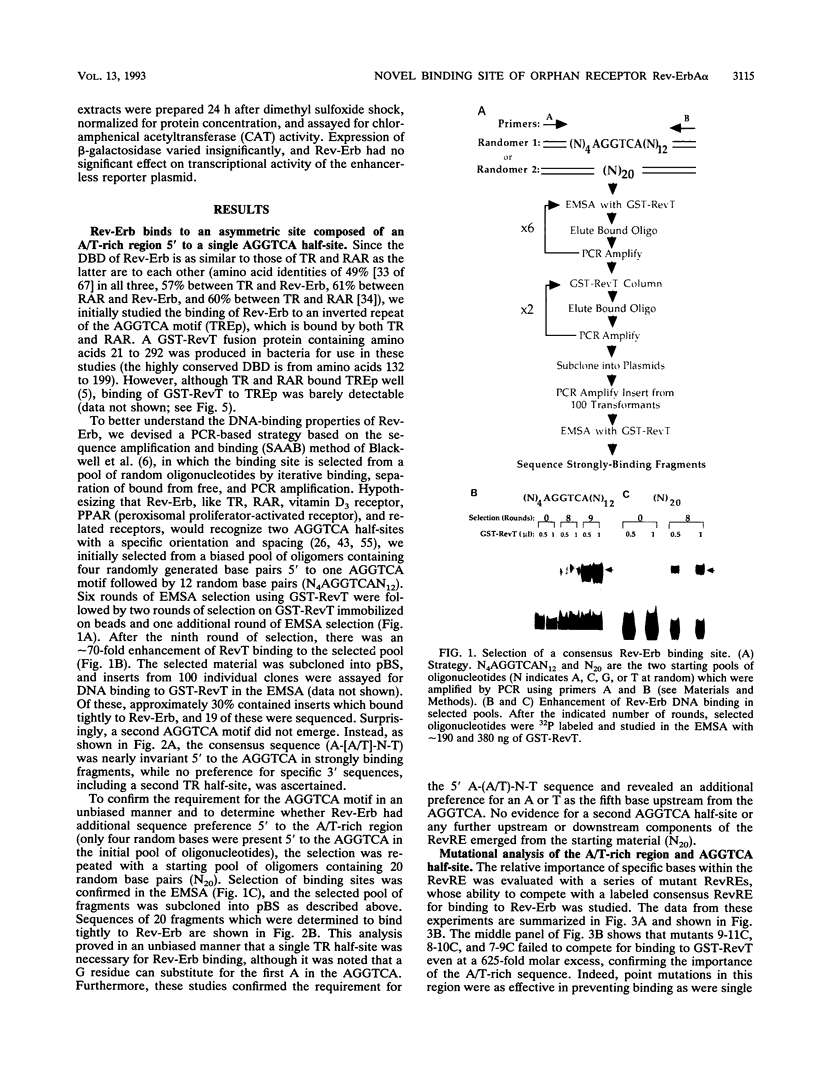
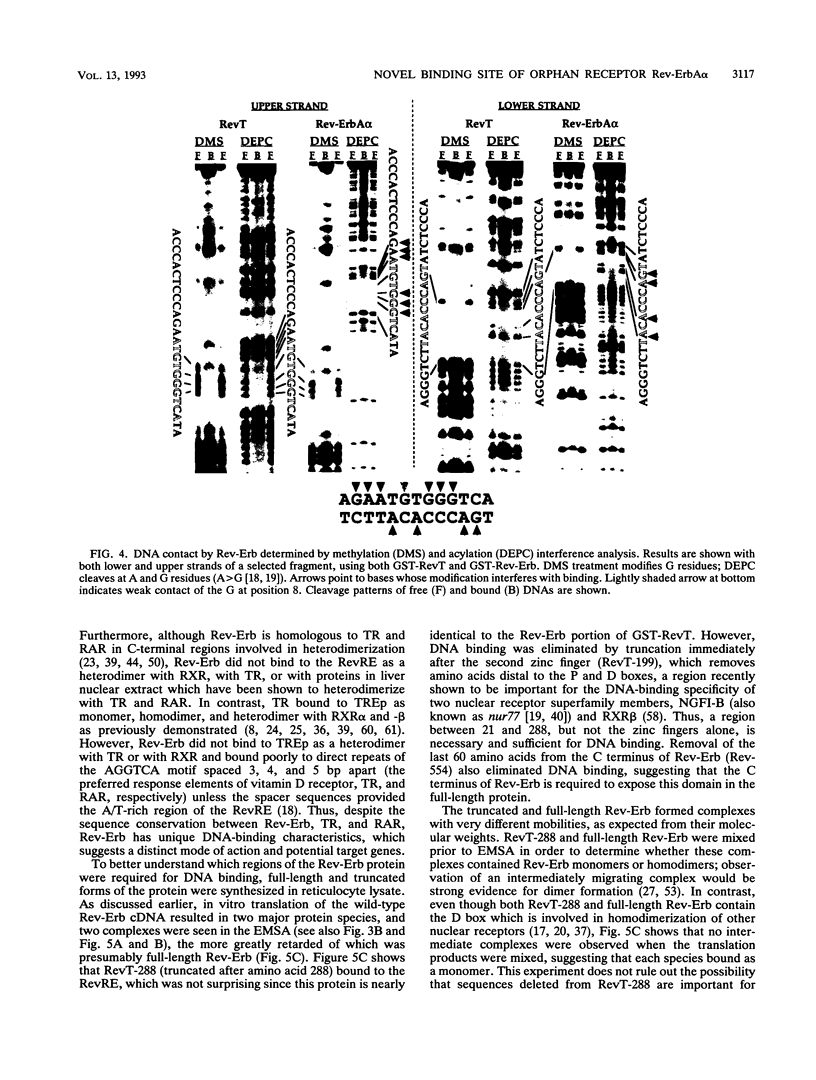
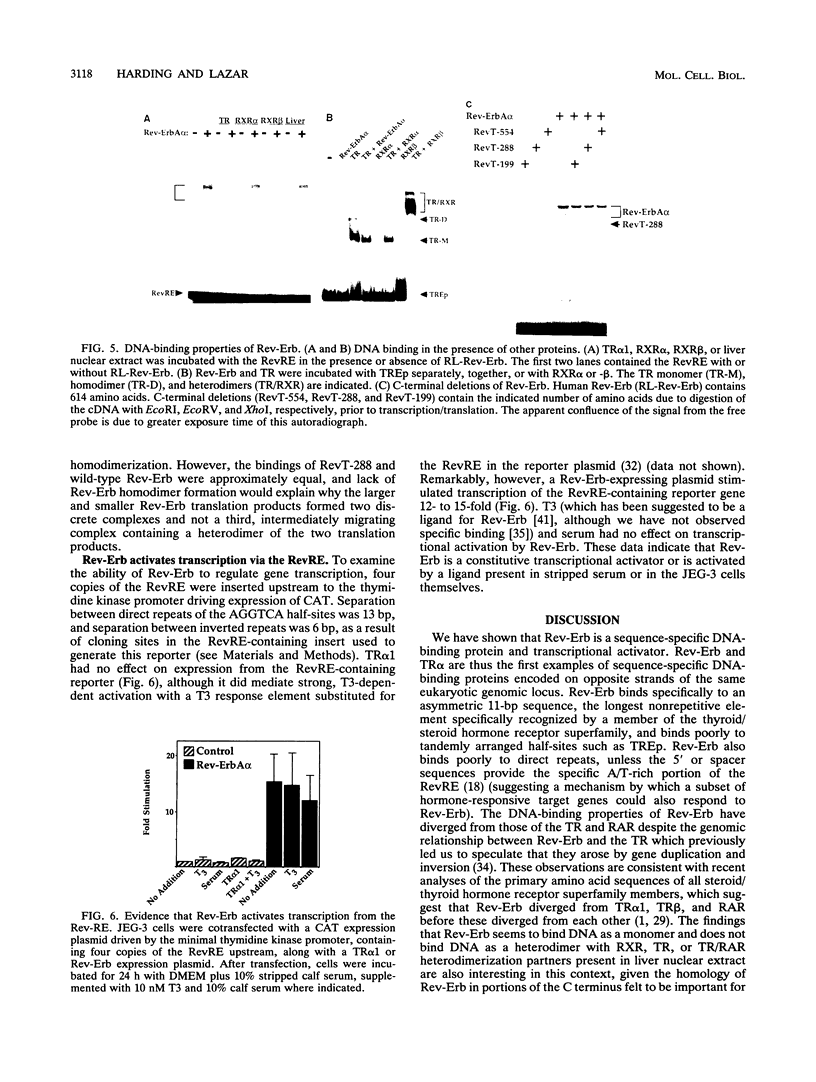
Abstract
Rev-ErbA alpha (Rev-Erb) is a nuclear hormone receptor-related protein encoded on the opposite strand of the alpha-thyroid hormone receptor (TR) gene. This unusual genomic arrangement may have a regulatory role, but the conservation of human and rodent Rev-Erb amino acid sequences suggests that the protein itself has an important function, potentially as a sequence-specific transcriptional regulator. However, despite its relationship to the TR, Rev-Erb bound poorly to TR binding sites. To determine its DNA-binding specificity in an unbiased manner, Rev-Erb was synthesized in Escherichia coli, purified, and used to select specific binding-sites from libraries of random double-stranded DNA sequences. We found that Rev-Erb binds to a unique site consisting of a specific 5-bp A/T-rich sequence adjacent to a TR half-site. Rev-Erb contacts this entire asymmetric 11-bp sequence, which is the longest nonrepetitive element specifically recognized by a member of the thyroid/steroid hormone receptor superfamily, and mutations in either the A/T-rich or TR half-site regions abolished specific binding. The binding specificity of wild-type Rev-Erb was nearly identical to that of C- and N-terminally truncated forms. This binding was not enhanced by retinoid X receptor, TR, or other nuclear proteins, none of which formed heterodimers with Rev-Erb. Rev-Erb also appeared to bind to the selected site as a monomer. Furthermore, Rev-Erb activates transcription through this binding site even in the absence of exogenous ligand. Thus, Rev-Erb is a transcriptional activator whose properties differ dramatically from those of classical nuclear hormone receptors, including the TR encoded on the opposite strand of the same genomic locus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amero S. A., Kretsinger R. H., Moncrief N. D., Yamamoto K. R., Pearson W. R. The origin of nuclear receptor proteins: a single precursor distinct from other transcription factors. Mol Endocrinol. 1992 Jan;6(1):3–7. doi: 10.1210/mend.6.1.1738368. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Berrodin T. J., Marks M. S., Ozato K., Linney E., Lazar M. A. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Mol Endocrinol. 1992 Sep;6(9):1468–1478. doi: 10.1210/mend.6.9.1331778. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Bugge T. H., Pohl J., Lonnoy O., Stunnenberg H. G. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992 Apr;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992 Sep;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Hinck L., Ringold G. M. Two amino acids within the knuckle of the first zinc finger specify DNA response element activation by the glucocorticoid receptor. Cell. 1989 Jun 30;57(7):1131–1138. doi: 10.1016/0092-8674(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Davis I. J., Hazel T. G., Lau L. F. Transcriptional activation by Nur77, a growth factor-inducible member of the steroid hormone receptor superfamily. Mol Endocrinol. 1991 Jun;5(6):854–859. doi: 10.1210/mend-5-6-854. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Hamada K., Gleason S. L., Levi B. Z., Hirschfeld S., Appella E., Ozato K. H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8289–8293. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel T. G., Nathans D., Lau L. F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M. A., Hinck L., Danielsen M., Ringold G. M. Discrimination of DNA response elements for thyroid hormone and estrogen is dependent on dimerization of receptor DNA binding domains. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5527–5531. doi: 10.1073/pnas.89.12.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwung Y. P., Crowe D. T., Wang L. H., Tsai S. Y., Tsai M. J. The COUP transcription factor binds to an upstream promoter element of the rat insulin II gene. Mol Cell Biol. 1988 May;8(5):2070–2077. doi: 10.1128/mcb.8.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härd T., Kellenbach E., Boelens R., Maler B. A., Dahlman K., Freedman L. P., Carlstedt-Duke J., Yamamoto K. R., Gustafsson J. A., Kaptein R. Solution structure of the glucocorticoid receptor DNA-binding domain. Science. 1990 Jul 13;249(4965):157–160. doi: 10.1126/science.2115209. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Krek W., Sellers W. R., DeCaprio J. A., Ajchenbaum F., Fuchs C. S., Chittenden T., Li Y., Farnham P. J., Blanar M. A. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992 Jul 24;70(2):351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Katz D., Berrodin T. J., Lazar M. A. The unique C-termini of the thyroid hormone receptor variant, c-erbA alpha 2, and thyroid hormone receptor alpha 1 mediate different DNA-binding and heterodimerization properties. Mol Endocrinol. 1992 May;6(5):805–814. doi: 10.1210/mend.6.5.1318505. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992 Aug 27;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Berrodin T. J. Thyroid hormone receptors form distinct nuclear protein-dependent and independent complexes with a thyroid hormone response element. Mol Endocrinol. 1990 Nov;4(11):1627–1635. doi: 10.1210/mend-4-11-1627. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Cardona G., Chin W. W. Gene expression from the c-erbA alpha/Rev-ErbA alpha genomic locus. Potential regulation of alternative splicing by opposite strand transcription. J Biol Chem. 1990 Aug 5;265(22):12859–12863. [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Chin W. W. Human carboxyl-terminal variant of alpha-type c-erbA inhibits trans-activation by thyroid hormone receptors without binding thyroid hormone. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7771–7774. doi: 10.1073/pnas.86.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989 Mar;9(3):1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Jones K. E., Chin W. W. Isolation of a cDNA encoding human Rev-ErbA alpha: transcription from the noncoding DNA strand of a thyroid hormone receptor gene results in a related protein that does not bind thyroid hormone. DNA Cell Biol. 1990 Mar;9(2):77–83. doi: 10.1089/dna.1990.9.77. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988 May;1(3):183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Miyajima N., Horiuchi R., Shibuya Y., Fukushige S., Matsubara K., Toyoshima K., Yamamoto T. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989 Apr 7;57(1):31–39. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- Munroe S. H., Lazar M. A. Inhibition of c-erbA mRNA splicing by a naturally occurring antisense RNA. J Biol Chem. 1991 Nov 25;266(33):22083–22086. [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- O'Donnell A. L., Rosen E. D., Darling D. S., Koenig R. J. Thyroid hormone receptor mutations that interfere with transcriptional activation also interfere with receptor interaction with a nuclear protein. Mol Endocrinol. 1991 Jan;5(1):94–99. doi: 10.1210/mend-5-1-94. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Conneely O. M. Orphan receptors: in search of a unifying hypothesis for activation. Mol Endocrinol. 1992 Sep;6(9):1359–1361. doi: 10.1210/mend.6.9.1331771. [DOI] [PubMed] [Google Scholar]
- Prost E., Moore D. D. CAT vectors for analysis of eukaryotic promoters and enhancers. Gene. 1986;45(1):107–111. doi: 10.1016/0378-1119(86)90138-1. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Segraves W. A., Hogness D. S. The E75 ecdysone-inducible gene responsible for the 75B early puff in Drosophila encodes two new members of the steroid receptor superfamily. Genes Dev. 1990 Feb;4(2):204–219. doi: 10.1101/gad.4.2.204. [DOI] [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Spanjaard R. A., Darling D. S., Chin W. W. Ligand-binding and heterodimerization activities of a conserved region in the ligand-binding domain of the thyroid hormone receptor. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8587–8591. doi: 10.1073/pnas.88.19.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R., Baumruker T., Franza B. R., Jr, Herr W. A 100-kD HeLa cell octamer binding protein (OBP100) interacts differently with two separate octamer-related sequences within the SV40 enhancer. Genes Dev. 1987 Dec;1(10):1147–1160. doi: 10.1101/gad.1.10.1147. [DOI] [PubMed] [Google Scholar]
- Tran P., Zhang X. K., Salbert G., Hermann T., Lehmann J. M., Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992 Oct;12(10):4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Ing N. H., Tsai S. Y., O'Malley B. W., Tsai M. J. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991;1(3):207–216. [PMC free article] [PubMed] [Google Scholar]
- Wilson T. E., Fahrner T. J., Johnston M., Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991 May 31;252(5010):1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- Wilson T. E., Paulsen R. E., Padgett K. A., Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992 Apr 3;256(5053):107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Binder M., Funk W. Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Mol Cell Biol. 1991 Aug;11(8):4104–4110. doi: 10.1128/mcb.11.8.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]