Abstract
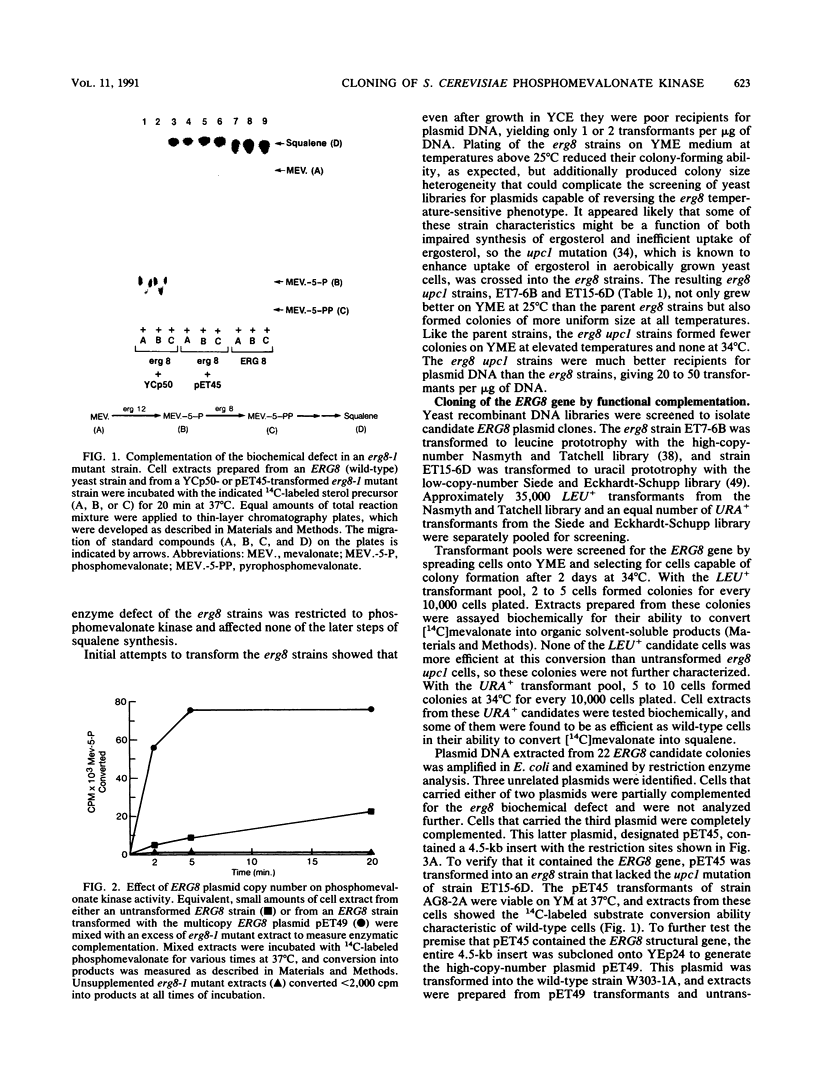
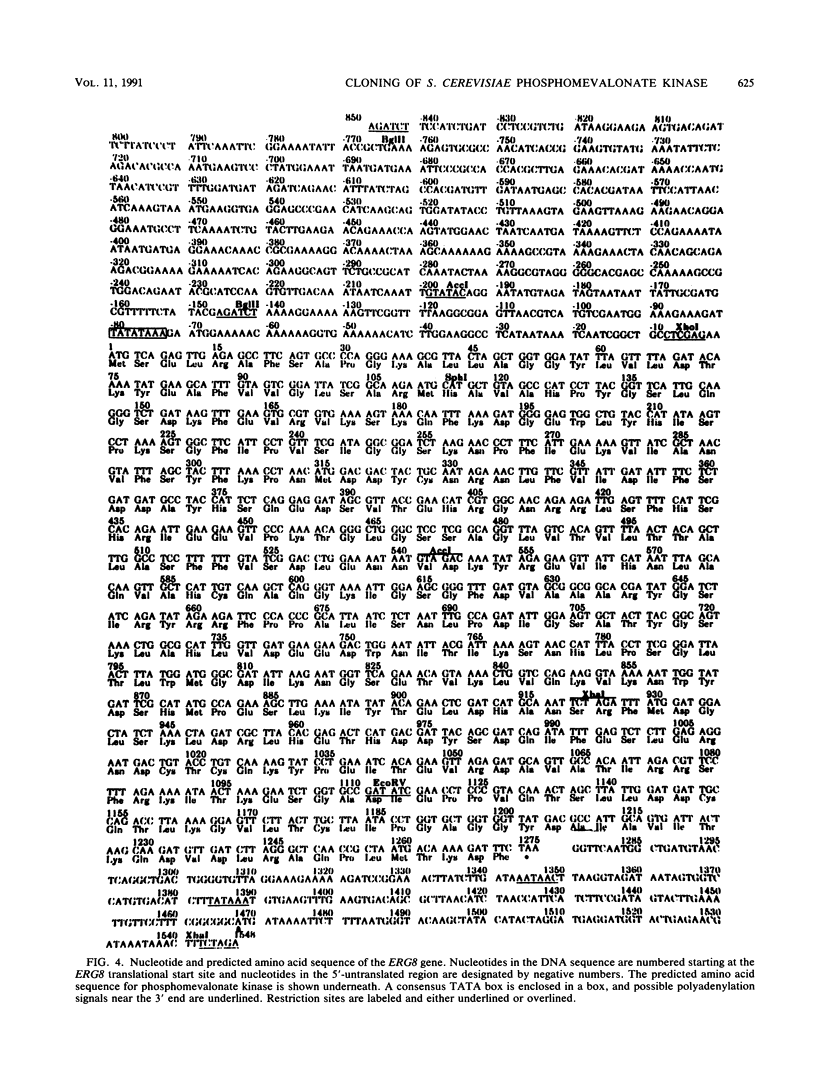
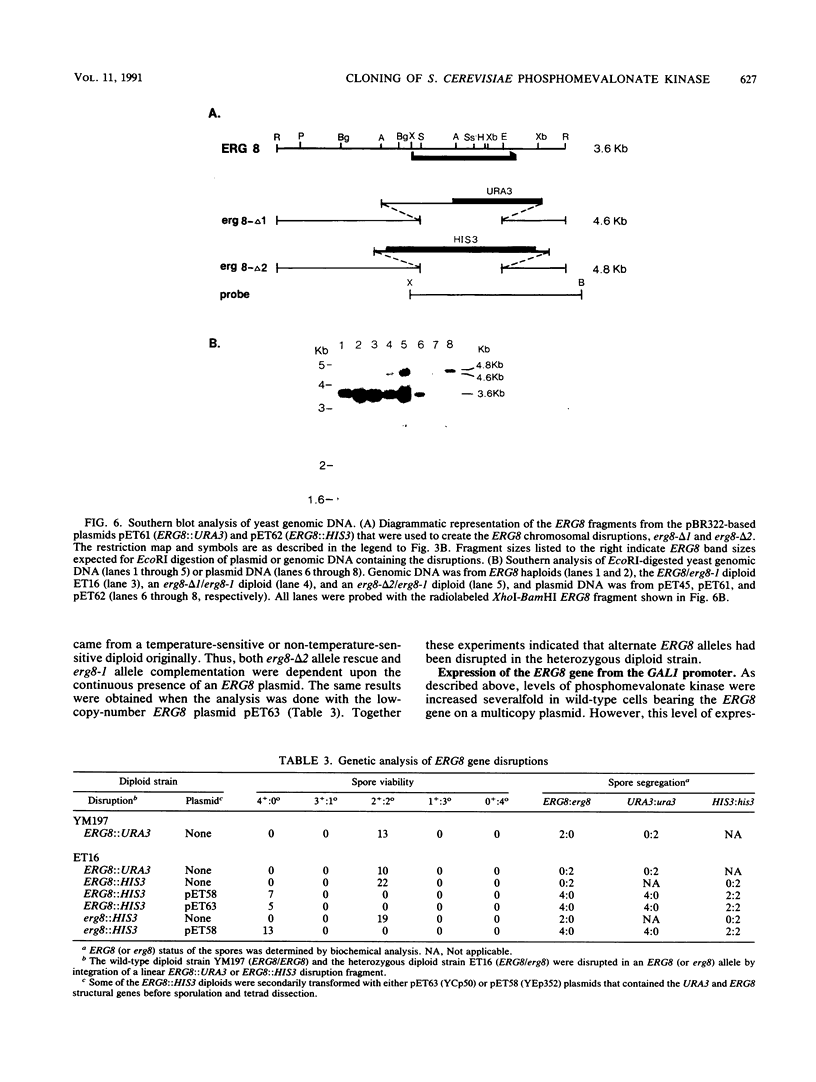
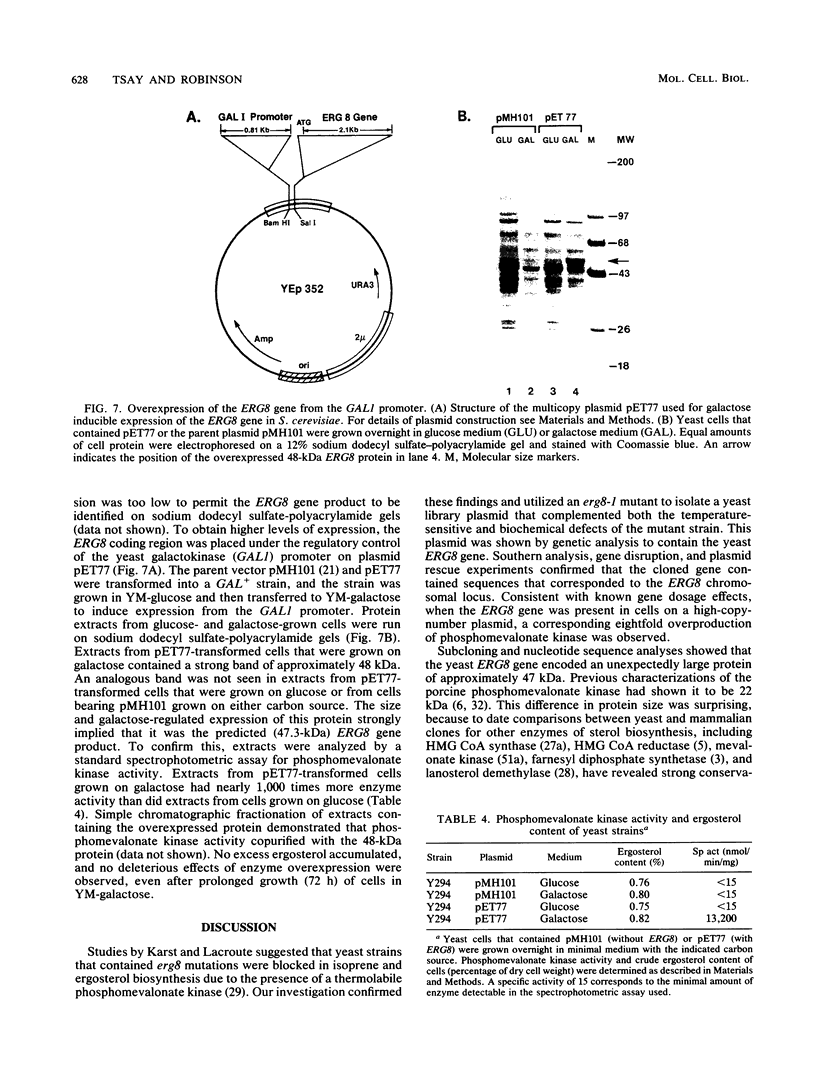
Saccharomyces cerevisiae strains that contain the ery8-1 mutation are temperature sensitive for growth due to a defect in phosphomevalonate kinase, an enzyme of isoprene and ergosterol biosynthesis. A plasmid bearing the yeast ERG8 gene was isolated from a YCp50 genomic library by functional complementation of the erg8-1 mutant strain. Genetic analysis demonstrated that integrated copies of an ERG8 plasmid mapped to the erg8 locus, confirming the identity of this clone. Southern analysis showed that ERG8 was a single-copy gene. Subcloning and DNA sequencing defined the functional ERG8 regulon as an 850-bp upstream region and an adjacent 1,272-bp open reading frame. The deduced 424-amino-acid ERG8 protein showed no homology to known proteins except within a putative ATP-binding domain present in many kinases. Disruption of the chromosomal ERG8 coding region by integration of URA3 or HIS3 marker fragments was lethal in haploid cells, indicating that this gene is essential. Expression of the ERG8 gene in S. cerevisiae from the galactose-inducible galactokinase (GAL1) promoter resulted in 1,000-fold-elevated levels of phosphomevalonate kinase enzyme activity. Overproduction of a soluble protein with the predicted 48-kDa size for phosphomevalonate kinase was also observed in the yeast cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Anderson M. S., Muehlbacher M., Street I. P., Proffitt J., Poulter C. D. Isopentenyl diphosphate:dimethylallyl diphosphate isomerase. An improved purification of the enzyme and isolation of the gene from Saccharomyces cerevisiae. J Biol Chem. 1989 Nov 15;264(32):19169–19175. [PubMed] [Google Scholar]
- Anderson M. S., Yarger J. G., Burck C. L., Poulter C. D. Farnesyl diphosphate synthetase. Molecular cloning, sequence, and expression of an essential gene from Saccharomyces cerevisiae. J Biol Chem. 1989 Nov 15;264(32):19176–19184. [PubMed] [Google Scholar]
- BLOCH K., CHAYKIN S., PHILLIPS A. H., DE WAARD A. Mevalonic acid pyrophosphate and isopentenylpyrophosphate. J Biol Chem. 1959 Oct;234:2595–2604. [PubMed] [Google Scholar]
- Basson M. E., Thorsness M., Finer-Moore J., Stroud R. M., Rine J. Structural and functional conservation between yeast and human 3-hydroxy-3-methylglutaryl coenzyme A reductases, the rate-limiting enzyme of sterol biosynthesis. Mol Cell Biol. 1988 Sep;8(9):3797–3808. doi: 10.1128/mcb.8.9.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaes S., Beytía E., Jabalquinto A. M., Solís de Ovando F., Gómez I., Eyzaguirre J. Pig liver phosphomevalonate kinase. 2. Participation of cysteinyl and lysyl groups in catalysis. Biochemistry. 1980 May 27;19(11):2305–2310. doi: 10.1021/bi00552a004. [DOI] [PubMed] [Google Scholar]
- Bazaes S., Beytía E., Jabalquinto A. M., Solís de Ovando F., Gómez I., Eyzaguirre J. Pig liver phosphomevalone kinase. 1. Purification and properties. Biochemistry. 1980 May 27;19(11):2300–2304. doi: 10.1021/bi00552a003. [DOI] [PubMed] [Google Scholar]
- Beier D. R., Sledziewski A., Young E. T. Deletion analysis identifies a region, upstream of the ADH2 gene of Saccharomyces cerevisiae, which is required for ADR1-mediated derepression. Mol Cell Biol. 1985 Jul;5(7):1743–1749. doi: 10.1128/mcb.5.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Boll M., Löwel M., Still J., Berndt J. Sterol biosynthesis in yeast. 3-Hydorxy-3-methylglutaryl-Coenzyme A reductase as a regulatory enzyme. Eur J Biochem. 1975 Jun;54(2):435–444. doi: 10.1111/j.1432-1033.1975.tb04154.x. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Dihanich M. E., Najarian D., Clark R., Gillman E. C., Martin N. C., Hopper A. K. Isolation and characterization of MOD5, a gene required for isopentenylation of cytoplasmic and mitochondrial tRNAs of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jan;7(1):177–184. doi: 10.1128/mcb.7.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Synthesis of ubiquinone and cholesterol in human fibroblasts: regulation of a branched pathway. Arch Biochem Biophys. 1979 Jan;192(1):86–99. doi: 10.1016/0003-9861(79)90074-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kekwick R. G. The inhibition of plant mevalonate kinase preparations by prenyl pyrophosphates. Biochim Biophys Acta. 1972 Sep 15;279(2):290–296. doi: 10.1016/0304-4165(72)90145-6. [DOI] [PubMed] [Google Scholar]
- Haffey M. L., Stevens J. T., Terry B. J., Dorsky D. I., Crumpacker C. S., Wietstock S. M., Ruyechan W. T., Field A. K. Expression of herpes simplex virus type 1 DNA polymerase in Saccharomyces cerevisiae and detection of virus-specific enzyme activity in cell-free lysates. J Virol. 1988 Dec;62(12):4493–4498. doi: 10.1128/jvi.62.12.4493-4498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Hoar E. T., Guarente L. Each of three "TATA elements" specifies a subset of the transcription initiation sites at the CYC-1 promoter of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8562–8566. doi: 10.1073/pnas.82.24.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Woods C. W., Turi T. G., Dey C. R., Sutter T. R., Loper J. C. Primary structure of the P450 lanosterol demethylase gene from Saccharomyces cerevisiae. DNA. 1987 Dec;6(6):529–537. doi: 10.1089/dna.1987.6.529. [DOI] [PubMed] [Google Scholar]
- Karst F., Lacroute F. Ertosterol biosynthesis in Saccharomyces cerevisiae: mutants deficient in the early steps of the pathway. Mol Gen Genet. 1977 Sep 9;154(3):269–277. doi: 10.1007/BF00571282. [DOI] [PubMed] [Google Scholar]
- LEVY H. R., POPJAK G. Studies on the biosynthesis of cholesterol. 10. Mevalonic kinase from liver. Biochem J. 1960 Jun;75:417–428. doi: 10.1042/bj0750417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Klinz F. J., Donath C., Gallwitz D. Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell. 1984 Mar;36(3):645–653. doi: 10.1016/0092-8674(84)90344-1. [DOI] [PubMed] [Google Scholar]
- Lee C. S., O'Sullivan W. J. Improved procedures for the synthesis of phosphomevalonate and for the assay and purification of pig liver phosphomevalonate kinase. Biochim Biophys Acta. 1985 Mar 29;839(1):83–89. doi: 10.1016/0304-4165(85)90184-9. [DOI] [PubMed] [Google Scholar]
- Lewis T. A., Taylor F. R., Parks L. W. Involvement of heme biosynthesis in control of sterol uptake by Saccharomyces cerevisiae. J Bacteriol. 1985 Jul;163(1):199–207. doi: 10.1128/jb.163.1.199-207.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. Molecular cloning of eucaryotic genes required for excision repair of UV-irradiated DNA: isolation and partial characterization of the RAD3 gene of Saccharomyces cerevisiae. J Bacteriol. 1982 Oct;152(1):323–331. doi: 10.1128/jb.152.1.323-331.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. Saccharomyces cerevisiae RAD2 gene: isolation, subcloning, and partial characterization. Mol Cell Biol. 1984 Feb;4(2):290–295. doi: 10.1128/mcb.4.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Servouse M., Karst F. Regulation of early enzymes of ergosterol biosynthesis in Saccharomyces cerevisiae. Biochem J. 1986 Dec 1;240(2):541–547. doi: 10.1042/bj2400541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servouse M., Mons N., Baillargeat J. L., Karst F. Isolation and characterization of yeast mutants blocked in mevalonic acid formation. Biochem Biophys Res Commun. 1984 Sep 17;123(2):424–430. doi: 10.1016/0006-291x(84)90247-x. [DOI] [PubMed] [Google Scholar]
- Siede W., Eckardt-Schupp F. DNA repair genes of Saccharomyces cerevisiae: complementing rad4 and rev2 mutations by plasmids which cannot be propagated in Escherichia coli. Curr Genet. 1986;11(3):205–210. doi: 10.1007/BF00420608. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N., Kekwick R. G. The enzymes forming isopentenyl pyrophosphate from 5-phosphomevalonate (mevalonate 5-phosphate) in the latex of Hevea brasiliensis. Biochem J. 1971 Sep;124(2):407–417. doi: 10.1042/bj1240407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R. D., Lee L. Y., Schafer B. L., Kratunis V. J., Mohler W. A., Robinson G. W., Mosley S. T. Molecular cloning of mevalonate kinase and regulation of its mRNA levels in rat liver. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2872–2876. doi: 10.1073/pnas.87.8.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocha P. J., Sprinson D. B. Location and regulation of early enzymes of sterol biosynthesis in yeast. Arch Biochem Biophys. 1976 May;174(1):45–51. doi: 10.1016/0003-9861(76)90322-2. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R., Basson M., D'Ari L., Rine J. Increased amounts of HMG-CoA reductase induce "karmellae": a proliferation of stacked membrane pairs surrounding the yeast nucleus. J Cell Biol. 1988 Jul;107(1):101–114. doi: 10.1083/jcb.107.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum R. R., Hanley S., West R., Jr, Ptashne M. Use of lacZ fusions to delimit regulatory elements of the inducible divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Oct;4(10):1985–1998. doi: 10.1128/mcb.4.10.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]