Abstract
In eukaryotes, ribosome biogenesis involves the nucleolar transcription and processing of pre-ribosomal RNA molecules (pre-rRNA) in a complex pathway requiring the participation of myriad protein and ribonucleoprotein factors. Through efforts aimed at categorizing and characterizing these factors, at least 20 RNA helicases have been shown to interact with or participate in the activities of the major ribosome biogenesis complexes. Unfortunately, little is known about the enzymatic properties of most of these helicases, and less is known about their roles in ribosome biogenesis and pre-rRNA maturation. This chapter presents approaches for characterizing RNA helicases involved in ribosome biogenesis. Included are methods for depletion of specific protein targets, with standard protocols for assaying the typical ribosome biogenesis defects that may result. Procedures and rationales for mutagenic studies of target proteins are discussed, as well as several approaches for identifying protein–protein interactions in order to determine functional context and potential cofactors of RNA helicases.
1. Introduction
RNA helicases comprise a well-conserved class of NTP-dependent nucleic acid remodeling enzymes found throughout all kingdoms of life. In eukaryotes, these proteins have been shown to act in nearly every cellular processes involving RNA (Jankowsky, 2011; Tanner and Linder, 2001). RNA helicases use NTPs to bind to and act on RNA or ribonucleoprotein (RNP) substrates (Liu et al., 2008; Pyle, 2008). Although these enzymes tend to exhibit little or no substrate specificity in vitro, they demonstrate a high degree of specificity in the cell, and frequently play essential roles in important biological processes, including ribosome biogenesis (RB; Bernstein et al., 2006; Blum et al., 1992; Granneman et al., 2006a; Jankowsky, 2011; Wahl et al., 2009).
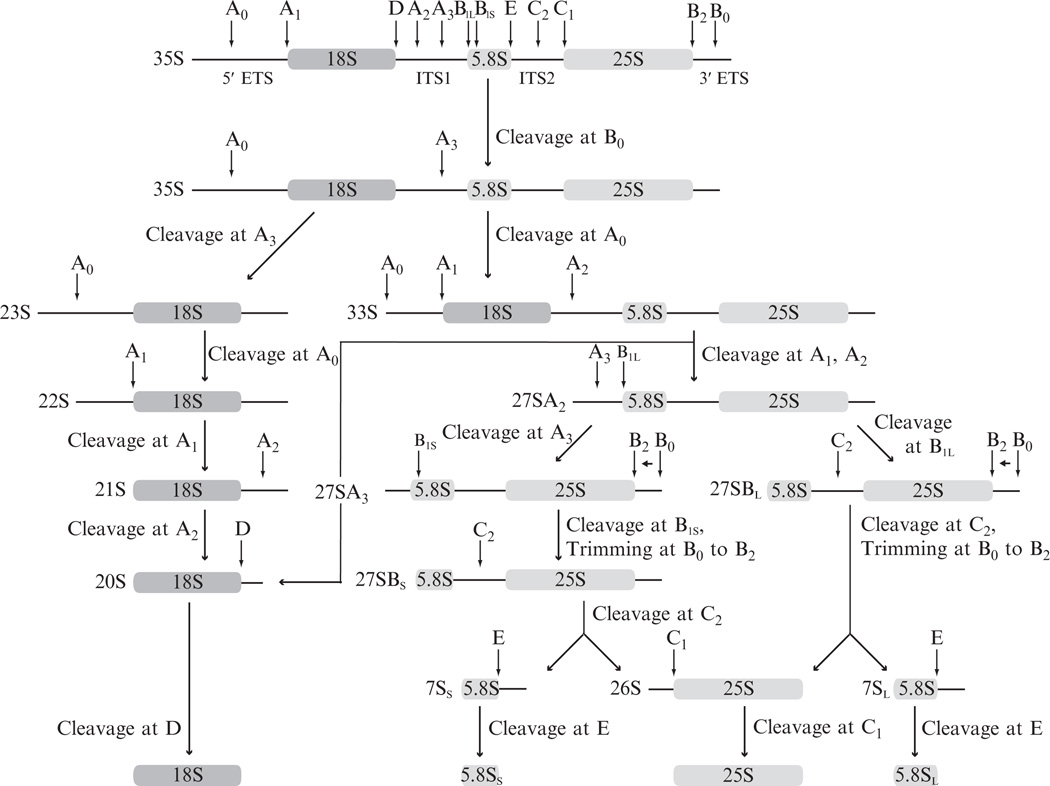
In eukaryotes, RB begins with the production of a large precursor transcript (pre-rRNA), which contains sequences destined for incorporation into both the small and large subunits (SSU and LSU) of the mature ribosome. This transcript is cleaved at several sites in order to separate the individual rRNA sequences, generating three fragments which are then further trimmed until only the rRNA sequences remain (Fig. 14.1; Granneman and Baserga, 2004; Henras et al., 2008; Kressler et al., 2010). The rRNA sequences also undergo a number of chemical modifications, mostly consisting of pseudouridylation and 2′-O-ribose methylation (Decatur and Fournier, 2002). Concomitant with the enzymatic modifications that take place on the nascent transcript, the individual rRNA sequences begin folding as they interact with a subset of ribosomal proteins, before both the pre-SSU and pre-LSU are exported from the nucleus for final maturation in the cytoplasm.
Figure 14.1.
Pre-rRNA processing in Saccharomyces cerevisiae. Boxed regions of the transcript represent the rRNA sequences. Cleavage sites are designated by vertical arrows below their common designations. Endonucleolytic events are indicated with horizontal arrows pointing in the direction of degradation. The sedimentation designation of each pre-rRNA species is indicated adjacent to its 5′ terminus.
To date, at least 20 RNA helicases have been implicated as eukaryotic RB factors (Bleichert and Baserga, 2007; Jankowsky, 2011; Kressler et al., 2010). Given the size and complexity of rRNA and the plethora of proteins and RNPs involved in its maturation, it is easy to envision a variety of potential roles for RNA helicases in the RB process. For instance, a number of the cleavage and chemical modification events that take place in the nucleolus require hybridization of the pre-rRNA to the snoRNA factors that mediate these events, and it has been shown that the depletion of several RNA helicases—Dbp4 (Kos and Tollervey, 2005), Has1 (Liang and Fournier, 2006), and Rok1 (Bohnsack et al., 2008)—results in accumulation of snoRNPs in large pre-rRNA-containing particles. Another RNA helicase, Mtr4, aids in pre-rRNA “trimming” by preparing the transcript for degradation by the nuclear exosome (de la Cruz et al., 1998a,b). Indeed, most RB helicases identified thus far have been shown to be essential (Table 14.1; Bernstein et al., 2006; Granneman et al., 2006a), highlighting their importance in this fundamental system. However, despite continued efforts aimed at better understanding these enzymes, a great deal remains to be learned about their specific roles in the ribosome maturation process, as well as their individual enzymatic behaviors. The following chapter presents methods for evaluating the role(s) played by RNA helicases in the pre-rRNA maturation process in vivo. Additionally, methods for assaying protein–protein interactions are discussed as a means of identifying potential cofactors for these enzymes and determining where a target enzyme may be acting in the context of the RB machinery.
Table 14.1.
RNA helicases involved in ribosome biogenesis
| Gene designation (S. cerevisiae) |
Essential? | Helicase family |
Associated pre-rRNA processing defects | References | Human homolog |
|---|---|---|---|---|---|
| Small subunit (SSU) | |||||
| Dhr1 | Y | DEAH | Deficient in cleavage at A0, A1, and A2 | Colley et al. (2000), Granneman et al. (2006a) | DHX37 |
| Dhr2 | Y | DEAH | Deficient in cleavage at A0, A1, and A2 | Colley et al. (2000), Granneman et al. (2006a) | DHX32 |
| Dbp4 | Y | DEAD | Deficient in cleavage at A2, likely due to non-productive accumulation of U14 snoRNP on pre-rRNA transcript | Kos and Tollervey (2005) | DDX10 |
| Dbp8 | Y | DEAD | Deficient in cleavage at A0, A1, and A2 | Daugeron and Linder (2001), Granneman et al. (2006a) | DDX49 |
| Fal1 | Y | DEAD | Deficient in cleavage at A0, A1, and A2 | Kressler et al. (1997), Granneman et al. (2006a) | DDX48 |
| Rok1 | Y | DEAD | Deficient in cleavage at A0, A1, and A2, possibly due to non-productive accumulation of snR30 on pre-rRNA transcript | Venema et al. (1997), Bohnsack et al. (2008) | DDX52 |
| Rrp3 | Y | DEAD | Deficient in cleavage at A1 and A2 | O’Day et al. (1996), Granneman et al. (2006a) | DDX47 |
| Large subunit (LSU) | |||||
| Dbp3 | N | DEAD | Deficient in cleavage at A3 | Weaver et al. (1997) | N/A |
| Dbp6 | Y | DEAD | Reduction in 27S and 7S pre-rRNA levels | Kressler et al. (1998) | DDX51 |
| Dbp7 | N | DEAD | Reduction in 27S and 7S pre-rRNA levels | Daugeron and Linder (1998) | DDX31 |
| Dbp9 | Y | DEAD | Reduction in 27S and 7S pre-rRNA levels | Daugeron et al. (2001) | DDX56 |
| Dbp10 | Y | DEAD | Deficient in processing 27SB intermediates | Burger et al. (2000) | DDX54 |
| Drs1 | Y | DEAD | Reduction in mature 25S rRNA levels | Ripmaster et al. (1992), Bernstein et al. (2006) | DDX27 |
| Mak5 | Y | DEAD | Deficient in cleavage at A0, A1, and A2 | Bernstein et al. (2006) | DDX24 |
| Spb4 | Y | DEAD | Deficient in cleavage at A0, A1, A2, C1, and C2 | de la Cruz et al. (1998a,b), Bernstein et al. (2006) | DDX55 |
| Mtr4 | Y | Ski2-like | 7S pre-rRNA accumulation; deficient in cleavage at A0, A1, A2, and C2 | de la Cruz et al. (1998), Bernstein et al. (2006) | SKIL2 |
| Both subunits | |||||
| Has1 | Y | DEAH | Deficient in cleavage at A0, A1, and A2; Deficient in processing 27SA3 and 27SB intermediates; Accumulation of snoRNAs in pre-rRNA particles | Emery et al. (2004), Liang and Fournier (2006) | DDX18 |
| Prp43 | Y | DEAH | Deficient in processing 35S, 27S, and 20S intermediates | Combs et al. (2006), Leeds et al. (2006) | DHX15 |
2. Experimental Strategies Used to Evaluate RB Helicases
In determining whether an RNA helicase plays a role in RB, a first step should be to determine whether this protein localizes to the nucleolus. Nucleolar localization is a strong indicator that a target enzyme may be involved in the ribosome maturation process, and because nucleolar localization has already been demonstrated for a number of putative RNA helicases in both yeast and mammalian systems, it may be possible to identify potential targets by querying a public database. In yeast, protein localization has been investigated for much of the proteome (Huh et al., 2003; Kumar et al., 2002; Ross-Macdonald et al., 1999), and localization information for helicases identified in Saccharomyces cerevisiae can be obtained through the Saccharomyces Genome Database at (http://www.yeastgenome.org). Several studies have also identified a number of proteins present in the human nucleolus (Anderson et al., 2002, 2005; Scherl et al., 2002), including many RNA helicases that exhibit homology to those implicated in yeast RB. The results of these studies have been compiled by Leung et al. (2006) and are available at the Nucleolar Protein Database (http://www.lamondlab.com/NOPdb).
Although the presence of a protein in the nucleolus is suggestive of a potential role in RB, it is by no means a definitive indicator. In order to firmly establish an enzyme as acting in the RB pathway, two complementary strategies are frequently employed. The first involves perturbing the target protein through either depletion or mutagenesis and measuring changes in levels of mature rRNA molecules as well as in levels of intermediate precursors that may accumulate due to defects in pre-rRNA processing (Colley et al., 2000; Combs et al., 2006; Daugeron and Linder, 2001; Leeds et al., 2006). In this way, it is possible to determine whether the enzyme in question is required for efficient production of mature rRNA, and whether its activity is required for transcription, early cleavage events, or later events in the maturation of the SSU, the LSU, or both.
A second, orthogonal approach used to definitively place a helicase in the RB pathway involves identifying the protein or RNP factors with which the helicase associates. A number of the proteins involved in the production and processing of pre-rRNA have already been identified in yeast and mammals. Some of these proteins can serve as markers for a RB pathway (LSU or SSU processing), or, in the case of several LSU proteins, for a particular stage of processing the pathway. Association with these factors can be assayed in either an ensemble or binary manner, yielding information about the super molecular context of the protein and the specific binding partner, respectively (Colley et al., 2000). Examining the interaction network of a target helicase will likely provide enough information to determine whether it acts predominantly in LSU or SSU maturation and may even serve to identify potential cofactors for the enzyme. Unlike the first approach, however, studying binding partners will provide relatively little information about the particular pre-rRNA maturation events in which the target helicase participates.
In conjunction, these strategies make it possible to broadly define the role of a target helicase by elucidating the steps of pre-rRNA processing that are perturbed by disruption of the enzyme’s activity and by providing a context for the protein among the large, dynamic cellular machinery involved in RB. In addition, binding experiments may identify cofactors that can modulate the catalytic activities of the helicase and/or aid in substrate binding. This information can then be used to formulate testable hypotheses regarding the biochemical properties and mechanistic behavior of the target enzyme.
Work done on the yeast DEAH-box protein Prp43 represents an excellent example of experimental development which can serve as a guide for future studies of putative RB helicases. Prp43 was initially identified as a spliceosome disassembly factor (Arenas and Abelson, 1997), but was later shown to be present in preribosome particles as well (Lebaron et al., 2005). Further work showed that Prp43 was required for efficient processing of both SSU and LSU components of pre-rRNA by demonstrating that several rRNA precursors accumulated when a mutant form of the enzyme was introduced into a cell depleted of the wild-type enzyme (Combs et al., 2006; Leeds et al., 2006). Based on the enrichment of the Prp43 binding partner, Pfa1, observed by Lebaron et al. and published in their 2005 study, this group went on to more thoroughly characterize the biochemical consequences of this interaction, eventually demonstrating that Pfa1 stimulates the ATPase and helicase activities of the Prp43 enzyme (Lebaron et al., 2009).
Most recently, a modified version of the high-throughput sequencing with cross linking assisted immunoprecipitation (HITS-CLIP) (Licatalosi et al., 2008; Ule et al., 2005) protocol was developed and optimized for use in yeast (Granneman et al., 2009). This procedure, termed CRAC for cross-linking and cDNA analysis, was used to identify putative target sites for Prp43 on the pre-rRNA in an effort to determine more precisely how its activity contributes to proper pre-rRNA maturation (Bohnsack et al., 2009; see also Chapter 13).
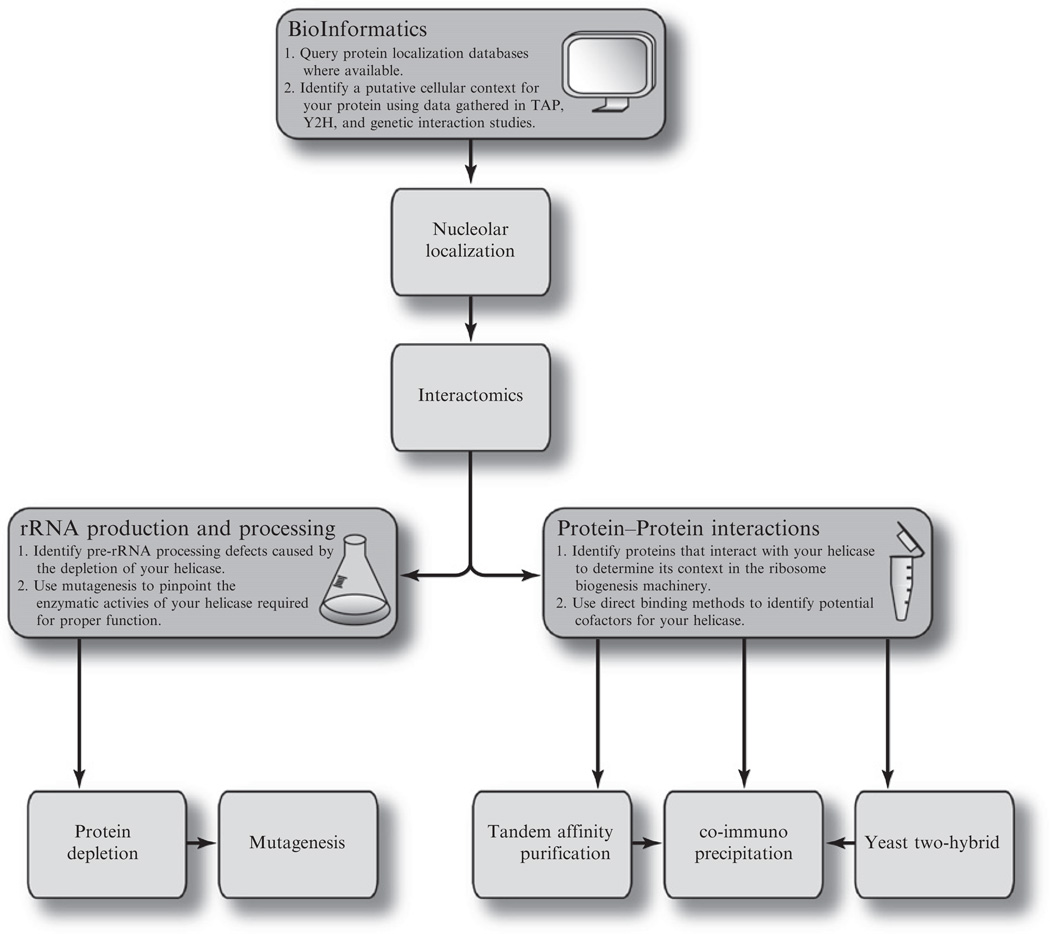
In this work, we will outline a typical experimental workflow designed to elucidate the biological and biochemical properties of a putative RNA helicase involved in RB (Fig 14.2). A number of the methods employed in these experiments have been thoroughly described elsewhere; in these cases, citations for appropriate protocols will be included; however, the discussion presented will focus on considerations that are particular to working with nucleolar helicases and the RB system. RNA helicases involved in eukaryotic RB have been most thoroughly characterized in budding yeast and humans, therefore the majority of the logical and practical considerations presented in this work will refer to and be derived from methods employed in these systems, with an emphasis on those described for S. cerevisiae. Nevertheless, the general reasoning and experimental strategy discussed here will be broadly applicable to eukaryotic systems, though actual protocols may differ significantly depending upon the organism in question.
Figure 14.2.
Flowchart of an experimental strategy designed to efficiently elucidate the role of a putative helicase protein in eukaryotic ribosome biogenesis. Tandem affinity purification is denoted as TAP. Yeast two-hybrid is denoted as Y2H.
3. Determining Where an RNA Helicase Acts in the RB Pathway
In yeast, RB begins with RNA polymerase I-mediated transcription of a 35S precursor that contains the 18S, 5.8S, and 25S rRNA sequences, in addition to two external transcribed spacer (ETS) and two internal transcribed spacer (ITS) sequences. This transcript undergoes a number of site specific nucleolytic cleavage events that are mediated by RB machinery and result in the stepwise production of several well-defined pre-rRNA processing intermediates (Fig. 14.1; Granneman and Baserga, 2004; Henras et al., 2008; Kressler et al., 2010; Venema and Tollervey, 1999). Normally, these intermediates are relatively short lived and are present at low levels during productive ribosome synthesis. Interfering with the normal activity of RB helicases often leads to diminished activity at one or more of the nucleolytic stages in the ribosome maturation process, which therefore results in the accumulation of one or more pre-rRNA precursors concomitant with a decrease in levels of mature rRNA (Combs et al., 2006; de la Cruz et al., 1998a,b; Emery et al., 2004; Kressler et al., 1997; Lee et al., 1992; Leeds et al., 2006; Ripmaster et al., 1992, and others).
Monitoring cellular levels of mature rRNA and pre-rRNA intermediates has become a standard tool used to evaluate RB defects. Because the process of pre-rRNA trimming has been exceptionally well characterized, it is possible to associate a target helicase with a specific stage in ribosome maturation by interfering with its enzymatic activity and observing any changes in rRNA and pre-rRNA levels (Bernstein et al., 2006; Granneman et al., 2006a). Methods for monitoring cellular RNA levels will be discussed, followed by methods for depleting a target helicase and introducing a mutagenized version into the cell, including important considerations for targeting mutagenesis.
3.1. Evaluating RB by monitoring rRNA production
In yeast, collecting and evaluating rRNA from a strain of interest is a fairly straightforward procedure that involves four basic steps: (i) the selected strain is grown to an optimal optical density, (ii) cells are harvested and total RNA is extracted, (iii) RNA species are separated by gel electrophoresis, and (iv) rRNA levels are quantified using either ethidium bromide staining or northern analysis. Important considerations will be discussed and protocols presented for each of these steps.
3.1.1. Selecting and culturing a yeast strain
In selecting a yeast strain, it is important to consider the availability of various metabolic markers for use in subsequent experiments in which the successful transformation of a plasmid or proper chromosomal insertion will have to be selected. A typical example of such a strain is YPH499 (Sikorski and Hieter, 1989), a mating type a strain auxotrophic for uridine, adenine, tryptophan, histidine, lysine, and leucine (denoted MATa ura3–52 lys2–80 ade2–101 trp1-Δ63 his3-Δ200 leu2-Δ1), although a wide variety of backgrounds are acceptable as long as they are compatible with plasmids chosen for use in future experiments. General guidelines for working with yeast are presented below:
Prior to culturing, a new strain should be struck out from a frozen stock onto rich media, as well as media lacking one of each of the purported selectable markers and incubated at 30 °C. Cells should grow only on the rich media. This procedure is used to verify the genotype of a new or freshly thawed strain. If cells grow on media lacking a purported marker, the strain may be contaminated or the genotype may be incorrect.
-
From a frozen stock or freshly struck plate, prepare an overnight culture by collecting a small amount of yeast on the tip of a sterile pipette and inoculating 10 ml liquid YPD media under sterile conditions. Incubate the culture on a shaking platform overnight at 30 °C.
Prepare YPD media by dissolving 20 g/l peptone and 10 g/l Yeast extract in water. Adjust the pH of the solution to 6.5 if necessary and autoclave. After autoclaving, allow the solution to cool to approximately 55 °C. Add glucose to a final concentration of 2% (w/v) from a sterile 20% (w/v) stock solution. Note that in this context, glucose is usually referred to by the antiquated designation dextrose (hence, YPD). This convention has been retained so that media containing galactose, another carbon source used in yeast culture, can be denoted YPG.
The following morning, assay the optical density at 600 nm (OD600) of the culture. Note that the culture may need to be diluted in order to achieve a reading in the linear range of the spectrophotometer.
Dilute an aliquot of the overnight culture into 25 ml of sterile YPD media in a new flask such that the final OD600 = 0.1 AU. Note that the doubling time for most strains of S. cerevisiae in liquid YPD is approximately 2 h at 30 °C; however it is recommended that doubling time be determined experimentally under the precise conditions in which cultures will be grown.
Continue incubating the cells at 30 °C. Allow at least two doubling events to take place, monitoring the optical density occasionally during incubation.
-
Once the cells have reached an OD600 between 0.4 and 0.5 AU, cells should be harvested by centrifugation. The amount of culture harvested depends on the number of cells needed for the particular application. In order to maintain consistency, an equal number of optical density units (ODU) are harvested for each sample. ODU can be calculated by multiplying the volume harvested in ml by the optical density in AU, for example, 10 ml of cells at OD600 = 0.5 AU yields 5 ODU.
For RB studies, cells must not be allowed to grow beyond an OD600 = 0.5, as ribosome synthesis becomes attenuated at this phase of growth, which can significantly alter experimental results (Ju and Warner, 1994).
Resuspend pelleted cells in 1 ml sterile water and transfer to 1.5 ml microcentrifuge tube. Pellet cells again and remove supernatant. Pellet can be flash frozen and stored at −80 °C, although it is recommended that the RNA extraction process be initiated prior to storage; see below.
3.1.2. Total RNA extraction from yeast cells
Due to the inherent instability of RNA and the high possibility of RNase contamination, it is recommended that equipment to be used for RNA extraction is kept free of total cellular material and/or wiped clean using 70% ethanol prior to use. All solutions should be prepared using RNase-free water that has been autoclaved or passed through a 0.22-µm filter. Where possible, work should be performed in a fume hood or designated RNase-free area.
Resuspend 10 ODU of cells in 400 µl TES solution (10 mM Tris (pH 7.5), 10 mM EDTA, 0.5% SDS).
Add 400 µl acid phenol and vortex suspension for 10 s at maximum intensity.
Incubate suspension at 65 °C for 45 min, vortexing briefly every 10 min to homogenize sample.
Centrifuge sample at maximum speed for 5 min at 4 °C.
After centrifugation, two layers will be discernable in the microcentrifuge tube. The top layer is aqueous, while the bottom will be composed of the organic phenol. Remove the aqueous phase and transfer to a clean 1.5 ml tube.
Add 400 µl acid phenol, briefly vortex the tube and centrifuge as in step 5; transfer the aqueous phase to a clean 1.5 ml microcentrifuge tube.
Add 400 µl chloroform, briefly vortex the tube and centrifuge as in step 5; transfer the aqueous phase to a 1.5-ml microcentrifuge tube.
-
The RNA is precipitated to concentrate the sample and remove residual phenol/chloroform. Add 1 ml of ice cold 100% ethanol and 40 µl of 3 M NaOAc.
At this point the sample can be stored at − 80 °C with less potential for sample loss or degradation than if frozen in the total cell pellet after harvesting.
If the sample is not stored at − 80 °C, incubate in a dry ice/ethanol bath for 30 min.
Pellet the RNA by centrifugation at max speed for 20 min at 4 °C.
Remove the supernatant, being careful to disturb the pellet as little as possible.
Add 1 ml ice cold 70% ethanol to the pellet, microfuge for an additional 2 min at max speed.
Remove the supernatant as thoroughly as possible without disturbing the pellet.
Allow the remaining ethanol to evaporate off the pellet by leaving the tube open on the bench for 10 min.
Resuspend the pellet in 50–100 µl of RNase-free water.
RNA can be quantified by measuring the absorbance of the sample at 260 nm, where an A260 of 1 represents 40 µg or RNA per ml of solution.
3.1.3. Evaluating ribosomal RNA levels by gel electrophoresis and northern blotting
Depending on the application, rRNA can be readily visualized by either ethidium bromide staining with UV detection, or by northern blotting and densitometry. The mature 18S and 25S rRNA species can easily be resolved and visualized using a 1.25% agarose TAE gel with ethidium bromide staining (Freed and Baserga, 2010); however, this method is not sufficiently sensitive for accurate determination of the levels of rRNA processing intermediates. To monitor accumulation of various pre-rRNA species, samples should be subjected to electrophoresis in a 1.25% agarose/formaldehyde gel then transferred to a nylon membrane for northern analysis with appropriate radiolabeled oligonucleotides.
3.1.3.1. Gel electrophoresis and transfer
To prepare a 100 ml, 1.25% agarose/formaldehyde gel, begin by dissolving 1.25 g high grade agarose into 72 ml water by heating in a microwave.
Working in a fume hood, allow the agarose solution to cool to the touch. Add 18 ml of 37% formaldehyde solution and 10 ml 10 × MOPS buffer (0.4 M MOPS (pH 7.0), 0.1 M NaOAc, 10 mM EDTA); mix well and pour gel.
Prepare sample loading buffer by combining 1 µl 10 × MOPS buffer, 10 µl formamide, and 3.5 µl 37% formaldehyde per sample. Add 15–20 µg of total RNA in no more than 3 µl total volume to the sample buffer.
Denature RNA by incubating the sample at 65 °C for 15 min.
Add 2 µl loading dye (50% glycerol, 10 mM EDTA, 0.25% (w/v), bromophenol blue) to each sample.
Load samples on 1.25% agarose/formaldehyde gel and run for 22–24 h at 59 V in 1 × MOPS buffer.
Once running is complete, transfer the gel to a clean tray and incubate in 75 mM NaOH for 20 min on an orbital shaker, ensuring that the gel is immersed in the solution.
Wash the gel 2 × with water, then incubate in 10 × SSC buffer (0.3 M NaCl, 30 mM sodium citrate, pH 7.0) for 45 min.
Transfer overnight to a Hybond N+ nylon membrane (www.gelifesciences.com) equilibrated in 10 × SSC using a standard capillary blotting apparatus.
Cross-link RNA to the membrane by exposure to UV light at 0.125 J for 30 s on each side of the membrane.
The blot can be stored at − 20 °C in aluminum foil until use in northern analysis.
3.1.3.2. Northern analysis
Oligonucleotide sequences to be used as probes in northern blotting depend greatly on the particular rRNA species under consideration. In general, levels of 18S and 25S rRNA will be considerably greater than levels of any pre-rRNA; therefore, while a probe complementary to a sequence within the 18S rRNA could theoretically be used to visualize a number of processing intermediates, exposure times necessary to observe pre-rRNA transcripts on a film will result in excessive exposure in the region corresponding to the mature rRNAs. Additionally, certain intermediates—such as 27SA and 27SB—may not be well resolved on the gel, and it might therefore be necessary to select a probe unique to one of these transcripts to definitively locate it on the gel. Probes have already been designed, which can distinguish between many of the important pre-rRNA species and may serve as a useful starting point for further analysis (Table 14.2).
Prepare a 5 × SSPE solution (0.9 M NaCl, 50 mM NaH2PO4 (pH 7.7), 1 mM EDTA).
Place cross-linked membrane containing RNA samples into a hybridization tube with the RNA facing the center. Immediately prior to use, add 20% (w/v) SDS solution to an aliquot of 5 × SSPE to achieve a final concentration of 0.1% SDS. Wet the membrane with this solution and remove any air bubbles using a sterile pipette.
Add between 10 and 25 ml of 5 × SSPE − 0.1% SDS solution depending on the size of the membrane; ensure that the portion of the membrane at the nadir of the tube will be sufficiently immersed.
Place the tube in a hybridization oven at 60 °C and allow it to rotate for 4 h.
Denature 106 cpm of 5′-end-labeled oligonucleotide(s) per ml of 5 × SSPE solution by incubating at 95 °C for 3 min.
Dilute labeled oligonucleotides(s) in 500 µl of 5 × SSPE to ensure better dispersion the hybridization tube.
Add probe mixture to the hybridization tube and incubate in the hybridization oven at 60 °C overnight.
The following day, remove the hybridization tubes from the oven and discard the hybridization buffer, taking care to observe all pertinent radioactive safety protocols.
Wash the blot by adding an equal volume of 5 × SSPE − 0.1% SDS back to the tube and incubating at 60 °C for 15 min.
Remove the wash buffer and repeat the wash.
Remove the wash buffer and add an equal volume of 1 × SSPE − 0.1% SDS to the tube. Incubate at 60 °C for 5 min.
Carefully remove the blot from the hybridization tube and wrap it in clear plastic wrap.
Expose the blot using an appropriate film or intensifying screen for densitometric analysis.
Table 14.2.
Oligonucleotide probes used to detect pre-rRNA processing intermediates
| Designation as published |
Sequence (shown 5′ to 3′) | Region of the pre-rRNA detected |
References |
|---|---|---|---|
| a | CATGGCTTAATCTTTGAGAC | 18S rRNA | |
| b | GCTCTTTGCTCTTGCC | ITS1 between the 18S and A2 | |
| c | ATGAAAACTCCACAGTG | ITS1 between A2 and A3 | Berges et al. (1994) |
| d | CCAGTTACGAAAATTCTTG | ITS1 at A2 | |
| e | GGCCAG CAATTT CAAGT | ITS2 between E and C2 | |
| f | AGATTAGCCGCAGTTGG | ITS2 between C2 and C1 | |
| g | CTCCGCTTATTGATATGC | 25S rRNA | |
| 1 | TCGGGTCTCTCTGCTGC | 5′ ETS before A0 | Venema and Tollervery (1996) |
| 3 | CATGGCTTAATCTTTGAGAC | 5′ region of the 18S rRNA | |
| 4 | CTCCGCTTATTGATATGC | 5′ region of the 25S rRNA | |
| 5 | GCTCTTTGCTCTTGCC | ITS1 between 18S and A2 | |
| 6 | TGTTACCTCTGGGCCC | ITS1 between A2 and A3 | |
| 7 | TTTCGCTGCGTTCTTCATC | 5.8S 3′ of B1S | |
| 8 | AACAGAATGTTTGAGAAGG | ITS2 3′ of E | |
| 9 | GGCCAGCAATTTCAAGTTA | ITS2 5′ of C2 | |
| 1 | GGTCTCTCTGCTGCCGG | 5′ ETS before A0 | Kressler et al. (1997) |
| 2 | CATGGCTTAATCTTTGAGAC | 18S rRNA | |
| 3 | CGGTTTTAATTGTCCTA | ITS1 between D and A2 | |
| 4 | TGTTACCTCTGGGCC | ITS1 between A2 and A3 | |
| 5 | AATTTCCAGTTACGAAAATTCTTG | ITS1 between A3 and B1 | |
| 6 | TTTCGCTGCGTTCTTCAT | 5.8S rRNA | |
| 7 | GGCCAGCAATTTCAAGTT | ITS2 between E and C2 | |
| 8 | GAACATTGTTCGCCTAGA | ITS2 between C1 and C2 | |
| 9 | CTCCGCTTATTGATATGC | 25S rRNA | |
| p18S | CGTCCTATTCTATTATTCCATG | 18S rRNA | Benard et al. (1998) |
| p5.8S | TTTCGCTGGGTTCTTCATC | 5.8S rRNA | |
| p25S | GCCCGTTCCCTTGGCTGTG | 25S rRNA nucleotides 2359 to 2377 | |
| p25S5′ | GCGGGTACTCCTACCTGATTTGAGGTC | 25S rRNA nucleotides 5 to 31 | |
| p25S3′ | CAGCAGATCGTAACAACAAGGCTACTCTAC | 25S rRNA nucleotides 3336 to 3365 | |
| y | GCCCGTTCCCTTGGCTGTG | Mid-25S rRNA | Wehner et al. (2002) |
By running samples from the same conditions on multiple gels and blotting with different probes, alone or in combinations, it is possible to monitor the levels of rRNA processing intermediates and to make direct comparisons between the levels of different pre-rRNA species. Additionally, samples can be taken at intervals subsequent to helicase depletion (see below) to monitor the change in levels of each species of rRNA over time.
3.2. Perturbation of helicase activity for RB studies
Of the 19 RNA helicases implicated as RB factors in yeast, 18 belong to the DExD/H-box family of enzymes (with one Ski2-like helicase, Mtr4) (Bleichert and Baserga, 2007; Jankowsky, 2011; Kressler et al., 2010). The following discussion will therefore be focused on studies, methods, and applications that pertain to this group of proteins.
As mentioned previously, the basic principle governing the standard methodology for investigating RB helicases is simply to somehow abrogate the function of a target enzyme then to determine how this perturbation has affected the quantity and maturation state of rRNA within the cell. Because the majority of RB helicases are essential (Table 14.1), it is necessary to deplete the target enzyme conditionally using genetic manipulation, as simply disrupting the gene would be lethal. Once a strain is developed in which the chromosomal copy of the enzyme can be conditionally depleted successfully, the gene can be reintroduced into the cell on a plasmid, making it possible to perform mutagenic studies in vivo.
3.2.1. Chromosomal insertion of a GAL1 promoter
In order to conditionally reduce the levels of protein in a yeast cell without excessively perturbing the system at large, a gene of interest can be placed under the control of an alternative endogenous promoter that can be activated or deactivated without drastically altering the cellular environment. In yeast, several promoters have been successfully adapted to this task (Belli et al., 1998; Etcheverry, 1990; Longtine et al., 1998; Mumberg et al., 1995), but as the methods employed to put a target gene under the control of any of these promoters are essentially identical, only the GAL1 promoter system will be discussed here. The GAL1 promoter normally controls transcription of genes necessary to metabolize galactose when glucose is no longer available as a carbon source. Taking advantage of this promoter is particularly convenient because activation/deactivation of genes under its control simply requires changing the carbon source with which a culture has been supplemented, a relatively simple and inexpensive process.
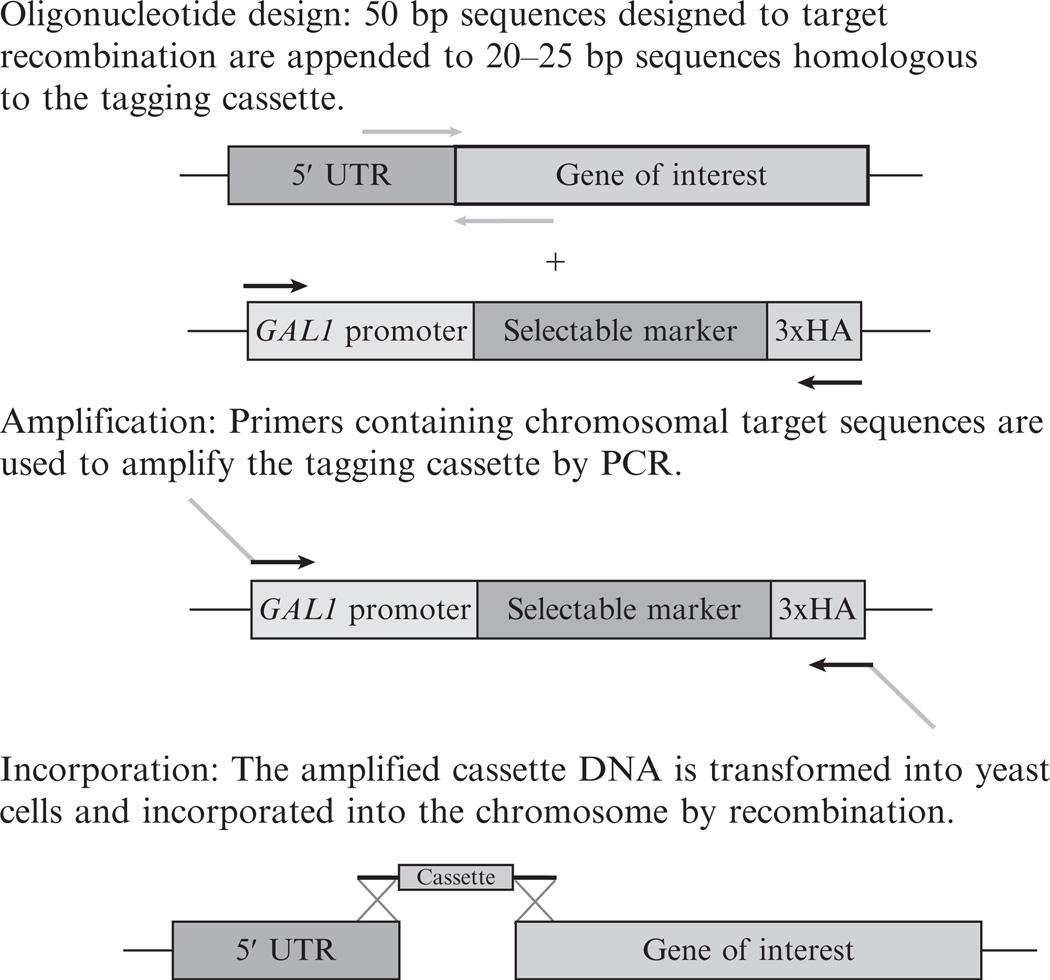
Placing a gene under the control of the GAL1 promoter is accomplished by transforming yeast cells with linear, double stranded DNA encoding the promoter, and a selectable marker flanked by sequences that are homologous to a target site in the yeast chromosome (Fig. 14.3). Once in the cell, the linear DNA undergoes a homologous recombination event, resulting in a chromosomal insertion at a location determined by the flanking sequences (Baudin et al., 1993; Schneider et al., 1995). DNA encoding the promoter and selectable marker can be amplified from plasmids produced by Longtine et al. which have been designed to allow amplification of a “cassette” containing a selectable marker and optional epitope tag in addition to the GAL1 promoter sequence (Longtine et al., 1998).
PCR primers should be designed with the forward primer containing a 50-bp segment homologous to the sense strand of chromosomal DNA immediately 5′ to the gene of interest, followed by 20–30 bp of sequence designed to anneal to the most 5′ portion of the template cassette.
The reverse primer should be the reverse complement of a 50-bp sequence homologous to the 5′ portion of the gene followed by 20–30 bp homologous to the 3′ portion of the template cassette (see Fig. 14.3 and Longtine et al., 1998).
Double stranded DNA for transformation should be generated using standard PCR techniques with a high fidelity polymerase in a 200-µl total reaction volume.
PCR product should be pooled and ethanol precipitated as described in Section 3.1.2. Precipitated DNA should be resuspended in 5–10 µl of sterile water depending on the amount of PCR product produced.
To transform the desired yeast strain, begin by growing a 25-ml culture in YPD media to an OD600 of 0.3–0.6 AU.
Harvest the cells by centrifugation, resuspend in 1 ml sterile water, and transfer to a 1.5-ml microcentrifuge tube.
Pellet the cells again and remove the supernatant.
Prepare a fresh LiAc/TE solution (150 µl 1 M LiAc, 150 µl TE, 1.2 ml sterile water) and LiAc/TE/PEG solution (150 µl 1 M LiAc, 150 µl TE, 1.2 ml 80% PEG).
Resuspend cell pellet in 250 µl LiAc/TE solution, and separate into 50 µl aliquots.
Denature a stock sample of sonicated salmon sperm DNA by incubating at 95 °C for 3 min.
To a single aliquot, add 2.5 µg of DNA (approximately 3‒5 µl of the precipitated PCR product), 5 µl sonicated salmon sperm DNA, and 300 µl of the LiAc/TE/PEG solution.
Incubate the mixture at 30 °C for 30 min.
Move the sample to 42 °C and incubate for a further 15 min.
Pellet the cells by centrifugation and remove the supernatant.
Resuspend the pellet in 150 µl of sterile water and plate on an appropriate restrictive media.
Incubate the plate at 30 °C until colonies appear.
Figure 14.3.
Chromosomal insertion of a GAL1 promoter. Sequences to be selected for incorporation into final oligonucleotide PCR primers are represented by arrows pointing in a 5′ to 3′ direction in relation to the primer sequence. Regions of the primer where the sequence is homologous to the chromosomal target site are shaded gray, while regions of the primer homologous to the tagging cassette are black. The final primer containing sequences from both regions is denoted as a chimera of both gray and black arrows. The final panel represents a recombination event mediated by the homologous sequences in the amplified product and the chromosomal target site.
3.2.2. Depleting a protein under control of the GAL1 promoter
After successful insertion of the GAL1 promoter is accomplished, a gene product can be readily depleted from the cell by growing the cell in a media lacking galactose. Normally, yeast are cultured in a 2% dextrose solution for optimal growth; however, a strain containing an essential gene that has been placed under the control of the GAL1 promoter must be grown in galactose (Gal) prior to protein depletion. Because galactose is such a poor carbon source, Gal media is usually supplemented with 2% raffinose (Raf). Upon commencement of a depletion experiment, cells can be moved from a Gal/Raf medium to a dextrose medium, and protein levels can be monitored by western analysis. Protein depletion is accomplished as follows:
Recall that for RB studies, cells should not be allowed to reach an OD600 > 0.5 AU less than two doubling events prior to analysis. To begin a depletion experiment, start a 10-ml overnight culture in YPG/R media from yeast grown on a medium selecting for proper chromosomal insertion of the GAL1 promoter. Incubate the culture on a shaking platform overnight at 30 °C.
The following morning, measure the OD600 of the culture. If the optical density is < 0.5 AU, proceed to step 4, if not, dilute the cells into a new 25 ml culture of YPG/R media to a final OD600 = 0.1 AU. Continue incubating the culture at 30 °C.
Monitor the OD600 of the culture over the course of the day. Once the culture is approaching an optical density of 0.5, harvest the cells by centrifugation. Dilute the cells into a new 25 ml overnight culture of YPG/R such that the final optical density is 0.01 AU. The cells should not exceed an optical density of 0.5 AU for at least 12–14 h, although this will need to be optimized experimentally. If carried out properly, this will ensure that the cells can be used the following morning without requiring additional dilution and doubling events.
Harvest 5 ODU from the YPG/R culture by centrifugation.
Wash the cells by resuspending the pellet in 1 ml of sterile water. Pellet the cells again.
Resuspend the cells in 1 ml of sterile water. The OD600 of these cells is now 5 AU.
Dilute 250 µl of the cells into a fresh 25 ml culture of YPD, resulting in a final OD600 = 0.01 AU. Incubate the cells on a shaking platform at 30 °C.
Protein depletion can be monitored by removing equal ODU from the culture at several time points over a 24-h period to be analyzed by western blot. If no antibody is available for the protein of interest, an epitope tag should be appended when transforming the target with the GAL1 chromosomal insertion.
Once the protocol has been optimized for a target helicase, protein-depleted cells can be analyzed for rRNA production and processing as described in Section 3.1.
3.2.3. Protein mutagenesis and expression in a native-protein-depleted background
Depletion of a target helicase is designed to show that this protein plays some role in some RB event or events, but it gives little insight into the mechanistic behavior of the enzyme. Because DExD/H-box helicases have been so thoroughly characterized and because they share such striking homology among their conserved motifs, a number of potential residues can be readily identified as targets for site directed mutagenesis studies designed to elucidate the particular enzymatic activities required for productive action. The roles and consensus sequences for each of the motifs present in any DExD/H-box protein have been reviewed extensively elsewhere (Cordin et al., 2006; Fairman-Williams et al., 2010; Linder and Jankowsky, 2011, and others). Homologous residues on any protein of interest can be identified by a combination of homology mapping (www.ncbi.nlm.nih.gov/BLAST) and sequence inspection.
Mutagenized proteins can be introduced into a cell depleted of its native protein in order to assay for complementation. If a particular activity of the protein can be perturbed without loss of complementation, it is reasonable to conclude that this activity is not essential for RB. Several studies have already been undertaken to identify mechanistically important motifs in a number of yeast RB helicases, and these can serve as starting points for further studies as well as examples of how these studies are accomplished (e.g., Bernstein et al., 2006, Daugeron and Linder, 2001; Granneman et al., 2006a; Kos and Tollervey, 2005; Rocak et al., 2005). The majority of techniques required for the introduction of a mutagenized protein have been discussed elsewhere in this manuscript, therefore only a few essential considerations will be detailed here.
Standard cloning techniques should be used to insert the gene of interest into a yeast expression vector compatible with the parental strain as discussed in Section 3.1.1.
For convenience, the vector chosen should express the gene under the control of a constitutive promoter, allowing concomitant depletion of the endogenous copy of a target gene and expression of the exogenous copy. Note that a mutagenized protein may confer a dominant negative phenotype when constitutively expressed in the cell. In these cases, protein expression must be placed under the control of an inducible promoter that can be activated subsequent to native protein depletion.
Using the material cited above as a guide, one or more residues should be selected for mutagenesis based on the enzymatic activities of the protein for which they are required.
Mutagenesis can be carried out using commercially available kits (e.g., GeneArt® Site Directed Mutagenesis Kit, www.invitrogen.com). Mutagenized enzymes can then be screened for the requirement of each activity individually (e.g., RNA binding, ATP hydrolysis, etc.).
Plasmids containing mutagenized proteins can be transformed into yeast cells and successful transformants selected as described for the linear recombination cassette in Section 3.2.1.
Protein depletion and rRNA processing studies can be carried out as previously described.
4. Elucidating the Supermolecular Context of an RB Helicase: Protein–Protein Interaction Studies
Processing of the pre-rRNA transcript is accomplished primarily in the context of large protein or RNP machines in which numerous factors act together to properly facilitate the many cleavage, modification, and folding events that comprise RB. The macromolecular complexes that mediate pre-rRNA maturation are broadly defined by their participation in small ribosomal subunit processing (i.e., the SSU processome), large ribosomal subunit processing, or chemical modification of the rRNA transcripts (e.g., snoRNPs). Many of the protein and snoRNA components involved in these tasks have already been identified and grouped based on their copurification with larger complex(es) known to mediate one of the above activities (Bassler et al., 2001; Dragon et al., 2002; Gallagher et al., 2004; Harnpicharchai et al., 2001; Krogan et al., 2004; Nissan et al., 2002; Pérez-Fernández et al., 2007). It is therefore possible to determine the general pathway in which a target enzyme participates by identifying the factors that are associated with that enzyme and comparing them to those that have previously been categorized.
Association with a given protein can occur directly—through a binary protein—protein interaction—or indirectly—via an intermediary protein or nucleic acid. It is important to recognize what kind of interactions will be observed using a chosen technique as the implications of a protein–protein association can vary drastically depending on whether the association is direct or indirect. This is particularly true when working with RB factors, as these factors are almost always found associated with extremely large complexes and can therefore coprecipitate many proteins that they do not interact with directly. This is inconsequential when attempting to determine whether a protein acts in SSU or LSU biogenesis but becomes important when attempting to identify potential cofactors for a target enzyme.
A variety of strategies exist for assaying both direct and indirect protein–protein interactions. Some of the most powerful and frequently applied methods will be discussed here, including Tandem Affinity Purification (TAP), co-immunoprecipitation (co-IP), and yeast two-hybrid (Y2H) analysis. TAP analysis is frequently coupled to protein identification by mass spectrometry and is most often used to select and identify members of multiprotein complexes, yielding results that include both direct and indirect binding partners (Hoang et al., 2005; Lebaron et al., 2005). Co-IP experiments can provide information on direct or indirect interactions depending on experimental conditions (Champion et al., 2008; Charette and Baserga, 2010; Yoshikawa et al., 2011). Interpretation of co-IP results involving RB proteins requires careful analysis and a thorough understanding of the macromolecular complexes involved in this system. Finally, the Y2H approach has been employed with great success as a high-throughput system for screening and identifying protein pairs involved in direct binding interactions (Champion et al., 2008; Gallagher and Baserga, 2004; Granneman et al., 2006b; Lebaron et al., 2005).
4.1. Tandem affinity purification
The TAP method was designed for the selection and identification of large protein complexes. TAP protocols involve relatively gentle conditions designed to minimize perturbation of native protein complexes while maintaining a high level of selectivity by employing a multistep approach to purification.
4.1.1. Epitope tagging target proteins
In order to purify a protein complex containing a helicase or cofactor of interest using the TAP method, an appropriate fusion construct of the target protein must be introduced into the host cell. In yeast, this type of tagging is frequently accomplished by incorporating a sequence into the chromosome at a position flanking either terminus of the target gene (Baudin et al., 1993; Knop et al., 1999; Schneider et al., 1995). This procedure involves transforming an appropriate yeast strain with double stranded DNA containing sequences that encode the desired tag and a selectable marker flanked by 50 bp sequences homologous to regions of the chromosome where the insertion event is to take place, and the method is discussed in detail in Section 3.2.1 (Fig. 14.3). Cells which have successfully incorporated the tagging cassette are selected via the marker contained therein. The presence of the fusion protein in the cell can be assayed by western blot.
Amplification of the TAP sequence in conjunction with a selectable marker can be achieved using one of two plasmids designed by Puig et al. (2001). These vectors—designated pBS1479 and pBS1539—are designed to accommodate either C- or N-terminal tagging, respectively. In designing primers, it is important to ensure that the cassette will be incorporated in frame with the target gene, and that in the case of C-terminal fusions, the native stop codon is not present in the primer sequence so that translation can proceed through the tag. It is also important to note that while the TAP epitope can be fused to either terminus of the target protein, the choice of terminus may be important when examining higher order complex formation. It is conceivable that tagging a particular terminus may perturb or abrogate important protein–protein interactions, resulting in low yields of native binding partners after purification.
4.1.2. TAP protocols
Protocols for TAP have been published in extensive detail elsewhere. Several large scale, genome-wide studies of protein complex formation have been conducted in yeast using the TAP methodology (Gavin et al., 2002, 2006; Krogan, et al., 2006), and the refined protocol developed through these studies has been exhaustively described in Babu et al. (2009).
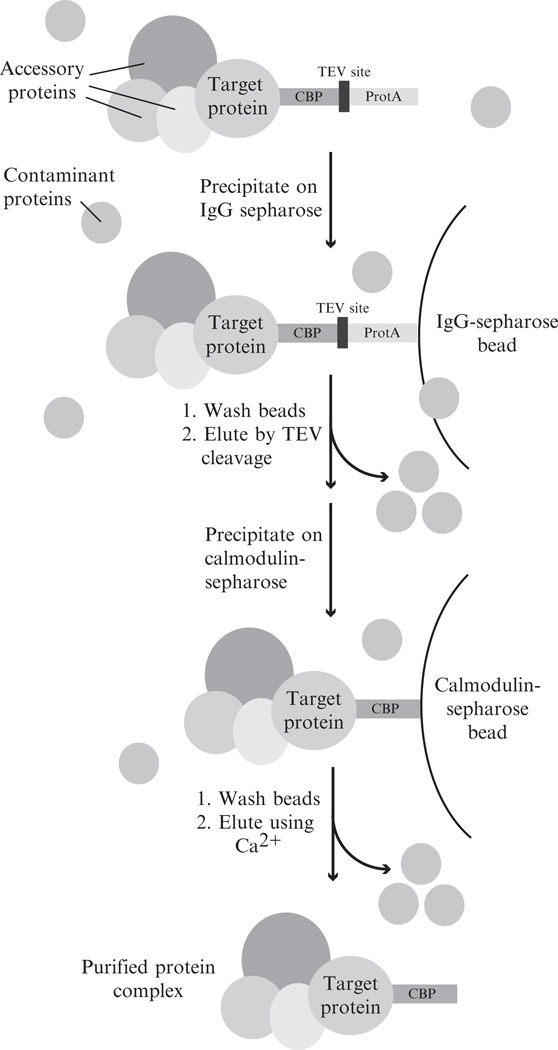
The TAP tag, as originally described in Puig et al. (2001) and Rigaut et al. (1999), consists of two IgG binding domains of protein A (ProtA) from Staphylococcus aureus and a calmodulin binding peptide (CBP) with a TEV protease cleavage site separating these two motifs. The TAP method involves selective immunoprecipitation of tagged proteins using IgG-Sepharose beads, followed by washing in experimentally optimized conditions. Bound complexes are eluted from the bead by TEV protease cleavage which separates the ProtA portion of the tag (bound to the IgG beads) from the remaining CBP portion and the target protein. This eluate is then precipitated on calmodulin sepharose beads, washed, and eluted using increasing concentrations of Ca2+ (Fig. 14.4).
Figure 14.4.
Tandem affinity purification procedure. A fusion protein construct is expressed in vivo and purified from contaminating cell lyste. The fusion protein in complex with associate factors is precipitated on IgG-Sepharose beads and washed prior to specific elution by TEV cleavage. In a second purification step, the fusion protein and associate factors are precipitated on calmodulin-Sepharose beads, washed, and eluted with by increasing the concentration of Ca2+
Despite the existence of a somewhat standardized approach to TAP, truly optimal experimental conditions can vary greatly depending on the nature of the target protein and the system in which it acts. RNA helicases can be particularly problematic in TAP studies due to the fact that interactions between these proteins and their binding partners can often be weak or transient. Indeed, a number of TAP studies using tagged constructs of other SSU processome proteins have failed to identify more than a small subset of the RNA helicases that have been shown to act in this system by alternative techniques (Dragon et al., 2002; Gavin et al., 2006; Krogan, et al., 2006).
The most successful examples of identifying protein–protein interactions involving these enzymes have relied on tagging the helicase (Bernstein et al., 2006; Granneman et al., 2006a; Lebaron et al., 2005) or its cofactor (Hoang et al., 2005) directly. It is therefore recommended that when assaying protein complex formation among RNA helicases in this system, the helicase should always be tagged, and particularly permissive conditions should be used in binding and washing buffers (e.g., salt concentrations at or below 100 mM, less than 0.1% detergent). It is also important to attempt each experiment in a variety of conditions, as it is probable that optimal conditions for any protein tested will differ significantly. Finally, as mentioned previously, it is crucial that cultures grown for these studies are never allowed to exceed an OD600 Of greater than 0.5 AU, as RB in S. cerevisiae is significantly attenuated when cells reach this phase of growth (Ju and Warner, 1994). Allowing cells to grow beyond this point induces dissipation of the RB machinery and might result in false negatives, as the helicase of interest is no longer partaking in the synthesis of ribosomes and may therefore not be in physical contact with other RB factors.
It should be noted that in addition to canonical TAP protocols, alternative procedures have been developed to provide different tagging options and more robust purification and recovery of target complexes. Several modified systems have been designed to improve yields and specificity when working in mammalian systems (Burckstummer et al., 2006; Drakas et al., 2005; Gloeckner et al., 2009). Most recently, a new three tag system consisting of a CBP, a streptavidin-binding peptide, and a 6 × histidine tag optimized for use in mammalian cells was described in Li et al. (2011). In addition to alternative tagging strategies, new protocols have been developed to improve the sensitivity and selectivity of current TAP systems. A notable example comes from Oeffinger et al. in which antibody-conjugated magnetic beads are used rather than a conjugated resin such as Sepharose, resulting in recovery of larger complexes with fewer nonspecifically bound contaminants (Oeffinger et al., 2007). In cases where typical procedures fail to produce quality results turning to a more specific or highly optimized method may provide a successful alternative.
4.2. Co-IP of tagged proteins
The general strategy employed in performing a co-IP experiment in yeast consists of growing cells in liquid culture to an optimal density, harvesting and lysing the cells, and incubating the soluble lysate with an IP media conjugated to an appropriate antibody to select for proteins containing the conjugate epitope tag (Steitz, 1989). The precipitated proteins can be eluted from the media and separated by SDS-PAGE, then detected by western blotting.
From a glycerol stock or freshly struck plate, prepare an overnight culture by collecting a small amount of yeast on the tip of a sterile pipette and inoculating 10 ml liquid YPD media under sterile conditions. Incubate the culture on a shaking platform overnight at 30 °C.
Also prepare IP beads by incubating 3 mg of ProtA-sepharose beads per sample in 500 µl NET-2 buffer with a saturating amount of IP antibody overnight at 4 °C. Before use, pellet and wash the beads 3 × with 1 ml NET-2 per 3 mg beads.
The following morning, measure the OD600 of the culture. Dilute an appropriate amount of the overnight culture into 25 ml of fresh YPD media such that the final optical density will be 0.1 AU. Continue incubating the culture at 30 °C.
Monitor the optical density, allowing the cells to grow to an OD600 of 0.4–0.5 AU.
Harvest at least 10 ODU of culture (e.g., 20 ml of culture at OD600 = 0.5 AU) by centrifugation.
Resuspended pelleted cells in 1 ml sterile water and transfer the sample to a 1.5-ml microcentrifuge tube. Pellet the cells again and remove the supernatant by pippetting.
Resuspend the cells in 600 µl NET-2 buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.05% Nonidet P-40) containing the appropriate dilution of a protease inhibitor cocktail [Roche]
Break the cells by adding 100 µl 0.5 mm glass beads and vortexing at 4 °C for five times at 45 s intervals. Place the cells on ice for 45 s between each round of vortexing.
Pellet the cell debris by centrifugation at maximum speed for 20 min in a microcentrifuge at 4 °C. Retain a 20-µl sample of the resulting supernatant for subsequent analysis.
Add 500 µl of the remaining lysate to 3 mg ProtA-sepharose conjugated to the IP antibody.
Incubate the sample for 1–2 h at 4 °C, preferably on a rocking or nutating platform.
Pellet the media by spinning at max speed for 1 min in a microcentrifuge. Remove the supernatant and discard.
Wash the beads by adding 1 ml NET-2 to the sample. Repeat steps 12 and 13 four more times. At this point, the protein selected by the antibody conjugated to the media will be precipitated on the resin. If the target protein acts in RB, a portion of the RB marker protein will also be precipitated via its interaction with the target.
To elute bound proteins, resuspend the media in 10 µl 5 × SDS Loading Buffer (200 mM Tris–HCl (pH 6.8), 10% (w/v) SDS, 20% (v/v) glycerol, 10 mM β-mercaptoethanol, 0.05% (w/v) bromophenol blue) and incubate the sample 3 min at 95 °C.
Perform SDS-PAGE with the total input and eluate samples in adjacent positions on the gel.
Bound proteins can be detected by western analysis.
4.3. Yeast two-hybrid analysis
Y2H analysis can be used as a high-throughput assay for detecting direct protein–protein interactions among large numbers of potential binding partners. Y2H analysis is made possible by the modular nature of many eukaryotic transcription factors. In these proteins, the domains responsible for binding DNA and recruiting transcription machinery can be physically separated and their activity reconstituted in trans. Y2H analysis consists of fusing one protein to the DNA binding domain of a transcription factor, and another to the activation domain, then coexpressing the fusion constructs in a yeast strain containing a reporter gene under the control of the parsed transcription factor. If the proteins interact with each other, that interaction will reconstitute the transcription factor, resulting in transcription of the reporter gene (Fields and Song, 1989).
As in the case of the TAP protocol, Y2H procedures vary widely and are largely dependent on the type of equipment available for use in the study, therefore a specific protocol will not be detailed here. Instead, a general framework for constructing a Y2H system will be discussed, with emphasis on important considerations for screening in systems with known protein components. Descriptions of high-throughput protocols employing various mechanical and biological techniques have been published and can be found in Cagney et al. (2000) and Rajagopala and Uetz (2009, 2011).
Because many of the factors involved in RB have already been identified, it is possible to perform experiments using a directed approach, in which a tractable list of potential targets can be compiled prior to analysis. Proteins that should be included in the study of a RB helicase will depend on results of other analyses. For RNA helicases involved in yeast ribosome maturation, the pathway in which each protein acts has been elucidated; hence, a number of ribosome synthesis factors can be immediately eliminated from consideration for a number of these proteins. Information gained in TAP or other binding partner studies can be used to further focus a study to a small group of potential binding partners.
Genes corresponding to all proteins of interest must be cloned into expression plasmids designed to produce a fusion construct containing either the DNA binding or activating domain of an appropriate transcription factor. The GAL4-responsive promoters are frequently used as the control element for various reporters within common Y2H strains, including Y2H Gold and Y187 (www.clontech.com), and the conjugate plasmids will therefore contain either the GAL4 DNA binding domain (GAL4 DNA-BD) or the GAL4 activating domain (GAL4 AD). Fusion proteins containing the DNA-BD are referred to as baits, while those containing the AD are considered preys. The choice of plasmid will depend on the markers available in the selected strain, as well as the desired cloning strategy; multiple vectors are available.
-
To facilitate screening of large numbers of proteins, it is preferable to use a yeast mating system, in which plasmids expressing one set of fusion proteins—for example, all GAL4 AD fusions—are transformed individually into a mating type a (MATa) version of the Y2H strain, while the other set of fusion plasmids is transformed into mating type alpha (MATα) cells. In this way, cultures of each transformed strain can be separately grown and mated in a one-to-one fashion until all possible pairings have been accomplished.
Previously, pooling strategies have been employed to screen large libraries of potential interactors (Ito et al., 2001; Uetz et al., 2000; Yu et al., 2008); however, this strategy should be avoided when assaying smaller cohorts, as it introduces bias into the system, selecting the most robust or least cytotoxic interacting partners over other potential positives (Koegl and Uetz, 2007; Lim et al., 2011; Rajagopala and Uetz, 2009).
Successful mating can be determined by growth on media selective for both plasmid markers. Once mating has been accomplished, cells containing both plasmids can be deposited on media selective for one or more of the reporter genes under control of the GAL4 promoter. Growth on this media implies successful reconstitution of the transcription factor and, by extension, a physical interaction between the two fusion proteins.
A number of selectable markers are available for use in most Y2H strains. Common markers used include but are not limited to his3 and ade2, which select for the ability to grow on media lacking histidine and adenine, respectively, as well as mel1, which metabolizes X-α-Gal resulting in blue colonies. In order to improve confidence in positive results, screens can be replicated on different markers or a combination of markers can be used in a single screen.
Histidine selection is somewhat leaky and a minimal amount of histidine biosynthesis can still occur in the cell without activation of the reporter gene. To minimize spurious positives related to this effect, an experimentally optimized amount (usually to a final concentration between 3 and 6 mM) of 3-aminotriazole is added to the media to dampen background histidine production.
REFERENCES
- Anderson JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M, Lamond AI. Directed proteomic analysis of the human nucleolus. Curr. Biol. 2002;12:1–11. doi: 10.1016/s0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, Mann M. Nucleolar proteome dynamics. Nature. 2005;433:77–83. doi: 10.1038/nature03207. [DOI] [PubMed] [Google Scholar]
- Arenas JE, Abelson JN. Prp43: An RNA helicase-like factor involved in spliceosome disassembly. Proc. Natl. Acad. Sci. USA. 1997;94:11798–11802. doi: 10.1073/pnas.94.22.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu M, Krogan NJ, Awrey DE, Andre E, Greenblatt JF. Systematic characterization of the protein interaction network and protein complexes in Saccharomyces cerevisiae using tandem affinity purification and mass spectrometry. Methods Mol. Biol. 2009;548:187–207. doi: 10.1007/978-1-59745-540-4_11. [DOI] [PubMed] [Google Scholar]
- Bassler J, Grandi P, Gadal O, Lessmann T, Petfalski E, Tollervey D, Lechner J, Hurt E. Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol. Cell. 2001;8:517–529. doi: 10.1016/s1097-2765(01)00342-2. [DOI] [PubMed] [Google Scholar]
- Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Culin C. A simple and efficient method for direct gene deletion in Saccaromyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli G, Gari E, Piedrafita L, Aldea M, Herrero E. An activator/repressor dual system allows tight tetracycline-regulated gene expression in budding yeast. Nucleic Acids Res. 1998;26:942–947. doi: 10.1093/nar/26.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard L, Carroll K, Valle RC, Wickner RB. Ski6p is a homolog of RNA-processing enzymes that affects translation of non-poly(A) mRNAs and 60S ribosomal subunit biogenesis. Mol. Cell. Biol. 1998;18:2688–2696. doi: 10.1128/mcb.18.5.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges T, Petfalski E, Tollervey D, Hurt EC. Synthetic lethality with fibrillarin identifies NOP77p, a nucleolar protein required for pre-rRNA processing and modification. EMBO J. 1994;13:3136–3148. doi: 10.1002/j.1460-2075.1994.tb06612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein KA, Granneman S, Lee AV, Manickman S, Baserga SJ. Comprehensive mutational analysis of yeast DEXD/H box RNA helicases involved in large ribosomal subunit biogenesis. Mol. Cell. Biol. 2006;26:1195–1208. doi: 10.1128/MCB.26.4.1195-1208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleichert F, Baserga SJ. The long unwinding road of RNA helicases. Mol. Cell. 2007;27:339–352. doi: 10.1016/j.molcel.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Blum S, Schmid SR, Pause A, Buser P, Linder P, Sonenberg N, Trachsel H. ATP hydrolysis by initiation factor 4A is required for translation initiation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1992;89:7664–7668. doi: 10.1073/pnas.89.16.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack MT, Kos M, Tollervey D. Quantitative analysis of snoRNA association with pre-ribosomes and release of snR30 by Rok1 helicase. EMBO Rep. 2008;9:1230–1236. doi: 10.1038/embor.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack MT, Martin R, Granneman S, Ruprecht M, Schleiff E, Tollervey D. Prp43 bound at different sites on the pre-rRNA performs distinct functions in ribosome synthesis. Mol. Cell. 2009;36:583–592. doi: 10.1016/j.molcel.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckstummer T, Bennett KL, Preradovic A, Schutze G, Hantschel O, Superti-Furga G, Bauch A. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat. Methods. 2006;3:1013–1019. doi: 10.1038/nmeth968. [DOI] [PubMed] [Google Scholar]
- Burger F, Daugeron MC, Linder P. Dbp10, a putative RNA helicase from Saccharomyces cerevisiae, is required for ribosome biogenesis. Nucleic Acids Res. 2000;28:2315–2323. doi: 10.1093/nar/28.12.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney G, Uetz P, Fields S. High-throughput screening for protein-protein interactions using two-hybrid assay. Methods Enzymol. 2000;328:3–14. doi: 10.1016/s0076-6879(00)28386-9. [DOI] [PubMed] [Google Scholar]
- Champion EA, Lane BH, Jackrel ME, Regan L, Baserga SJ. A direct interaction between the Utp6 half-a-tetratricopeptide repeat domain and a specific peptide in Utp21 is essential for efficient pre-rRNA processing. Mol. Cell. Biol. 2008;28:6547–6556. doi: 10.1128/MCB.00906-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette JM, Baserga SJ. The DEAD-box RNA helicase-like Utp25 is an SSU processome component. RNA. 2010;16:2156–2169. doi: 10.1261/rna.2359810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley A, Beggs JD, Tollervey D, Lafontaine DL. Dhr1p, a putative DEAH-box RNA helicase, is associated with the box C+D snoRNP U3. Mol. Cell. Biol. 2000;20:7238–7246. doi: 10.1128/mcb.20.19.7238-7246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs DJ, Nagel RJ, Ares M, Jr, Stevens SW. Prp43p is a DEAH-box spliceosome disassembly factor essential for ribosome biogenesis. Mol. Cell. Biol. 2006;26:523–534. doi: 10.1128/MCB.26.2.523-534.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordin O, Banroques J, Tanenr NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;15:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Daugeron MC, Linder P. Dbp7p, a putative ATP-dependent RNA helicase from Saccharomyces cerevisiae, is required for 60S ribosomal subunit assembly. RNA. 1998;4:566–581. doi: 10.1017/s1355838298980190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugeron MC, Linder P. Characterization and mutational analysis of yeast Dbp8p, a putative RNA helicase involved in ribosome biogenesis. Nucleic Acids Res. 2001;29:1144–1155. doi: 10.1093/nar/29.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugeron MC, Kressler D, Linder P. Dbp9p, a putative ATP-dependent RNA helicase involved in 60S-ribosomal-subunit biogenesis, functionally interacts with Dbp6p. RNA. 2001;7:1317–1334. doi: 10.1017/s1355838201010640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Rojo M, Tollervey D, Linder P. Spb4, an essential putative RNA helicase, is required for a late step in the assembly of 60S ribosomal subunits in Saccharomyces cerevisiae. RNA. 1998a;4:1268–1281. doi: 10.1017/s1355838298981158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Tollervey D, Linder P. Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J. 1998b;17:1128–1140. doi: 10.1093/emboj/17.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem. Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- Dragon F, Gallagher JE, Compagnone-Post PA, Mitchell BM, Porwancher KA, Wehner KA, Wormsley S, Settlage RE, Shabanowitz J, Osheim Y, Beyer AL, Hunt DF, et al. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature. 2002;417:967–970. doi: 10.1038/nature00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakas R, Prisco M, Baserga R. A modified tandem affinity purification tag technique for the purification of protein complexes in mammalian cells. Proteomics. 2005;5:132–137. doi: 10.1002/pmic.200400919. [DOI] [PubMed] [Google Scholar]
- Emery B, de la Cruz J, Rocak S, Deloche O, Linder P. Has1p, a member of the DEAD-box family, is required for 40S ribosomal subunit biogenesis in Saccharomyces cerevisiae. Mol. Microbiol. 2004;52:141–158. doi: 10.1111/j.1365-2958.2003.03973.x. [DOI] [PubMed] [Google Scholar]
- Etcheverry T. Induced expression using yeast copper metallothionein promoter. Methods Enzymol. 1990;185:319–329. doi: 10.1016/0076-6879(90)85028-m. [DOI] [PubMed] [Google Scholar]
- Fairman-Williams ME, Guenther UP, Jankowsky E. SF1 and SF2 helicases: Family matters. Curr. Opin. Struct. Biol. 2010;20:313–324. doi: 10.1016/j.sbi.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S, Song O. A Novel genetic system to detect protein–protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Freed EF, Baserga SJ. The C-terminus of Utp4, mutated in childhood cirrhosis, is essential for ribosome biogenesis. Nucleic Acids Res. 2010;38:4798–4806. doi: 10.1093/nar/gkq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JE, Baserga SJ. Two-hybrid Mpp 10p interaction-defective Imp4 proteins are not interaction defective in vivo but do confer specific pre-rRNA processing defects in Saccharomyces cerevisiae. Nucleic Acids Res. 2004;32:1404–1413. doi: 10.1093/nar/gkh318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J, Dunbar D, Granneman S, Mitchess B, Osheim Y, Beyer A, Baserga SJ. RNA polymerase I transcritption and pre-rRNA processing are linked by specific SSU processome components. Genes Dev. 2004;18:2506–2517. doi: 10.1101/gad.1226604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, Remor M, Hofert C, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, Marzioch M, Rau C, Jensen LJ, Bastuck S, Dumpelfeld B, Edelmann A, Heurtier MA, et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, Boldt K, Schumacher A, Ueffing M. Tandem affinity purification of protein complexes from mammalian cells by the Strep/FLAG (SF)-TAP tag. Methods Mol. Biol. 2009;564:359–372. doi: 10.1007/978-1-60761-157-8_21. [DOI] [PubMed] [Google Scholar]
- Granneman S, Baserga SJ. Ribosome biogenesis: Of knobs and RNA processing. Exp. Cell Res. 2004;15:43–50. doi: 10.1016/j.yexcr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Granneman S, Bernstein KA, Bleichert F, Baserga SJ. Comprehensive mutational analysis of yeast DEXD/H box RNA helicases required for small ribosomal subunit synthesis. Mol. Cell. Biol. 2006a;26:1183–1194. doi: 10.1128/MCB.26.4.1183-1194.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman S, Lin C, Champion EA, Nandineni MR, Zorca C, Baserga SJ. The nucleolar protein Esf2 interacts directly with the DExD/H box RNA helicase, Dbp8, to stimulate RNA hydrolysis. Nuc. Acids Res. 2006b;34:3189–3199. doi: 10.1093/nar/gkl419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman S, Kudla G, Petfalski E, Tollervery D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc. Natl. Acad. Sci. USA. 2009;106:9613–9618. doi: 10.1073/pnas.0901997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnpicharchai P, Jakovljevic J, Horsey E, Miles T, Roman J, Rout M, Meacher D, Imai B, Guo Y, Brame CJ, Shabanowitz J, Hunt DF, et al. Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol. Cell. 2001;8:505–515. doi: 10.1016/s1097-2765(01)00344-6. [DOI] [PubMed] [Google Scholar]
- Henras AK, Soudet J, Gerus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell. Mol. Life Sci. 2008;65:2334–2359. doi: 10.1007/s00018-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Peng WT, Vanrobays E, Krogan N, Hiley S, Beyer AL, Osheim YM, Breenblatt J, Hughes TR, Lafontaine DL. Esf2p, a U3-associated factor required for small-subunit processome assembly and compaction. Mol. Cell. Biol. 2005;25:5523–5534. doi: 10.1128/MCB.25.13.5523-5534.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Ito T, Chiba T, Ozawa R, Yoshida M, Hattori M, Sakaki Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA. 2001;98:4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky E. RNA helicases at work: Binding and rearranging. Trends Biochem. Sci. 2011;36:19–29. doi: 10.1016/j.tibs.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Q, Warner JR. Ribosome synthesis during the growth cycle of Saccharomyces cerevisiae. Yeast. 1994;10:151–157. doi: 10.1002/yea.320100203. [DOI] [PubMed] [Google Scholar]
- Knop M, Siegers K, Pereira G, Zachariae W, Winsor B, Nasmyth K, Schiebel E. Epitope tagging of yeast genes using a PCR-based strategy: More tags and improved practical routines. Yeast. 1999;15:963–972. doi: 10.1002/(SICI)1097-0061(199907)15:10B<963::AID-YEA399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Koegl M, Uetz P. Improving yeast two-hybrid screening systems. Brief. Funct. Genomic. Proteomic. 2007;6:302–312. doi: 10.1093/bfgp/elm035. [DOI] [PubMed] [Google Scholar]
- Kos M, Tollervey D. The Putative RNA Helicase Dbp4p is Required for Release of the U14 snoRNA from Preribosomes in Saccharomyces cerevisiae. Mol. Cell. 2005;20:53–64. doi: 10.1016/j.molcel.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Kressler D, de la Cruz J, Rojo M, Linder P. Fal1p is an essential DEAD-box protein involved in 40S-ribosomal-subunit biogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997;17:7283–7294. doi: 10.1128/mcb.17.12.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D, de la Cruz J, Rojo M, Linder P. Dbp6p is an essential putative ATP-dependent RNA helicase required for 60S-ribosomal-subunit assembly in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998;18:1855–1865. doi: 10.1128/mcb.18.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim. Biophys. Acta. 2010;1803:673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Peng WT, Cagney G, Robinson MD, Haw R, Zhong G, Guo X, Zhang X, Canadien V, Richards DP, Beattie BK, Lalev A, et al. High-definition macromolecular composition of yeast RNA-processing complexes. Mol. Cell. 2004;13:225–239. doi: 10.1016/s1097-2765(04)00003-6. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, Punna T, Peregrin-Alvarez JM, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- Kumar A, Agarwal S, Heyman JA, Matson S, Heidtman M, Piccirillo S, Umansky L, Drawid A, Jansen R, Liu Y, Cheung KH, Miller P, et al. Subcellular localization of the yeast proteome. Genes Dev. 2002;16:707–719. doi: 10.1101/gad.970902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaron S, Froment C, Fromont-Racine M, Rain J, Monsarrat B, Caizergues-Ferrer M. The splicing ATPase Prp43p is a component of multiple preribosomal particles. Mol. Cell. Biol. 2005;25:9269–9282. doi: 10.1128/MCB.25.21.9269-9282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaron S, Papin C, Capeyrou R, Chen YL, Froment C, Monsarrat B, Caizergues-Ferrer M, Grigoriev M, Henry Y. The ATPase and helicase activities of Prp43p are stimulated by the G-patch protein Pfa1p during yeast ribosome biogenesis. EMBO J. 2009;28:3808–3819. doi: 10.1038/emboj.2009.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Zabetakis D, Melese T. NSR1 is required for pre-rRNA processing and for the proper maintenance of steady-state levels of ribosomal subunits. Mol. Cell. Biol. 1992;12:3865–3871. doi: 10.1128/mcb.12.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds NB, Small EC, Hiley SL, Hughes TR, Staely JP. The splicing factor Prp43p, a DEAH box ATPase, functions in ribosome biogenesis. Mol. Cell. Biol. 2006;26:513–522. doi: 10.1128/MCB.26.2.513-522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Trinkle-Mulcahy L, Lam YW, Andersen JS, Mann M, Lamond AI. NOPdb: Nucleolar proteome database. Nucleic Acids Res. 2006;34:D218–D220. doi: 10.1093/nar/gkj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Franklin S, Zhang MJ, Vondriska TM. Highly efficient purification of protein complexes from mammalian cells using a novel streptavidin-binding peptide and hexahistidine tandem tag system: Application to Bruton’s tyrosine kinase. Protein Sci. 2011;20:140–149. doi: 10.1002/pro.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Fournier MJ. The helicase Has1p is required for snoRNA release from pre-rRNA. Mol. Cell. Biol. 2006;26:7437–7450. doi: 10.1128/MCB.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, Darnell JC, Darnell RB. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456:464–469. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YH, Charette JM, Baserga SJ. Assembling a protein–protein interaction map of the SSU processome from existing datasets. PLoS One. 2011;6:e17701. doi: 10.1371/journal.pone.0017701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder P, Jankowsky E. From unwinding to clamping—The DEAD box RNA helicase family. Nat. Rev. Mol. Cell Biol. 2011;12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- Liu F, Putnam A, Jankowsky E. ATP hydrolysis is required for DEAD-box protein recycling but not for duplex unwinding. Proc. Natl. Acad. Sci. USA. 2008;105:20209–20214. doi: 10.1073/pnas.0811115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mumberg D, Mailer R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Nissan TA, Bassler J, Petfalski E, Tollervey D, Hurt E. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 2002;21:5539–5547. doi: 10.1093/emboj/cdf547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Day CL, Chavanikamannil F, Abelson J. 18S rRNA processing requires the RNA helicase-like protein Rrp3. Nucleic Acids Res. 1996;24:3201–3207. doi: 10.1093/nar/24.16.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger M, Wei KE, Rogers R, DeGrasse JA, Chait BT, Aitchison JD, Rout MP. Comprehensive analysis of diverse ribonucleoprotein complexes. Nat. Methods. 2007;4:951–956. doi: 10.1038/nmeth1101. [DOI] [PubMed] [Google Scholar]
- Pérez-Fernández J, Román A, De Las Rivas J, Bustelo X, Dosil M. The 90S preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol. Cell. Biol. 2007;27:5415–5429. doi: 10.1128/MCB.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B. The tandem affinity purification (TAP) method: A general procedure of protein complex purification. Methods. 2001;24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- Pyle AM. Translocation and unwinding mechanisms of RNA and DNA helicases. Annu. Rev. Biophys. 2008;37:317–336. doi: 10.1146/annurev.biophys.37.032807.125908. [DOI] [PubMed] [Google Scholar]
- Rajagopala SV, Uetz P. Analysis of protein-protein interactions using array-based yeast two-hybrid screens. Methods Mol. Biol. 2009;548:223–245. doi: 10.1007/978-1-59745-540-4_13. [DOI] [PubMed] [Google Scholar]
- Rajagopala SV, Uetz P. Analysis of protein-protein interactions using high-throughput yeast two-hybrid screens. Methods Mol. Biol. 2011;781:1–29. doi: 10.1007/978-1-61779-276-2_1. [DOI] [PubMed] [Google Scholar]
- Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seaphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- Ripmaster TL, Vaughn GP, Wooldford JL., Jr A putative ATP-dependent RNA helicase involved in Saccharomyces cerevisiae ribosome assembly. Proc. Natl. Acad. Sci. USA. 1992;89:11131–11135. doi: 10.1073/pnas.89.23.11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocak S, Emery B, Tanner NK, Linder P. Characterization of the ATPase and unwinding activities of the yeast DEAD-box protein Has1p and the analysis of the roles of the conserved motifs. Nucleic Acids Res. 2005;33:999–1009. doi: 10.1093/nar/gki244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Macdonald P, Coelho PS, Roemer T, Agarwal S, Kumar A, Jansen R, Cheung KH, Sheehan A, Symoniatis D, Umansky L, Heidtman M, Nelson FK, et al. Large-scale analysis of the yeast genome by transposon tagging and gene disruption. Nature. 1999;402:413–418. doi: 10.1038/46558. [DOI] [PubMed] [Google Scholar]
- Scherl A, Coute Y, Deon C, Calle A, Kindbeiter K, Sanchez JC, Greco A, Hochstrasser D, Diaz JJ. Functional proteomic analysis of human nucleolus. Mol. Biol. Cell. 2002;13:4100–4109. doi: 10.1091/mbc.E02-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BL, Seufert W, Steiner B, Yang QH, Futcher AB. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast. 1995;11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz JA. Immunoprecipitation of ribonucleoproteins using autoantibodies. Methods Enzymol. 1989;180:468–481. doi: 10.1016/0076-6879(89)80118-1. [DOI] [PubMed] [Google Scholar]
- Tanner NK, Linder P. DExD/H box RNA helicases: From generic motors to specific dissociation functions. Mol. Cell. 2001;8:251–262. doi: 10.1016/s1097-2765(01)00329-x. [DOI] [PubMed] [Google Scholar]
- Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Ule J, Jensen K, Mele A, Darnell RB. CLIP: A method for identifying protein-RNA interaction sites in living cells. Methods. 2005;37:376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Venema J, Tollervery D. RRP5 is required for formation of both 18S and 5.8S rRNA in yeast. EMBO J. 1996;15:5701–5714. [PMC free article] [PubMed] [Google Scholar]
- Venema J, Tollervey D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999;33:261–311. doi: 10.1146/annurev.genet.33.1.261. [DOI] [PubMed] [Google Scholar]
- Venema J, Bousquet-Antonelli C, Gelugne JP, Caizerques-Ferrer M, Tollervey D. Rok1p is a putative RNA helicase required for rRNA processing. Mol. Cell. Biol. 1997;17:3398–3407. doi: 10.1128/mcb.17.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Lührmann R. The spliceosome: Design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Weaver PL, Sun C, Chang TH. Dbp3p, a putative RNA helicase in Saccharomyces cerevisiae is required for efficient pre-rRNA processing predominantly at site A3. Mol. Cell. Biol. 1997;17:1354–1365. doi: 10.1128/mcb.17.3.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner KA, Gallagher JE, Baserga SJ. Components of an interdependent unit within the SSU processome regulate and mediate its activity. Mol. Cell. Biol. 2002;22:7258–7267. doi: 10.1128/MCB.22.20.7258-7267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Komatsu W, Hayano T, Miura Y, Homma K, Izumikawa K, Ishikawa H, Miyazawa N, Tachikawa H, Yamauchi Y, Isobe T, Takahashi N. Splicing factor 2-associated protein p32 participates in ribosome biogenesis by regulating the binding of Nop52 and Fibrillarin to preribosome particles. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.006148. M110.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, Sahalie J, Hirozane-Kishikawa T, Gebreab F, Li N, Simonis N, Hao T, Rual JF, et al. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322:104–110. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]