations comparing means from two independent samples using published data from Kobayashi et al. (30).
Table 2.
Example sample size calculations for a study comparing immune infiltrates in cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3) to normal cervical tissue using mean and standard deviation (σ) data.
As shown in Table 2, both the effect size and the number of comparisons made are important considerations when calculating sample size. However, note that when the standard deviation is large compared to the mean, as in the case of the Kobayashi study, a test for a difference in means may not be the best approach. If the data are not normally distributed, the Wilcoxon-Mann-Whitney test for a difference in medians may be more appropriate (60). Other hypotheses about differences in the distribution of immune markers between groups can also be considered, such as mixture models where one group might have a higher proportion with values above or below a biologically important threshold. For example, mixture models have been used to demonstrate that the overall breast cancer population consists of two separate groups, patients with early-onset breast cancer and patients with late-onset breast cancer (61), and that early-onset versus late-onset breast cancer vary by race (62). Such findings indicate biologic heterogeneity in the overall breast cancer population that may offer clues as to the underlying etiology for these tumor subtypes (61, 62). Similarly, tumor heterogeneity in immune profiles may also exist (6). Useful testing approaches include the standard χ2 test of homogeneity if a priori cut-points are available or the Kolmogorov-Smirnov test otherwise, although these approaches require larger samples sizes than testing for a shift in mean or median.
Although concerns over multiple comparisons are more important for the discovery of new markers rather than for the validation of previously identified markers, many published studies include fewer than 25 subjects per group and examine multiple immune markers. Therefore, reproducibility across studies is lacking due to inadequate sample sizes and multiple comparisons; as the number of comparisons increases, the number of false positives also increases. Another important point is that these sample size calculations assume no measurement error, but in actuality, some degree of measurement error is nearly always present. Traditional sample size calculations represent the best case scenario; reality may require larger sample sizes.
Potential confounders
Studies of immune response must also consider potential confounders. For example, age may be associated with both immunity and cancer. In our previous example of immune markers in precursor lesions, precursors typically occur in individuals at a younger age than cancer, and immunity declines with age (63). Thus, age should be accounted for carefully. Immunity also differs for men and women (64), so it is important to consider sex for non-sex-specific cancers. Race/ethnicity has been associated with a number of immune markers, such as immunoglobulin A and γ-globulin levels, CD8 cell counts, and antibodies against infections (65-70). Alcohol or substance use can also affect immune function (71). Many other factors might also influence interpretation of immune responses (e.g., recent immunization, contraceptive use) (72). Each cancer and research question must be considered carefully to adequately address potential confounders.
Tissue-based immune markers and the natural history of cancer
An important goal is to establish how immune response contributes to the development of cancer. Immune markers that are present at the time of cancer diagnosis may reflect the immune function that contributed to the development of cancer or may be the result of the cancer itself. Therefore, disease effects may be best addressed in subjects with precursor conditions. Comparisons of tissue-based immune markers from low-grade precursor conditions to cancer, as well as the range of stages among cancer cases, can provide evidence that immune responses are relevant for cancer development.
Of course, cancers for which screening of precursor lesions is relatively non-invasive and for which biopsies may be collected from preneoplastic lesions are most conducive to this approach. Cervical cancer is a prime example because the natural history and histological changes that precede cancer are well described, yet many questions remain regarding the factors that drive progression from human papillomavirus (HPV) infection to cancer. When women become infected with HPV, the necessary cause of cervical cancer, they may develop mild histologic abnormalities like CIN grade 1 (73). Most women clear their HPV infections, suggesting that the immune system usually successfully prevents HPV from causing cancer. However, in a small proportion of women, HPV infection persists. These women are at risk for developing cervical pre-cancer and cancer (74), and inflammation from other exposures, such as other sexually transmitted infections, may contribute to the progression from persistent HPV infection to cancer. Studies of changes in tissue-based immune markers from across the spectrum of cervical cancer precursors and HPV infection may elucidate the role of immune responses in cervical carcinogenesis.
Another example of a cancer model with precursor conditions is esophageal cancer. In this model, histologic precursor lesions (mild, moderate, and severe dysplasia, and carcinoma in situ) can be observed and biopsied during cross-sectional endoscopic screening of high-risk populations (75). Prospective follow-up of these subjects provides insight into the association between increasing grades of dysplasia and the increasing risk of developing esophageal squamous cell carcinoma (76, 77). Examining immune infiltrates in tissues archived from baseline biopsies and the association with risk of esophageal squamous cell carcinoma may improve understanding of the role of immune function in effecting the progression to cancer.
An integrative approach
Although tissue-based measures are not without limitations, they contribute to our understanding of the role of immune factors in the development of cancer, especially by combining them with other studies of immune markers that have different strengths and limitations. In particular, genetic studies of polymorphisms in immune pathways provide information on the association between inherited differences in immune-related pathways and cancer risk. For example, polymorphisms in the IL1β and the TNFα genes, which encode key pro-inflammatory cytokines, as well as in the toll-like receptor genes have been implicated in gastric cancer risk [reviewed in (78, 79)]. A recent pooled analysis from the International Lymphoma Epidemiology Consortium identified polymorphisms in TNF and IL-10, an anti-inflammatory cytokine gene, associated with non-Hodgkin lymphoma (80). Polymorphisms in multiple immune genes, including IL-10, have been associated with gallbladder cancer (81). Genetic variation in HLA regions has been associated with increased or decreased risk of both non-Hodgkin lymphoma (82) and cervical cancer [reviewed in (83)], and there are many other examples.
A major advantage of evaluating inherited genes is that there is no potential for disease effects because germline DNA is clearly present prior to the development of cancer. Another major advantage is that germline DNA is extracted from blood and is easily available from both cases and controls. A disadvantage is that this type of genetic evaluation does not provide information about the functionality of the identified genes.
Functionality may be evaluated by examining gene expression using reverse-transcription PCR-based gene expression analysis, which can provide information about the transcription of immune-related genes. This approach is valuable because it can be applied in both tissue and blood, if the samples are collected to preserve RNA. Thus, it can provide information about immune responses in the local tumor and non-tumor environment, and also systemically. For example, analyses from a population-based case control study of lung cancer found that immune-related gene expression in non-tumor tissue could differentiate smokers and non-smokers, suggesting that smoking affects immune-related gene expression (84), and cases and controls could be distinguished by differential T-cell receptor gene expression in peripheral whole blood, providing additional evidence of immune involvement in lung carcinogenesis (85). Such analyses of local and systemic gene expression can provide a more comprehensive assessment of immune changes in the patient as a whole.
Although gene expression can provide some information about the functionality of immune-related pathways, it does not provide information about the end product of that pathway: proteins. Measurement of circulating immune markers such as cytokines can provide information about immune-related proteins. As demonstrated by a recent evaluation in the Prostate, Lung, Colorectal, and Ovarian Cancer Trial, immune markers can be measured reliably with multiplexed technology in both serum and plasma (86). However, systemic levels of immune-related proteins may or may not reflect the immune response occurring in the tissue where the cancer arose. Directly examining these proteins through IHC in the tissue can thus provide important local-level information that compliments other approaches to measuring immune response.
By combining these biological measures, it is possible to take advantage of their strengths while addressing their limitations. Such an integrative approach can provide a more complete picture of how the immune response contributes to carcinogenesis.
Conclusions
In summary, tissue-based studies can be used to examine associations between immune markers and cancer outcomes, evaluate and possibly discover new tumor subtypes, and identify clues to the biological mechanisms that contribute to the development of cancer. For each of these approaches, it is important to consider the characteristics of the study population, choose the most suitable study design, use proper techniques to select study participants and their biological samples, employ validated, reliable assays, and appropriately assess confounding. Using the principles and tools of molecular epidemiology, we can improve our understanding of immune factors and how they relate to cancer development and progression.
Acknowledgements
We thank Drs. Brad Nelson and Ruth Pfeiffer for their critical review of this manuscript and Drs. Ruth Pfeiffer and Philip Rosenberg for their statistical expertise. This paper is based on a symposium entitled “Tissue-based immune markers and cancer” presented at the 3rd Congress of Epidemiology held in Montreal, Canada, on June 22, 2011.
Abbreviations
- (CCL)
chemokine (C-C motif) ligand
- (CXCL)
chemokine (C-X-C motif) ligand
- (CIN)
cervical intraepithelial neoplasia
- (ER)
estrogen receptor
- (Foxp3)
forkhead box P3
- (HLA)
human leukocyte antigen
- (HPV)
human papillomavirus
- (IDO)
indoleamine 2,3-dioxygenase
- (IF)
immunofluorescence
- (IHC)
immunohistochemistry
- (IFN)
interferon
- (IL)
interleukin
- (MDSCs)
myeloid-derived suppressive cells
- (NK)
natural killer
- (PCR)
polymerase chain reaction
- (PR)
progesterone receptor
- (Tregs)
regulatory T cells
- (TMA)
tissue microarray
- (TGF)
transforming growth factor
- (TAMs)
tumor-associated macrophages
- (TNF)
tumor necrosis factor
- (M1)
type 1 macrophages
- (M2)
type 2 macrophages
References
- 1.Burnet M. Cancer: A Biological Approach. The British Medical Journal. 1957;1:779–86. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz TF. Cancer and viral infections in immunocompromised individuals. International Journal of Cancer. 2009;125:1755–63. doi: 10.1002/ijc.24741. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 5.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 6.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 8.Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson SK, Kerr KM, Chapman AD, Kennedy MM, King G, Cockburn JS, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27:27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 10.Ladányi A, Kiss J, Somlai B, Gilde K, Fejös Z, Mohos A, et al. Density of DC-LAMP+ dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunology, Immunotherapy. 2007;56:1459–69. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of Breast Cancer Risk Factors With Tumor Subtypes: A Pooled Analysis From the Breast Cancer Association Consortium Studies. Journal of the National Cancer Institute. 2010 doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365–7. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Febbo PG, Ladanyi M, Aldape KD, De Marzo AM, Hammond ME, Hayes DF, et al. NCCN Task Force report: Evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9(Suppl 5):S1–32. doi: 10.6004/jnccn.2011.0137. quiz S3. [DOI] [PubMed] [Google Scholar]
- 15.PR2085a. [cited 2011 July 7]; High-density (114 cases/208 cores) prostate adenocarcinoma (I-4 grade) and normal prostate (from autopsy and cancer adjacent) tissue array] Available from: http://www.biomax.us/tissue-arrays/Prostate/PR2085a.
- 16.Tissue Array Networks. [cited 2011 July 23]; Available from: http://www.tissue-array.net/
- 17.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–8. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 19.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived from Ly6C(high) Monocytes. Cancer Research. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 20.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–8. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 21.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends in immunology. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, Lei K-j, Li L, Marinos N, et al. Conversion of Peripheral CD4+CD25-Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. The Journal of Experimental Medicine. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 27.Littman DR, Rudensky AY. Th17 and Regulatory T Cells in Mediating and Restraining Inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic Significance of Activated CD8+ T Cell Infiltrations within Esophageal Carcinomas. Cancer Research. 2001;61:3932–6. [PubMed] [Google Scholar]
- 29.Haas M, Dimmler A, Hohenberger W, Grabenbauer G, Niedobitek G, Distel L. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterology. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, et al. Functional Attributes of Mucosal Immunity in Cervical Intraepithelial Neoplasia and Effects of HIV Infection. Cancer Research. 2004;64:6766–74. doi: 10.1158/0008-5472.CAN-04-1091. [DOI] [PubMed] [Google Scholar]
- 31.Kovacic MB, Katki HA, Kreimer AR, Sherman ME. Epidemiologic analysis of histologic cervical inflammation: relationship to human papillomavirus infections. Human Pathology. 2008;39:1088–95. doi: 10.1016/j.humpath.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Nedergaard BS, Nielsen K, Nyengaard JR, Ladekarl M. Stereologic estimation of the total numbers, the composition and the anatomic distribution of lymphocytes in cone biopsies from patients with stage I squamous cell carcinoma of the cervix uteri. APMIS. 2007;115:1321–30. doi: 10.1111/j.1600-0643.2007.00655.x. [DOI] [PubMed] [Google Scholar]
- 33.Shen Z, Zhou S, Wang Y, Li R-l, Zhong C, Liang C, et al. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. Journal of Cancer Research and Clinical Oncology. 2010;136:1585–95. doi: 10.1007/s00432-010-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan D, Gallagher W. Prognostic ability of a panel of immunohistochemistry markers - retailoring of an ‘old solution’. Breast Cancer Research. 2008;10:102. doi: 10.1186/bcr1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brennan M-L, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic Value of Myeloperoxidase in Patients with Chest Pain. New England Journal of Medicine. 2003;349:1595–604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 36.Whitcup SM, Belfort R, Jr, de Smet MD, Palestine AG, Nussenblatt RB, Chan C-C. Immunohistochemistry of the Inflammatory Response in Propionibacterium acnes Endophthalmitis. Arch Ophthalmol. 1991;109:978–9. doi: 10.1001/archopht.1991.01080070090041. [DOI] [PubMed] [Google Scholar]
- 37.Cregger M, Berger AJ, Rimm DL. Immunohistochemistry and Quantitative Analysis of Protein Expression. Archives of Pathology & Laboratory Medicine. 2006;130:1026–30. doi: 10.5858/2006-130-1026-IAQAOP. [DOI] [PubMed] [Google Scholar]
- 38.Brennan DJ, O’Connor DP, Rexhepaj E, Ponten F, Gallagher WM. Antibody-based proteomics: fast-tracking molecular diagnostics in oncology. Nat Rev Cancer. 2010;10:605–17. doi: 10.1038/nrc2902. [DOI] [PubMed] [Google Scholar]
- 39.Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, et al. Antibody validation. Biotechniques. 2010;48:197–209. doi: 10.2144/000113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beranek JT. CD68 is not a macrophage-specific antigen. Annals of the Rheumatic Diseases. 2005;64:342–4. [PMC free article] [PubMed] [Google Scholar]
- 41.Camp RL, Neumeister V, Rimm DL. A Decade of Tissue Microarrays: Progress in the Discovery and Validation of Cancer Biomarkers. Journal of Clinical Oncology. 2008;26:5630–7. doi: 10.1200/JCO.2008.17.3567. [DOI] [PubMed] [Google Scholar]
- 42.Dahlman A, Rexhepaj E, Brennan DJ, Gallagher WM, Gaber A, Lindgren A, et al. Evaluation of the prognostic significance of MSMB and CRISP3 in prostate cancer using automated image analysis. Mod Pathol. 2011;24:708–19. doi: 10.1038/modpathol.2010.238. [DOI] [PubMed] [Google Scholar]
- 43.Moeder CB, Giltnane JM, Moulis SP, Rimm DL. Quantitative, fluorescence-based in-situ assessment of protein expression. Methods Mol Biol. 2009;520:163–75. doi: 10.1007/978-1-60327-811-9_12. [DOI] [PubMed] [Google Scholar]
- 44.Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr Med Chem. 2011;18:2257–62. doi: 10.2174/092986711795656072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T, Jin R, Huang Z, Hong W, Chen Z, Wang J. The variation of expression of CD4+ CD25+ Foxp3+ regulatory T cells in patients with Helicobacter pylori infection and eradication. Hepatogastroenterology. 2010;57:430–5. [PubMed] [Google Scholar]
- 46.Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277–88. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Wilson EB, Brooks DG. The role of IL-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol. 2011;350:39–65. doi: 10.1007/82_2010_96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–12. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fowler DW, Copier J, Wilson N, Dalgleish AG, Bodman-Smith MD. Mycobacteria activate gammadelta T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmeyer KA, Jeon H, Zang X. The PD-1/PD-L1 (B7-H1) Pathway in Chronic Infection-Induced Cytotoxic T Lymphocyte Exhaustion. J Biomed Biotechnol. 2011;2011:451694. doi: 10.1155/2011/451694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engels EA. Cancer risk associated with receipt of vaccines contaminated with simian virus 40: epidemiologic research. Expert Rev Vaccines. 2005;4:197–206. doi: 10.1586/14760584.4.2.197. [DOI] [PubMed] [Google Scholar]
- 52.Koshiol J, Kreimer AR. Lessons from Australia: human papillomavirus is not a major risk factor for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:1889–92. doi: 10.1158/1055-9965.EPI-10-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koshiol J, Wang E, Zhao Y, Marincola F, Landi MT. Strengths and limitations of laboratory procedures for microRNA detection. Cancer Epidemiol Biomarkers Prev. 2010;19:907–11. doi: 10.1158/1055-9965.EPI-10-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Lippincott Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 55.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herrero R, Schiffman MH, Bratti C, Hildesheim A, Balmaceda I, Sherman ME, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Rev Panam Salud Publica. 1997;1:362–75. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 57.Safaeian M, Hildesheim A, Gonzalez P, Yu K, Porras C, Li JQ, et al. Single nucleotide polymorphisms in the PRDX3 and RPS19 and risk of HPV persistence and cervical precancer/cancer. doi: 10.1371/journal.pone.0033619. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang SS, Zuna RE, Wentzensen N, Dunn ST, Sherman ME, Gold MA, et al. Human Papillomavirus Cofactors by Disease Progression and Human Papillomavirus Types in the Study to Understand Cervical Cancer Early Endpoints and Determinants. Cancer Epidemiology Biomarkers & Prevention. 2009;18:113–20. doi: 10.1158/1055-9965.EPI-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wentzensen N, Schiffman M, Dunn ST, Zuna RE, Walker J, Allen RA, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. International Journal of Cancer. 2009;124:964–9. doi: 10.1002/ijc.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2nd ed. John Wiley & Sons; New York: 1999. [Google Scholar]
- 61.Anderson WF, Pfeiffer RM, Dores GM, Sherman ME. Comparison of age distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1899–905. doi: 10.1158/1055-9965.EPI-06-0191. [DOI] [PubMed] [Google Scholar]
- 62.Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100:1804–14. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grubeck-Loebenstein B, Wick G. Advances in Immunology. Academic Press; 2002. The aging of the immune system; pp. 243–84. [DOI] [PubMed] [Google Scholar]
- 64.Forbes MR. On sex differences in optimal immunity. Trends in Ecology & Evolution. 2007;22:111–3. doi: 10.1016/j.tree.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Lichtman MA, Vaughan JH, Hames CG. The distribution of serum immunoglobulins, anti-gamma-G globulins (“rheumatoid factors”) and antinuclear antibodies in White and Negro subjects in Evans County, Georgia. Arthritis Rheum. 1967;10:204–15. doi: 10.1002/art.1780100306. [DOI] [PubMed] [Google Scholar]
- 66.Mili F, Flanders WD, Boring JR, Annest JL, Destefano F. The associations of race, cigarette smoking, and smoking cessation to measures of the immune system in middle-aged men. Clin Immunol Immunopathol. 1991;59:187–200. doi: 10.1016/0090-1229(91)90017-5. [DOI] [PubMed] [Google Scholar]
- 67.Tollerud DJ, Brown LM, Blattner WA, Weiss ST, Maloney EM, Kurman CC, et al. Racial differences in serum immunoglobulin levels: relationship to cigarette smoking, T-cell subsets, and soluble interleukin-2 receptors. J Clin Lab Anal. 1995;9:37–41. doi: 10.1002/jcla.1860090107. [DOI] [PubMed] [Google Scholar]
- 68.de-The G, Day NE, Geser A, Lavoue MF, Ho JH, Simons MJ, et al. Sero-epidemiology of the Epstein-Barr virus: preliminary analysis of an international study - a review. IARC Sci Publ. 1975:3–16. [PubMed] [Google Scholar]
- 69.Piruzyan L, Mikhailovskii E. Metabolic, ethnic, constitutional specificity of antibacterial immunity. Human Physiology. 2009;35:357–68. [PubMed] [Google Scholar]
- 70.Wallace TA, Martin DN, Ambs S. Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabo G, Mandrekar P. A Recent Perspective on Alcohol, Immunity, and Host Defense. Alcoholism: Clinical and Experimental Research. 2009;33:220–32. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson BL, Cu-Uvin S. Clinical parameters essential to methodology and interpretation of mucosal responses. Am J Reprod Immunol. 2011;65:352–60. doi: 10.1111/j.1600-0897.2010.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. The Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 74.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent Human Papillomavirus Infection and Cervical Neoplasia: A Systematic Review and Meta-Analysis. American Journal of Epidemiology. 2008;168:123–37. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dawsey SM, Lewin KJ, Liu FS, Wang GQ, Shen Q. Esophageal morphology from Linxian, China. Squamous histologic findings in 754 patients. Cancer. 1994;73:2027–37. doi: 10.1002/1097-0142(19940415)73:8<2027::aid-cncr2820730803>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 76.Dawsey SM, Lewin KJ, Wang G-Q, Liu F-S, Nieberg RK, Yu Y, et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus.A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686–92. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 77.Wang G-Q, Abnet CC, Shen Q, Lewin KJ, Sun X-D, Roth MJ, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–92. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milne A, Carneiro F, O’Morain C, Offerhaus G. Nature meets nurture: molecular genetics of gastric cancer. Human Genetics. 2009;126:615–28. doi: 10.1007/s00439-009-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–20. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 80.Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, et al. Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: a report from the InterLymph Consortium. Lancet Oncol. 2006;7:27–38. doi: 10.1016/S1470-2045(05)70434-4. [DOI] [PubMed] [Google Scholar]
- 81.Hsing AW, Sakoda LC, Rashid A, Andreotti G, Chen J, Wang BS, et al. Variants in inflammation genes and the risk of biliary tract cancers and stones: a population-based study in China. Cancer Res. 2008;68:6442–52. doi: 10.1158/0008-5472.CAN-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang SS, Abdou AM, Morton LM, Thomas R, Cerhan JR, Gao X, et al. Human leukocyte antigen class I and II alleles in non-Hodgkin lymphoma etiology. Blood. 2010;115:4820–3. doi: 10.1182/blood-2010-01-266775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr. 2003:35–40. doi: 10.1093/oxfordjournals.jncimonographs.a003480. [DOI] [PubMed] [Google Scholar]
- 84.Landi MT, Dracheva T, Rotunno M, Figueroa JD, Liu H, Dasgupta A, et al. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS One. 2008;3:e1651. doi: 10.1371/journal.pone.0001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rotunno M, Hu N, Su H, Wang C, Goldstein AM, Bergen AW, et al. A gene expression signature from peripheral whole blood for stage I lung adenocarcinoma. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-10-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaturvedi AK, Kemp TJ, Pfeiffer RM, Biancotto A, Williams M, Munuo S, et al. Evaluation of Multiplexed Cytokine and Inflammation Marker Measurements: a Methodologic Study. Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Löb S, Königsrainer A, Zieker D, Brücher B, Rammensee H-G, Opelz G, et al. IDO1 and IDO2 are expressed in human tumors: levo-but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunology, Immunotherapy. 2009;58:153–7. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: Polarization towards pro-tumor immunity. Cytokine & growth factor reviews. 2010;21:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neumeister V, Agarwal S, Bordeaux J, Camp RL, Rimm DL. In Situ Identification of Putative Cancer Stem Cells by Multiplexing ALDH1, CD44, and Cytokeratin Identifies Breast Cancer Patients with Poor Prognosis. The American Journal of Pathology. 2010;176:2131–8. doi: 10.2353/ajpath.2010.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proceedings of the National Academy of Sciences. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagaraj S, Gabrilovich DI. Myeloid-Derived Suppressor Cells in Human Cancer. The Cancer Journal. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. 10.1097/PPO.0b013e3181eb358. [DOI] [PubMed] [Google Scholar]
- 92.Junankar SR, Eichten A, Kramer A, de Visser KE, Coussens LM. Analysis of immune cell infiltrates during squamous carcinoma development. J Investig Dermatol Symp Proc. 2006;11:36–43. doi: 10.1038/sj.jidsymp.5650024. [DOI] [PubMed] [Google Scholar]
- 93.Brant R. Inference for Means: Comparing Two Independent Samples. [cited; Available from: http://www.stat.ubc.ca/~rollin/stats/ssize/n2.html.