Key Points
MYD88 L265P is expressed in WM and IgM MGUS patients using AS-PCR assays with potential use in diagnostic discrimination and response assessment.
Abstract
By whole-genome and/or Sanger sequencing, we recently identified a somatic mutation (MYD88 L265P) that stimulates nuclear factor κB activity and is present in >90% of Waldenström macroglobulinemia (WM) patients. MYD88 L265P was absent in 90% of immunoglobulin M (IgM) monoclonal gammopathy of undetermined significance (MGUS) patients. We therefore developed conventional and real-time allele-specific polymerase chain reaction (AS-PCR) assays for more sensitive detection and quantification of MYD88 L265P. Using either assay, MYD88 L265P was detected in 97 of 104 (93%) WM and 13 of 24 (54%) IgM MGUS patients and was either absent or rarely expressed in samples from splenic marginal zone lymphoma (2/20; 10%), CLL (1/26; 4%), multiple myeloma (including IgM cases, 0/14), and immunoglobulin G MGUS (0/9) patients as well as healthy donors (0/40; P < 1.5 × 10−5 for WM vs other cohorts). Real-time AS-PCR identified IgM MGUS patients progressing to WM and showed a high rate of concordance between MYD88 L265P ΔCT and BM disease involvement (r = 0.89, P = .008) in WM patients undergoing treatment. These studies identify MYD88 L265P as a widely present mutation in WM and IgM MGUS patients using highly sensitive and specific AS-PCR assays with potential use in diagnostic discrimination and/or response assessment. The finding of this mutation in many IgM MGUS patients suggests that MYD88 L265P may be an early oncogenic event in WM pathogenesis.
Introduction
Waldenström macroglobulinemia (WM) is a distinct clinicopathological entity resulting from the accumulation, predominantly in the bone marrow (BM), of clonally related lymphocytes, lymphoplasmacytic cells, and plasma cells that secrete a monoclonal immunoglobulin M (IgM) protein.1 This condition is considered to correspond to the lymphoplasmacytic lymphoma (LPL) as defined by the World Health Organization lymphoma classification system.2 Most cases of LPL are WM, with <5% of cases made up of immunoglobulin A (IgA), immunoglobulin G (IgG), and nonsecreting LPL.
We recently described the finding of a highly recurrent somatic mutation (MYD88 L265P) in WM patients using whole-genome sequencing (WGS) and subsequently confirmed its presence by Sanger sequencing.3 Sanger sequencing also identified MYD88 L265P in non–IgM-secreting LPL patients. In total, 91% of LPL patients had the MYD88 L265P mutation in their tumor cells. Importantly, MYD88 L265P was either absent or rarely present in samples from patients with B-cell disorders with overlapping clinicopathological features including myeloma, marginal zone lymphoma, and IgM-secreting monoclonal gammopathy of unknown significance (MGUS). MYD88 L265P presence in tumor cells from 70% to 100% of WM patients has also recently been reported by other groups4-7 and has been found in lower frequencies in tumor samples from patients with ABC-type diffuse large B-cell lymphoma (DLBCL; 14%-29%), primary central nervous system lymphoma (33%), mucosa-associated lymphoid tissue lymphoma (9%), and chronic lymphocytic leukemia (CLL; 2.9%) by whole-genome, whole-exome, or Sanger sequencing.8-11
MYD88 is an adaptor molecule in Toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) signaling.12,13 Following TLR or IL-1R stimulation, MYD88 is recruited to the activated receptor complex as a homodimer, which then complexes with interleukin-1 receptor-associated kinase (IRAK) 4 and activates IRAK1 and IRAK2.14,15 Tumor necrosis factor receptor–associated factor 6 is then activated by IRAK1, leading to nuclear factor κB (NF-κB) activation via IκBα phosphorylation.16 Inhibition of MYD88 signaling in L265P-mutated WM cells blocks IκBα and NF-κB p65 phosphorylation and nuclear translocation of p65-NF-κB.3 Both IRAK1 and Bruton tyrosine kinase facilitate NF-κB activation in response to MYD88 in L265P-mutated WM cells, and NF-κB is essential for growth and survival of WM cells.17-19
As part of these efforts, we sought to develop reliable PCR-based assay systems for detection and quantification of MYD88 L265P mutation for use in WM/LPL patients and to look for the presence of MYD88 L265P in IgM MGUS patients using more sensitive detection assays. We also examined the use of real-time AS-PCR assays for MYD88 L265P for response assessment in WM patients undergoing therapy. The findings of these studies are reported herein.
Materials and methods
Patients and sample collection
A total of 104 WM patients, 24 IgM MGUS patients, 40 healthy donors, 26 CLL patients, 20 splenic marginal zone lymphoma (MZL) patients, 14 multiple myeloma (MM), including 3 IgM MM, and 9 IgG MGUS patients were included in this study. Patients with WM and IgM MGUS met the consensus criteria diagnosis as set forth at the Second International Workshop on WM and were untreated.1 Their participation was approved by the Institutional Review Board at the Dana-Farber Cancer Institute. Patients provided written consent in accordance with the Declaration of Helsinki using consent forms approved by each participating institution’s review board. BM mononuclear cells were sorted using magnetic beads as described previously (Miltenyi, Auburn, CA).3 CD19+-selected cells from BM aspirates of WM and MGUS patients, CD5+CD19+-selected cells from peripheral blood (PB) of CLL patients, CD19+-selected cells from tumor-involved BM or PB of splenic MZL patients, CD138+-selected cells from BM aspirates of MM patients (including 3 with IgM-secreting disease), as well as CD19+-selected cells from PB (n = 30) or BM (n = 10) of healthy donors were used for AS-PCR assays. DNA was extracted using Allprep DNA/RNA mini kit (QIAGEN, Valencia, CA). BCWM.1 (from our laboratory) and MWCL-1 cells (kindly provided by Dr Steve Ansell, Mayo Clinic, Rochester, MN) are WM cell lines that are heterozygous for MYD88 L265P whereas OCI-LY3 (homozygous for MYD88 L265P) and OCI-LY19 (wild-type for MYD88) are DLBCL cell lines kindly provided by Dr Mark Minden (University Health Network, Toronto, Canada).3,4,16,17 These cell lines were used for AS-PCR assay determination for MYD88 L265P.
Development of a conventional AS-PCR assay for MYD88 L265P assessment
Two reverse primers were designed to differentiate the mutant and wild-type allele of MYD88 L265P. To optimize the specificity, an internal mismatch in the third position from the 3′-end was introduced. The mutant-specific reverse primer was 5′-CCT TGT ACT TGA TGG GGA aCG-3′ and the wild-type-specific reverse primer was 5′-GCC TTG TAC TTG ATG GGG AaC A-3′. The common forward primer was 5′-AAT GTG TGC CAG GGG TAC TTA G-3′. PCR reaction was performed in a final volume of 25 μL with 50 nM of each primer and 50 ng DNA using PCR SuperMix High Fidelity (Life Technologies, Grand Island, NY). Thermal cycling conditions were: 2 minutes at 94°C, followed by 40 cycles of 94°C for 30 seconds, 57°C for 30 seconds, and 68°C for 30 seconds, with a final extension at 68°C for 5 minutes. The amplified PCR products (159 bp) were separated on 2% agarose gel. To confirm the sequence, PCR products were purified by QIA quick gel extraction kit (QIAGEN) and sequenced using both forward and reverse PCR primers.
Development of a real-time AS-PCR assay for MYD88 L265P assessment
Quantitative detection of the MYD88 L265P mutation was developed using the primers described above and Power SYBR Green PCR Master Mix according to the manufacturer’s instructions on the ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). Briefly, PCR reaction was performed in a final volume of 25 µL with 25 nM of each primer and 50 ng DNA. Thermal cycling conditions were: 10 minutes at 95°C, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Each sample was assayed in triplicate. The standard curve for MYD88 L265P was generated by a serial dilution of the mutant DNA with the wild-type DNA (50%, 10%, 2%, 0.4%, 0.08%, and wild-type). For the corresponding reference PCR, the forward primer is same as the one used for the AS-PCR (5′-AAT GTG TGC CAG GGG TAC TTA G-3′) and the reverse primer is located at 53 bp downstream of the AS-PCR primer (5′-TGG TGT AGT CGC AGA CAG TGA-3′). Levels of the mutant MYD88 L265P in patient samples were calculated based on the value of delta CT (ΔCT) and the standard curve.
Confirmatory Sanger sequencing
To confirm AS-PCR results, Sanger sequencing was used as previously reported.3 The forward PCR primer 5′-GGG ATA TGC TGA ACT AAG TTG CCA C-3′ and reverse PCR primer 5′-GAC GTG TCT GTG AAG TTG GCA TCT C-3′ were designed to amplify a 726-bp fragment covering the MYD88 L265P site. Amplified PCR products were isolated by QIA quick gel extraction kit (QIAGEN) and sequenced using the reverse PCR primer and a sequencing primer 5′-GCT GTT GTT AAC CCT GGG GTT GAA G-3′.
Statistical analysis
Statistical analysis was conducted using R (R Foundation for Statistical Computing, Vienna, Austria). Clinical correlations with MYD88 L265P mutational status were assessed using analysis of covariance (ANCOVA). Categorical associations were evaluated using Fisher’s exact test. Correlation of ΔCT and BM response was assessed using Pearson’s product-moment correlation. All other comparisons were assessed using the Mann-Whitney U test.
Results
Conventional AS-PCR assay for MYD88 L265P
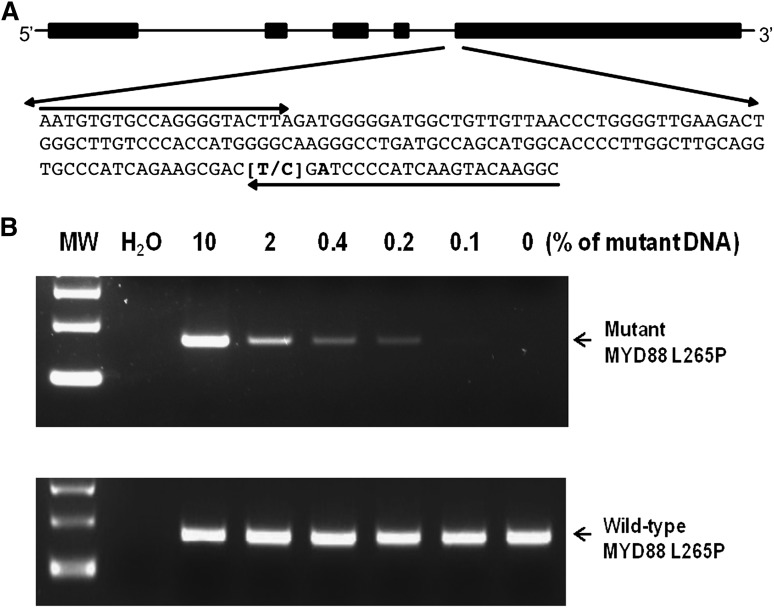
The somatic mutation L265P in the MYD88 gene is detectable in BM-isolated CD19+-selected samples by either WGS or Sanger sequencing in 90% of WM patients.3 Both techniques can be time consuming and costly and require ample quantities of DNA as well as tumor cell presence for detection. For more sensitive and efficient detection of MYD88 L265P, we sought to develop conventional and real-time AS-PCR assays that can be easily implemented in most clinical laboratories with conventional PCR technology. As shown in Figure 1, the AS primers are located in exon 5 whereas the common forward primer is located in intron 4. To enhance the specificity in the AS-PCR reaction, an additional mismatch (T>A) was introduced at the third position from the 3′ end of the AS primers. Sensitivity of the AS-PCR assay was assessed by serial dilution of DNA isolated from the DLBCL cell line OCI-LY3, which is homozygous for MYD88 L265P, with the DNA from DLBCL cell line OCI-LY19, which is wild-type MYD88. The MYD88 L265P status of these cell lines was previously confirmed by Sanger sequencing.4 Sensitivity assessments demonstrated that MYD88 L265P was detectable to a dilution of 0.1% (Figure 1). Reproducibility studies showed complete precision (100% agreement) among replicate aliquots, both within (intraassay) and between (intraassay) runs, in tumor DNA diluted to 0.1% concentration.
Figure 1.
Agarose gel-based conventional AS-PCR assay for detection of MYD88 L265P. (A) MYD88 L265P AS-PCR primer design. The reverse primers with an internal mismatch in the third position from the 3′-end and the common forward primer are indicated by arrows. (B) Sensitivity of the conventional AS-PCR assay was established by serial dilutions of DNA from OCI-LY3 against OCI-LY19 cells. The PCR products (159 bp) for this assay were separated on a 2% agarose gel as indicated by arrows. The mutant MYD88 L265P allele was detected to a dilution of 0.1%. MW, molecular weight.
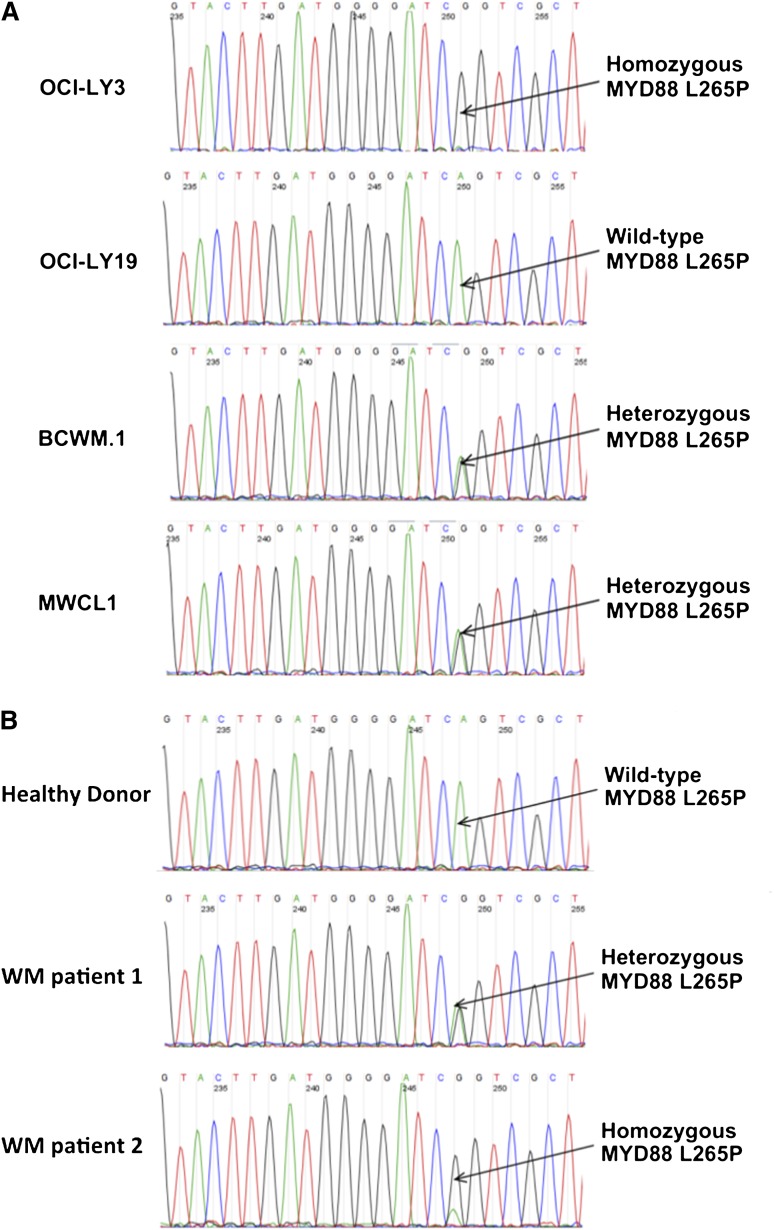
We next examined the use of the conventional AS-PCR MYD88 L265P assay in BM samples from 104 untreated WM patients. A total of 97 of 104 (93%) patients were positive for MYD88 L265P by this technique, with representative patients shown in Figure 2. To confirm these findings, the entire cohort was sequenced by Sanger sequencing at the MYD88 L265P position. Among the 97 patients positive for MYD88 L265P by the conventional AS-PCR assay, MYD88 L265P was detected in 93 patients by Sanger sequencing; for the 4 patients who were negative by Sanger sequencing, examination of the agarose gels used for conventional PCR testing showed a weak mutational signal. By contrast, all patients negative for MYD88 L265P by AS-PCR remained negative by Sanger sequencing. In addition, we assessed samples from healthy donors (HD) as well as patients with other B-cell disorders for the presence of MYD88 L265P by AS-PCR. MYD88 L265P was present in sorted CD19+ cells from 0/40 (0%) HD, 0/9 (0%) non-IgM MGUS, 13/24 (54%) IgM MGUS, 1/26 (4%) CLL, 2/20 (10%) MZL patients, and CD138+ cells from 0 of 14 MM patients (P < 1.5 × 10−5 for WM vs other cohorts; P = .005 for IgM MGUS vs IgG MGUS patients). Conventional AS-PCR demonstrated the expression of MYD88 L265P in OCI-Ly3, BCMW.1, and MWCL-1 but not OCI-Ly19 cells (Figure 2), consistent with previous Sanger sequencing results.3,4 Precision studies performed on 3 positive and 3 negative patient samples showed complete reproducibility among replicate aliquots, both within and between runs.
Figure 2.
MYD88 L265P in DLBCL and WM cell lines and primary WM patient cells using conventional AS-PCR assay. Detection of MYD88 L265P in OCI-LY3 and OCI-LY19 DLBCL, BCWM.1, and MWCL-1 WM cell lines (A) and primary WM patient tumor cells isolated from BM aspirates (B) by conventional AS-PCR assay. The depicted zygosity of MYD88 L265P in primary WM patient cells shown was confirmed by WGS. The position of MYD88 L265P locus is indicated by arrows. Complementary strand sequences are shown.
Quantitative detection of MYD88 L265P mutation
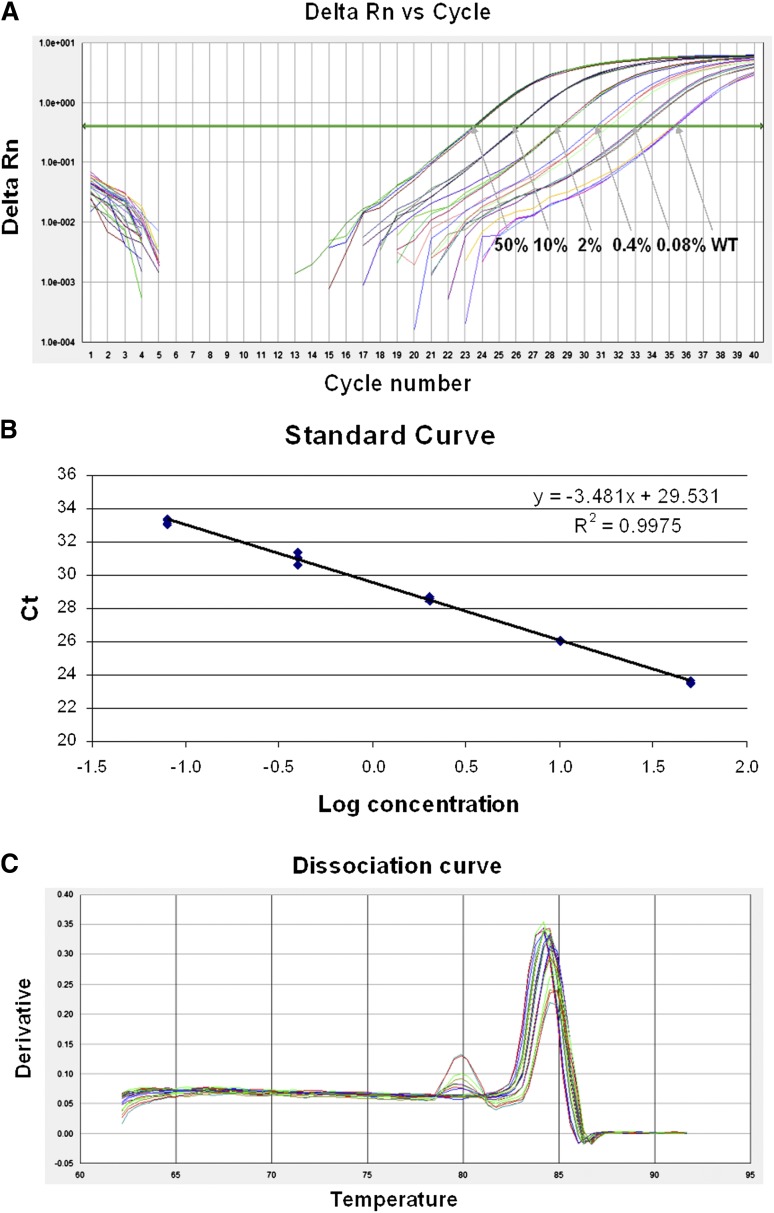
We next developed a SYBR green–based real-time AS-PCR to quantify MYD88 L265P allele burden. Given the high frequency of the MYD88 L265P mutation in WM, quantitative assessment of MYD88 L265P has potential to be developed as a robust biomarker for monitoring disease progression and response to treatment. Sensitivity and specificity of the real-time AS-PCR was determined by a serial dilution of the mutant DNA with the wild-type DNA as before. CT values were recorded for mutant-specific and reference PCR and the corresponding ΔCT values were calculated. As shown in Figure 3, this real-time AS-PCR can detect the MYD88 L265P mutation at a dilution of 0.08% with more than 2 cycle differences from the wild-type DNA background. Correlation coefficient of the standard curve was 0.998 with a slope value of −3.5 (Figure 3). The melting curve analysis revealed that the MYD88 L265P mutant-specific amplicon melted at 84°C (Figure 3). A minor nonspecific amplification was only found in the dilution of 0.4% or lower with a melting peak at 80°C. Precision studies performed on 12 replicates of tumor/normal DNA, on 5 separate assay runs, demonstrated high precision, both within and between runs. Interassay percent coefficients of variation (CV) for samples with 50% mutant DNA were 0.56% for mutant CT, 0.74% for wild-type CT, 7.53% for ΔCT. Samples with 2% mutant DNA performed equally well, with interassay CVs of 0.96% (mutant CT), 0.79% (wild type CT), and 4.18% (ΔCT). Intraassay CVs for ΔCT were 5.75% (50% mutant DNA) and 1.90% (2% mutant DNA).
Figure 3.
Sensitivity and specificity plots for real-time AS-PCR assay. (A) Δ reaction curve for real time AS-PCR assay. The MYD88 L265P mutant DNA (OCI-LY3) was diluted with the wild-type DNA (OCI-LY19) at the concentrations indicated in the amplification plot, with the MYD88 L265P allele detectable to a dilution of 0.08%. (B) Standard curve for real time AS-PCR assay. The correlation coefficient was 0.998 with a slope value of −3.48. (C) Dissociation curve for real time AS-PCR assay. Melting curve analysis revealed that the MYD88 L265P mutant-specific amplicon melted at 84°C. A minor nonspecific amplification was only found in the dilution of 0.4% or lower with a melting peak at 80°C. WT, wild-type.
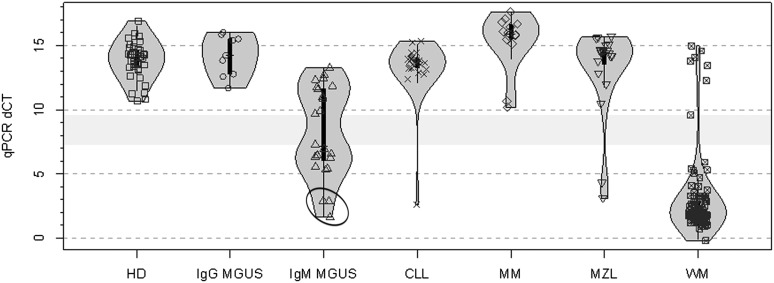
To gain further insight into the performance of the real-time assay, all samples analyzed by the conventional AS-PCR and Sanger sequencing were reanalyzed by the real-time AS-PCR assay. The quantitative AS-PCR results for these individuals are shown in Figure 4. Healthy donors displayed a median ΔCT value of 14.1 (range 10.7-16.9 cycles), whereas WM patients had a median ΔCT value of 1.9 (range −0.2 to 15 cycles; P = 2.2 × 10−16 vs healthy donors). There were 2 major clusters of WM patients separated by 3.1 cycles. A cluster of 7 WM patients had a median ΔCT value of 13.8 (range 9.6-15 cycles), which was similar to that observed in healthy donors, and were negative for the MYD88 L265P mutation by Sanger sequencing. Conversely, a cluster of 97 patients had ΔCT values with a median of 1.9 (range −0.2 to 5.9 cycles) and were deemed to be positive for the MYD88 L265P mutation. The results obtained in these studies showed complete concordance with those obtained using the conventional AS-PCR assay described above.
Figure 4.
Real-time AS-PCR results for MYD88 L265P in samples from patients with WM, IgM MGUS, and other B-cell lymphoproliferative disorders. Violin plot representing AS-PCR differences in cycle threshold (ΔCT). The span of grey area for each cohort represents the kernel density estimation of the sample distribution, and highlights the bimodal nature of the data. Box plots with interquartile ranges are shown in black with an overlay of the individual data points. Samples evaluated were from healthy donors (HD, n = 40); along with patients with IgG (n = 9) and IgM (n = 24) MGUS; CLL (n = 26); MM including 3 patients with IgM myeloma (n = 14); MZL (n = 20), and WM (n = 104). The light grey bar represents the distance between the highest positive (7.3), and lowest negative (9.6) sample ΔCT values. Circled area depicts results for 3 IgM MGUS patients who progressed to WM.
Predictive value for detection of MYD88 L265P by either conventional or real-time PCR
The sensitivity and specificity for MYD88 L265P detection by either conventional or real-time PCR in WM patient samples was 100% and 92.1%, respectively. The positive predictive and negative predictive values for either PCR based assay were 95.9% and 100%, respectively.
Determination of MYD88 L265P in IgM MGUS patients
IgM MGUS most commonly evolves to WM upon progression, though the progression rate for evolution is estimated at 2% to 2.5% per year. In our previous studies using Sanger sequencing, 2 of 22 (10%) IgM MGUS patients were determined to carry the MYD88 L265P mutation, including 1 patient who progressed to WM. Because the presence of clonal B cells is often very low in IgM MGUS patients, the use of more sensitive assays to determine MYD88 L265P could have both pathogenic and prognostic implications. Using BM-isolated CD19+ cells samples from 24 individuals with IgM MGUS, 13 (54%) were MYD88 L265P positive by either PCR assay. The quantitative AS-PCR results for these patients are shown in Figure 4. The median ΔCT value for all 24 IgM MGUS patients was 7.1 (range 1.6-13.3 cycles). For the 13 patients who were deemed to have the MYD88 L265P mutation, the median ΔCT value was 6.3 (range 1.6-7.3 cycles). Three of these patients, all with low ΔCT values (1.63, 2.85, 2.89), subsequently progressed to WM. These patients were also positive for MYD88 L265P by Sanger sequencing. For the 11 patients who were deemed to be negative for the MYD88 L265P mutation, the median ΔCT value was 11.8 (range 9.7-13.3 cycles, P = 3.9 × 10−5 vs MYD88 L265P patients). All IgM MGUS patients with the exception of the 3 patients with the low ΔCT values who progressed to WM were negative by Sanger sequencing. For 9 of these patients, sufficient DNA was available to determine presence of clonal immunoglobulin H (IgH) rearrangements. In support that sufficient clonal B cells were likely present in most samples for this analysis, 6 of these 9 (67%) patients demonstrated clonal IgH rearrangements. There was no correlation between the expression of MYD88 L265P and presence of clonal B cells by either flow cytometric analysis or an IgH rearrangement assay (data not shown), the latter of which has a lower limit of detection 2 orders of magnitude less sensitive than the AS-PCR assays described herein.18
Determination of MYD88 L265P in other B-cell lymphoproliferative disorders
To further evaluate the performance of the quantitative AS-PCR MYD88 L265P detection assay, ΔCT levels were determined for the same samples used in conventional AS-PCR evaluations for patients with other B-cell lymphoproliferative disorders. These studies were in complete concordance with results obtained by the quantitative AS-PCR assay and those obtained with the conventional AS-PCR assay. The quantitative AS-PCR results for these patients are shown in Figure 4. The median ΔCT for MYD88 L265P presence was 14.2 (range 11.7-16 cycles) for the 9 IgG MGUS patients who were negative, 15.9 (range 10.2-17.6 cycles) for the 14 MM patients who were negative, 13.8 (range 12.4-15.3 cycles) for the 25 CLL patients who were negative, 2.6 cycles for 1 CLL patient who was positive, 14.5 (range 10.5-15.7 cycles) for 18 splenic MZL patients who were negative, and 3.1 and 4.3 cycles for 2 splenic MZL patients who were positive.
Correlation between MYD88 L265P status and clinical characteristics
We next sought to evaluate any differences in clinical characteristics between WM patients based on the presence of the MYD88 L265P mutation by AS-PCR. ANCOVA analysis revealed that the MYD88 L265P-positive patients demonstrated greater median BM disease involvement (60% vs 15%, P = .01) and higher serum IgM levels (3010 vs 1130 mg/dL, P = .05). The median IgA (49 vs 155 mg/dL, P = .002) and IgG levels (665 vs 967 mg/dL, P = .46) were lower in MYD88 L265P mutated patients. Correspondingly, more MYD88 L265P patients had IgA (55/97 [57%] vs 0/7 [0%], P = .004) and IgG (49/97 [51%] vs 3/7 [42%], P = 1.0) hypogammaglobulinemia.
Response assessment by real-time MYD88 L265P AS-PCR
To explore the potential of using real-time AS-PCR for MYD88 L265P in response assessment, we examined concordance between readouts of BM involvement by histologic examination and the levels of mutant MYD88 L265P by real-time AS-PCR for 7 patients following therapy. The results of this analysis are summarized in Table 1. There was a strong correlation between the percentage changes of BM involvement and levels of MYD88 L265P determined by real-time AS-PCR (r = 0.89, P = .008), and this correlation was independent of treatment received in this small sample set. In one patient (A), the attainment of a complete response (ie, normalization of serum IgM level, no IgM monoclonal protein by serum protein electrophoresis and immunofixation, and undetectable BM disease involvement) was accompanied by undetectable MYD88 L265P levels by real time AS-PCR testing.
Table 1.
Comparison of MYD88 L265P before and after treatment using real-time AS-PCR in patients with WM
| Patient | Age (years) | Gender | Treatment | Pre-/posttherapy BM involved, % | Pre-/posttherapy MYD88 L265P levels | Change in BM involved, % | Change in MYD88 L265P levels, % |
|---|---|---|---|---|---|---|---|
| A | 61 | Male | Benda-R | 70 (negative) | 78.45 (negative) | −100 | −100 |
| B | 44 | Male | R-CD | 90 | 60.73 | −89 | −96.33 |
| 10 | 2.23 | ||||||
| C | 52 | Male | R-CD | 50 | 72.12 | −90 | −73.61 |
| 5 | 19.03 | ||||||
| D | 59 | Male | Everolimus | 95 | 99.15 | −47 | −45.39 |
| 50 | 54.15 | ||||||
| E | 63 | Male | Everolimus | 90 | 96.07 | −67 | −21.03 |
| 30 | 75.87 | ||||||
| F | 70 | Male | Everolimus | 95 | 95.93 | −37 | −8.61 |
| 60 | 87.67 | ||||||
| G | 63 | Male | Everolimus | 20 | 67.93 | 25 | 12.87 |
| 25 | 76.67 |
Changes in expression levels for MYD88 L265P were calculated from a standard curve.
Benda-R, bendamustine and rituximab; R-CD, rituximab, cyclophosphamide, and dexamethasone.
Discussion
The recent identification of MYD88 L265P as a highly recurrent somatic mutation in WM by WGS and Sanger sequencing prompted us to develop AS-PCR assays that could be used for both the detection and quantification of MYD88 L265P. PCR-based assays are expedient and require less DNA than WGS or Sanger sequencing. In addition, materials and time consumption are less for PCR-based testing, making such assay systems less expensive to run. The presence of MYD88 L265P could help discern diseases that overlap with WM, such as MZL and IgM-secreting myeloma, and may also provide insight into treatment management in the future with the development of MYD88 pathway inhibitors. In addition, a real-time–based assay for MYD88 L265P could help clarify disease response to treatment and also assess disease progression or relapse. We therefore sought the development and validation of conventional and real-time AS-PCR assays that could detect and quantitatively assess MYD88 L265P.
In this study, we report the development of 2 assays suitable for use in detection and quantitative assessment of MYD88 L265P, both of which were capable of detecting MYD88 L265P at a lower limit of 0.1%, thus providing a resolution level much lower than typically appreciated by routine BM pathological studies. Important in this regard, AS-PCR testing detected the presence of MYD88 L265P in 54% of IgM MGUS patients, much higher than the 10% detection frequency reported by us with Sanger sequencing. The detection rate for MYD88 L265P by Sanger sequencing in this series of IgM MGUS patients was also similar, with 3 of 24 positive patients (12.5%). These 3 IgM MGUS patients also had the lowest ΔCT values for MYD88 L265P detection by quantitative AS-PCR, which were in the range of ΔCT values observed for WM patients. All 3 of these IgM MGUS patients subsequently progressed to WM. Because IgM MGUS evolves to WM in most but not all cases upon progression,19-21 a longitudinal study to help clarify if the detection and/or levels of the MYD88 L265P mutation predict ultimate WM evolution will be of interest. These studies can be facilitated by the use of more sensitive AS-PCR testing for MYD88 L265P such as the ones described herein. The finding of MYD88 L265P in IgM MGUS patients is also of interest because its presence may constitute a “driver mutation,” one that might give the early WM clone a competitive growth advantage, and potentially predispose the expanding clone to other mutation(s) leading to symptomatic WM. Following MYD88 L265P, the next most common somatic mutations revealed by WGS in WM patients are those in CXCR4 (27%), ARID1A (17%), MUC16 (13%), TRAF2 (10%), and TRRAP (10%).3,22 The positioning of these mutations, as well as other mutations that may not yet be revealed in the context of IgM MGUS and WM, may help clarify which additional “hits,” if any, are needed for symptomatic WM progression.
In this population of WM patients who were unselected for any baseline characteristics, both the conventional and real-time AS-PCR assays detected MYD88 L265P in 93% of 104 WM patients and none of the 40 healthy donors examined. Results were confirmed by Sanger sequencing and demonstrated that the sensitivity and specificity for these assays was 100% and 92.1%, and positive and negative predictive values were 95.9% and 100%, respectively. These levels of detection are in line with those commonly used in molecular clinical diagnostic assays. The presence of MYD88 L265P using either conventional or real-time AS-PCR in WM patients was also associated with higher BM disease involvement (P = .01), serum IgM levels (P = .05), as well as subnormal IgA (P = .004) levels. The detection of MYD88 L265P may therefore help stratify those WM patients with high- vs low-risk disease. Due to small number of MYD88 L265P-negative patients present in this study, these observations will need to be confirmed in a larger cohort of WM patients.
The use of AS-PCR assays to delineate heterozygous vs homozygous MYD88 L265P presence may not always be possible. The patient depicted in Figure 1D demonstrated an acquired UPD at 3p22.2 by WGS establishing homozygous expression for MYD88 L265P.3 In this patient, the MYD88 L265P allele frequency was significantly greater than 50%, thereby supporting homozygous expression in at least a subset of the tumor population. In patients with lower levels of MYD88 L265P presence, such discrimination may not be possible due to contamination of samples with normal B cells.
The detection of MYD88 by AS-PCR L265P is also significant given interest for the development of targeted therapeutics for pathways activated by this mutation, including inhibitors blocking MYD88 homodimerization, MYD88-IRAK interactions, IRAK1 and/or IRAK4, as well as BTK activation. The detection of MYD88 L265P by AS-PCR may therefore help identify those patients who are more suitable for treatments targeting MYD88 L265P signaling. Additionally, the feasibility of using real-time PCR for the quantitative assessment of disease burden was also demonstrated in these studies in patients who underwent chemotherapy for symptomatic WM. A high rate of concordance between MYD88 L265P ΔCT and BM disease involvement was observed in these studies. The value of using real-time PCR for MYD88 L265P in response assessment will need to be validated against a large series of treated WM patients, and ideally across multiple therapeutic regimens.
Quantitative assessment of MYD88 L265P at baseline may also help clarify the prognostic value for attainment of a molecular complete response. Deeper categorical response attainment, particularly the attainment of very good partial response or complete response, is associated with longer progression-free survival in WM patients undergoing rituximab-based therapy.23 Improved progression-free survival in association with attainment of a molecular response has been described in multiple studies in patients with follicular lymphoma in which there is a disease-related genetic marker (BCL2/IgH fusion transcript), as well as for myeloma using tumor-related IgH rearrangements for quantitative PCR analysis.24-30 Quantitative PCR analysis of BM cells for BCL2/IgH at baseline also served as a prognostic indicator for response and disease control,24 and a similar study using AS-PCR for MYD88 L265P could also help establish its worthiness as a prognostic marker for WM.
In conclusion, MYD88 L265P is detectable in most patients with WM and in half of patients with IgM MGUS by use of conventional and real time real-time AS-PCR assays. The finding of this mutation in many IgM MGUS patients suggests that MYD88 L265P may be an early oncogenic event in WM pathogenesis. The detection of MYD88 L265P in WM and IgM MGUS by AS-PCR assays may aid in distinguishing these entities from overlapping B-cell disorders. In addition, real-time AS-PCR may help identify those patients with IgM MGUS likely to progress to WM and may also permit quantitative assessment of disease burden in WM patients undergoing chemotherapy. These studies provide molecular tools and a framework for the investigation of MYD88 L265P in the diagnosis and management of WM, IgM MGUS, and possibly other B-cell disorders in which MYD88 L265P is present.
Acknowledgments
The authors thank the WM patients who provided samples for their support of these studies.
This study was supported by the Peter and Helen Bing Foundation, the Coyote Fund for WM, the International Waldenstrom’s Macroglobulinemia Foundation, the Waldenstrom’s Cancer Fund, the Bailey Family Fund for WM, the D’Amato Family Fund for Genomic Discovery, the Edward and Linda Nelson Fund for WM Research, the Bauman Family Trust, and the Tannenhauser Family Foundation.
Footnotes
Presented at the Annual Meeting of the American Society for Clinical Oncology, Chicago, IL, June 1-5, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.X. and S.P.T. conceived and designed the experiments and wrote the manuscript. L.X., Z.R.H., and S.P.T. performed the data analysis. L.X., Y.C., C.K.T., E.M., A.T., A.G., L.A., M.V., J.R.B., Y.-T.T., K.C.A., N.C.M., and C.J.P. procured and/or prepared samples, and L.X. designed and performed PCR-based sequencing studies. L.X., G.Y., Y.Z., Y.C., and X.L. performed validation studies. S.P.T. and P.S. provided patient care and obtained consent and samples. R.J.M. collected patient data. N.I.L. provided input for development and validation of AS-PCR assays.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström’s Macroglobulinemia, Dana Farber Cancer Institute, M547, 45 Brookline Ave, Boston MA 02215; e-mail: steven_treon@dfci.harvard.edu.
References
- 1.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al., editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008. pp. 194–195. [Google Scholar]
- 3.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367(9):826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 4.Gachard N, Parrens M, Soubeyran I, et al. IGHV gene features and MYD88 L265P mutation separate the three marginal zone lymphoma entities and Waldenstrom macroglobulinemia/lymphoplasmacytic lymphomas. Leukemia. 2013;27(1):183–189. doi: 10.1038/leu.2012.257. [DOI] [PubMed] [Google Scholar]
- 5.Poulain S, Roumier C, Decambron A, et al. MYD88 L265P mutation in Waldenstrom’s macroglobulinemia [abstract]. Blood. 2012;120(21) doi: 10.1182/blood-2012-06-436329. Abstract 1307. [DOI] [PubMed] [Google Scholar]
- 6.Varettoni M, Arcaini L, Zibellini S, et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in patients with Waldenstrom macroglobulinemia, IgM monoclonal gammopathy of undetermined significance or other mature B-cell neoplasms. [published online ahead of print January 25, 2013]. Blood. 2013. [Google Scholar]
- 7.Ansell SM, Secreto FJ, Manske M, et al. MYD88 pathway activation in lymphoplasmacytic lymphoma drives tumor cell growth and cytokine expression [abstract]. Blood. 2012;120(21) Abstract 2699. [Google Scholar]
- 8.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43(9):830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montesinos-Rongen M, Godlewska E, Brunn A, et al. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122(6):791–792. doi: 10.1007/s00401-011-0891-2. [DOI] [PubMed] [Google Scholar]
- 12.Watters TM, Kenny EF, O’Neill LAJ. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85(6):411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 13.Loiarro M, Gallo G, Fantò N, et al. Identification of critical residues of the MyD88 death domain involved in the recruitment of downstream kinases. J Biol Chem. 2009;284(41):28093–28103. doi: 10.1074/jbc.M109.004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465(7300):885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawagoe T, Sato S, Matsushita K, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9(6):684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 16.Cohen L, Henzel WJ, Baeuerle PA. IKAP is a scaffold protein of the IkappaB kinase complex. Nature. 1998;395(6699):292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Zhou Y, Liu X, Cao Y, Hunter Z, Treon SP. Disruption of MYD88 pathway signaling leads to loss of constitutive IRAK1, NF-κβ and JAK/STAT Signaling and induces apoptosis of cells expressing the MYD88 L265P mutation in Waldenstrom’s macroglobulinemia [abstract]. Blood. 2011;118(21):274-275. Abstract 597. [Google Scholar]
- 18.Yang G, Xu L, Zhou Y, et al. Participation of BTK in MYD88 signaling in malignant cells expressing the L265P mutation in Waldenstrom’s macroglobulinemia, and effect on tumor cells with BTK-inhibitor PCI-32765 in combination with MYD88 pathway inhibitors. J Clin Oncol. 2012;30(15) Abstract 8106. [Google Scholar]
- 19.Leleu X, Eeckhoute J, Jia X, et al. Targeting NF-kappaB in Waldenstrom macroglobulinemia. Blood. 2008;111(10):5068–5077. doi: 10.1182/blood-2007-09-115170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ditzel Santos D, Ho AW, Tournilhac O, et al. Establishment of BCWM.1 cell line for Waldenström’s macroglobulinemia with productive in vivo engraftment in SCID-hu mice. Exp Hematol. 2007;35(9):1366–1375. doi: 10.1016/j.exphem.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Hodge LS, Novak AJ, Grote DM, et al. Establishment and characterization of a novel Waldenstrom macroglobulinemia cell line, MWCL-1. Blood. 2011;117(19):e190–e197. doi: 10.1182/blood-2010-12-326868. [DOI] [PubMed] [Google Scholar]
- 22.van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 23.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102(10):3759–3764. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 24.Morra E, Cesana C, Klersy C, et al. Clinical characteristics and factors predicting evolution of asymptomatic IgM monoclonal gammopathies and IgM-related disorders. Leukemia. 2004;18(9):1512–1517. doi: 10.1038/sj.leu.2403442. [DOI] [PubMed] [Google Scholar]
- 25.Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom’s macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;23(21):4662–4668. doi: 10.1200/JCO.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 26.Hunter ZR, Xu L, Yang G, et al. Use of whole genome sequencing to identify highly recurrent somatic mutations in Waldenström’s macroglobulinemia. J Clin Oncol. 2012;▪▪▪:30. [Abstract 8107] [Google Scholar]
- 27.Treon SP, Yang G, Hanzis C, et al. Attainment of complete/very good partial response following rituximab-based therapy is an important determinant to progression-free survival, and is impacted by polymorphisms in FCGR3A in Waldenstrom macroglobulinaemia. Br J Haematol. 2011;154(2):223–228. doi: 10.1111/j.1365-2141.2011.08726.x. [DOI] [PubMed] [Google Scholar]
- 28.Rambaldi A, Carlotti E, Oldani E, et al. Quantitative PCR of bone marrow BCL2/IgH+ cells at diagnosis predicts treatment response and long-term outcome in follicular non-Hodgkin lymphoma. Blood. 2005;105(9):3428–3433. doi: 10.1182/blood-2004-06-2490. [DOI] [PubMed] [Google Scholar]
- 29.Arcaini L, Montanari F, Alessandrino EP, et al. Immunochemotherapy with in vivo purging and autotransplant induces long clinical and molecular remission in advanced relapsed and refractory follicular lymphoma. Ann Oncol. 2008;19(7):1331–1335. doi: 10.1093/annonc/mdn044. [DOI] [PubMed] [Google Scholar]
- 30.Montoto S, Moreno C, Domingo-Doménech E, et al. Grup per l’Estudi dels Limfomes de Catalunya I Balears (GELCAB) Spain. High clinical and molecular response rates with fludarabine, cyclophosphamide and mitoxantrone in previously untreated patients with advanced stage follicular lymphoma. Haematologica. 2008;93(2):207–214. doi: 10.3324/haematol.11671. [DOI] [PubMed] [Google Scholar]