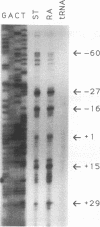
Abstract
The message for the zinc finger gene Rex-1 (Zfp-42) is expressed in undifferentiated murine F9 teratocarcinoma cells and embryonic stem cells. Expression of Rex-1 is reduced at the transcriptional level when F9 cells are induced by the addition of retinoic acid (RA) to differentiate. We have isolated genomic DNA for the Rex-1 gene (Zfp-42), characterized the gene's structure, and mapped the gene to mouse chromosome 8. Promoter elements contributing to the regulation of the Rex-1 promoter in F9 cells have been identified. A region required for Rex-1 promoter activity in F9 stem cells contains an octamer motif (ATTTGCAT) which is a binding site for octamer transcription factor members of the POU domain family of DNA-binding proteins. Rex-1 reporter plasmids including this octamer site also exhibited reduced expression in F9 cells treated with RA. Thus, the octamer motif is a regulatory element required for the activity of the Rex-1 promoter in F9 stem cells, and this motif contributes to the negative regulation by RA of the transcription of the Rex-1 gene. As an initial confirmation of the in vivo relevance of the isolated fragment, a larger Rex-1 promoter fragment, also containing the octamer site, was able to promote expression of the bacterial lacZ gene in mouse embryos at the morula stage.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson M. C., Silver J., Kozak C. A. The mouse homolog of the Gibbon ape leukemia virus receptor: genetic mapping and a possible receptor function in rodents. Virology. 1991 Aug;183(2):778–781. doi: 10.1016/0042-6822(91)91010-e. [DOI] [PubMed] [Google Scholar]
- Annweiler A., Müller-Immerglück M., Wirth T. Oct2 transactivation from a remote enhancer position requires a B-cell-restricted activity. Mol Cell Biol. 1992 Jul;12(7):3107–3116. doi: 10.1128/mcb.12.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth A., Skene B., Swift S., Lovell-Badge R. Zfa is an expressed retroposon derived from an alternative transcript of the Zfx gene. EMBO J. 1990 May;9(5):1529–1534. doi: 10.1002/j.1460-2075.1990.tb08271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth A., Williams B. P., Buchberg A. M., Goodfellow P. N., Solomon E., Potter J., Willison K. R. Chromosomal localization of zinc finger protein genes in man and mouse. Genomics. 1989 Apr;4(3):323–327. doi: 10.1016/0888-7543(89)90337-6. [DOI] [PubMed] [Google Scholar]
- Chowdhury K., Dietrich S., Balling R., Guenet J. L., Gruss P. Structure, expression and chromosomal localization of Zfp-1, a murine zinc finger protein gene. Nucleic Acids Res. 1989 Dec 25;17(24):10427–10438. doi: 10.1093/nar/17.24.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. R., Boam D. S., Docherty K. A new series of CAT expression vectors. Nucleic Acids Res. 1989 Dec 11;17(23):10130–10130. doi: 10.1093/nar/17.23.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatola A. M., Basilico C. Expression of the K-fgf proto-oncogene is controlled by 3' regulatory elements which are specific for embryonal carcinoma cells. Mol Cell Biol. 1990 Jun;10(6):2475–2484. doi: 10.1128/mcb.10.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Felli M. P., Vacca A., Meco D., Screpanti I., Farina A. R., Maroder M., Martinotti S., Petrangeli E., Frati L., Gulino A. Retinoic acid-induced down-regulation of the interleukin-2 promoter via cis-regulatory sequences containing an octamer motif. Mol Cell Biol. 1991 Sep;11(9):4771–4778. doi: 10.1128/mcb.11.9.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach G., Johnson M. H., Braude P. R., Taylor R. A., Bolton V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1(6):681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A., LePage D., Pons G., Mader S. L., Park K., Atchison M. L., Walsh K. Functional antagonism between YY1 and the serum response factor. Mol Cell Biol. 1992 Sep;12(9):4209–4214. doi: 10.1128/mcb.12.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Heilstedt-Williamson H., Gray T. A., Tarlé S. A., Shelton D. A., Tagle D. A., Slightom J. L., Goodman M., Collins F. S. Phylogenetic footprinting reveals a nuclear protein which binds to silencer sequences in the human gamma and epsilon globin genes. Mol Cell Biol. 1992 Nov;12(11):4919–4929. doi: 10.1128/mcb.12.11.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Lehmann J. M., Zhang X. K., Hermann T., Husmann M., Graupner G., Pfahl M. A retinoic acid receptor-specific element controls the retinoic acid receptor-beta promoter. Mol Endocrinol. 1990 Nov;4(11):1727–1736. doi: 10.1210/mend-4-11-1727. [DOI] [PubMed] [Google Scholar]
- Hosler B. A., LaRosa G. J., Grippo J. F., Gudas L. J. Expression of REX-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in F9 teratocarcinoma cells. Mol Cell Biol. 1989 Dec;9(12):5623–5629. doi: 10.1128/mcb.9.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Will H. Molecular biology of viral and nonviral retroelements. Trends Genet. 1989 Nov;5(11):357–359. doi: 10.1016/0168-9525(89)90151-0. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus 'immediate-early' gene transcription in vitro. Nature. 1985 Sep 12;317(6033):179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- Kothary R., Clapoff S., Darling S., Perry M. D., Moran L. A., Rossant J. Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development. 1989 Apr;105(4):707–714. doi: 10.1242/dev.105.4.707. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Peyser M., Krall M., Mariano T. M., Kumar C. S., Pestka S., Mock B. A. Molecular genetic markers spanning mouse chromosome 10. Genomics. 1990 Nov;8(3):519–524. doi: 10.1016/0888-7543(90)90039-w. [DOI] [PubMed] [Google Scholar]
- Kutoh E., Strömstedt P. E., Poellinger L. Functional interference between the ubiquitous and constitutive octamer transcription factor 1 (OTF-1) and the glucocorticoid receptor by direct protein-protein interaction involving the homeo subdomain of OTF-1. Mol Cell Biol. 1992 Nov;12(11):4960–4969. doi: 10.1128/mcb.12.11.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., Kim S. J., Angel P., Roberts A. B., Sporn M. B., Karin M., Wilder R. L. Interleukin-1 stimulates and all-trans-retinoic acid inhibits collagenase gene expression through its 5' activator protein-1-binding site. Mol Endocrinol. 1990 Jul;4(7):973–980. doi: 10.1210/mend-4-7-973. [DOI] [PubMed] [Google Scholar]
- Langston A. W., Gudas L. J. Identification of a retinoic acid responsive enhancer 3' of the murine homeobox gene Hox-1.6. Mech Dev. 1992 Sep;38(3):217–227. doi: 10.1016/0925-4773(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Shi Y., Schwartz R. J. Displacement of BrdUrd-induced YY1 by serum response factor activates skeletal alpha-actin transcription in embryonic myoblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9814–9818. doi: 10.1073/pnas.89.20.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. M., Zhang X. K., Pfahl M. RAR gamma 2 expression is regulated through a retinoic acid response element embedded in Sp1 sites. Mol Cell Biol. 1992 Jul;12(7):2976–2985. doi: 10.1128/mcb.12.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Staudt L., Robbins P., Kuang A., Mulligan R. C., Baltimore D. Repression of the IgH enhancer in teratocarcinoma cells associated with a novel octamer factor. Science. 1989 Jan 27;243(4890):544–546. doi: 10.1126/science.2536195. [DOI] [PubMed] [Google Scholar]
- Linney E. Retinoic acid receptors: transcription factors modulating gene regulation, development, and differentiation. Curr Top Dev Biol. 1992;27:309–350. doi: 10.1016/s0070-2153(08)60538-4. [DOI] [PubMed] [Google Scholar]
- Lu Q., Wallrath L. L., Allan B. D., Glaser R. L., Lis J. T., Elgin S. C. Promoter sequence containing (CT)n.(GA)n repeats is critical for the formation of the DNase I hypersensitive sites in the Drosophila hsp26 gene. J Mol Biol. 1992 Jun 20;225(4):985–998. doi: 10.1016/0022-2836(92)90099-6. [DOI] [PubMed] [Google Scholar]
- Luo Y., Fujii H., Gerster T., Roeder R. G. A novel B cell-derived coactivator potentiates the activation of immunoglobulin promoters by octamer-binding transcription factors. Cell. 1992 Oct 16;71(2):231–241. doi: 10.1016/0092-8674(92)90352-d. [DOI] [PubMed] [Google Scholar]
- MacLeod C. L., Finley K., Kakuda D., Kozak C. A., Wilkinson M. F. Activated T cells express a novel gene on chromosome 8 that is closely related to the murine ecotropic retroviral receptor. Mol Cell Biol. 1990 Jul;10(7):3663–3674. doi: 10.1128/mcb.10.7.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Yang X. Y., Folk W. R. Expression of a 91-kilodalton PEA3-binding protein is down-regulated during differentiation of F9 embryonal carcinoma cells. Mol Cell Biol. 1992 May;12(5):2213–2221. doi: 10.1128/mcb.12.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Okazawa H., Okamoto K., Ishino F., Ishino-Kaneko T., Takeda S., Toyoda Y., Muramatsu M., Hamada H. The oct3 gene, a gene for an embryonic transcription factor, is controlled by a retinoic acid repressible enhancer. EMBO J. 1991 Oct;10(10):2997–3005. doi: 10.1002/j.1460-2075.1991.tb07850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C. J., Shelly L. L., Rabin D. S., Chou J. Y. Inhibition of tyrosine aminotransferase gene expression by retinoic acid. Mol Endocrinol. 1992 Apr;6(4):572–580. doi: 10.1210/mend.6.4.1350056. [DOI] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A., Heguy A., Fujii H., Roeder R. G. Activation of octamer-containing promoters by either octamer-binding transcription factor 1 (OTF-1) or OTF-2 and requirement of an additional B-cell-specific component for optimal transcription of immunoglobulin promoters. Mol Cell Biol. 1990 Dec;10(12):6204–6215. doi: 10.1128/mcb.10.12.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. B., Hosler B. A., Gudas L. J. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 1991 Nov;113(3):815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- Rosner M. H., Vigano M. A., Ozato K., Timmons P. M., Poirier F., Rigby P. W., Staudt L. M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990 Jun 21;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Schoorlemmer J., Kruijer W. Octamer-dependent regulation of the kFGF gene in embryonal carcinoma and embryonic stem cells. Mech Dev. 1991 Dec;36(1-2):75–86. doi: 10.1016/0925-4773(91)90074-g. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Balling R., Hatzopoulos A. K., Suzuki N., Gruss P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J. 1989 Sep;8(9):2551–2557. doi: 10.1002/j.1460-2075.1989.tb08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Ciesiolka T., Gruss P. A nexus between Oct-4 and E1A: implications for gene regulation in embryonic stem cells. Cell. 1991 Jul 26;66(2):291–304. doi: 10.1016/0092-8674(91)90619-a. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Dressler G. R., Balling R., Rohdewohld H., Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990 Jul;9(7):2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R. Octamania: the POU factors in murine development. Trends Genet. 1991 Oct;7(10):323–329. doi: 10.1016/0168-9525(91)90422-m. [DOI] [PubMed] [Google Scholar]
- Schüle R., Evans R. M. Cross-coupling of signal transduction pathways: zinc finger meets leucine zipper. Trends Genet. 1991 Nov-Dec;7(11-12):377–381. doi: 10.1016/0168-9525(91)90259-s. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Shi Y., Shenk T. YY1 is an initiator sequence-binding protein that directs and activates transcription in vitro. Nature. 1991 Nov 21;354(6350):241–245. doi: 10.1038/354241a0. [DOI] [PubMed] [Google Scholar]
- Shapiro M., Kozak C. A. Genetic mapping in the mouse of four loci related to the jun family of transcriptional activators. Somat Cell Mol Genet. 1991 Jul;17(4):341–347. doi: 10.1007/BF01233059. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Rohdewohld H., Neuman T., Gruss P., Schöler H. R. Oct-6: a POU transcription factor expressed in embryonal stem cells and in the developing brain. EMBO J. 1990 Nov;9(11):3723–3732. doi: 10.1002/j.1460-2075.1990.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wieland S., Döbbeling U., Rusconi S. Interference and synergism of glucocorticoid receptor and octamer factors. EMBO J. 1991 Sep;10(9):2513–2521. doi: 10.1002/j.1460-2075.1991.tb07791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. H., Cowie A., Lachance P., Hassell J. A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992 Mar;6(3):481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Zhang X. K., Graupner G., Tzukerman M., Sakamoto B., Karin M., Pfahl M. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 1991 Dec;3(12):1206–1219. [PubMed] [Google Scholar]
- Zheng Z. S., Polakowska R., Johnson A., Goldsmith L. A. Transcriptional control of epidermal growth factor receptor by retinoic acid. Cell Growth Differ. 1992 Apr;3(4):225–232. [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- el-Baradi T., Pieler T. Zinc finger proteins: what we know and what we would like to know. Mech Dev. 1991 Nov;35(3):155–169. doi: 10.1016/0925-4773(91)90015-x. [DOI] [PubMed] [Google Scholar]
- von Melchner H., DeGregori J. V., Rayburn H., Reddy S., Friedel C., Ruley H. E. Selective disruption of genes expressed in totipotent embryonal stem cells. Genes Dev. 1992 Jun;6(6):919–927. doi: 10.1101/gad.6.6.919. [DOI] [PubMed] [Google Scholar]