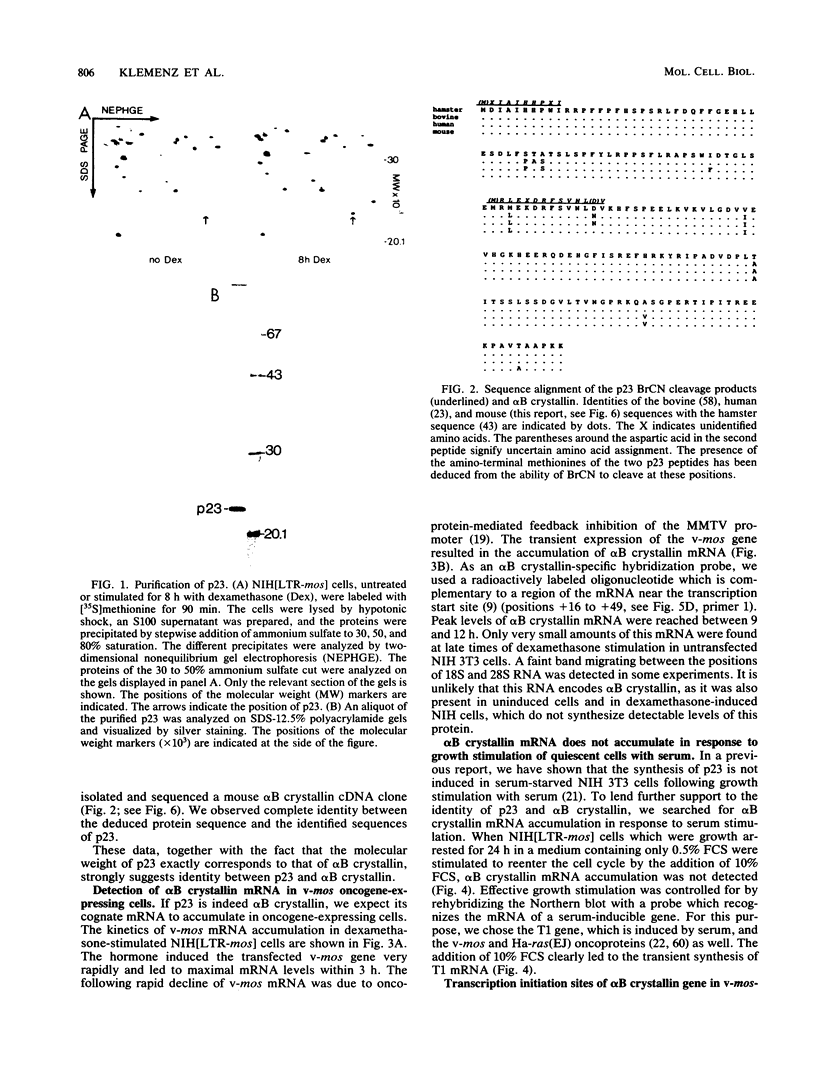
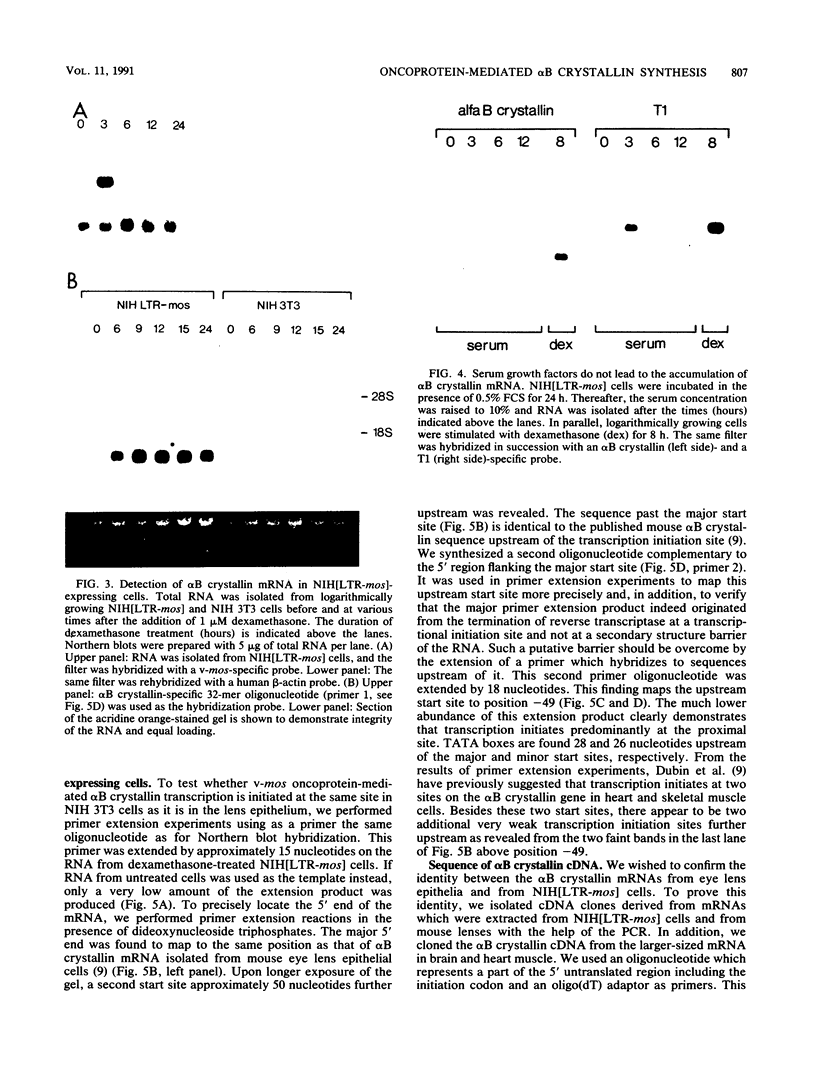
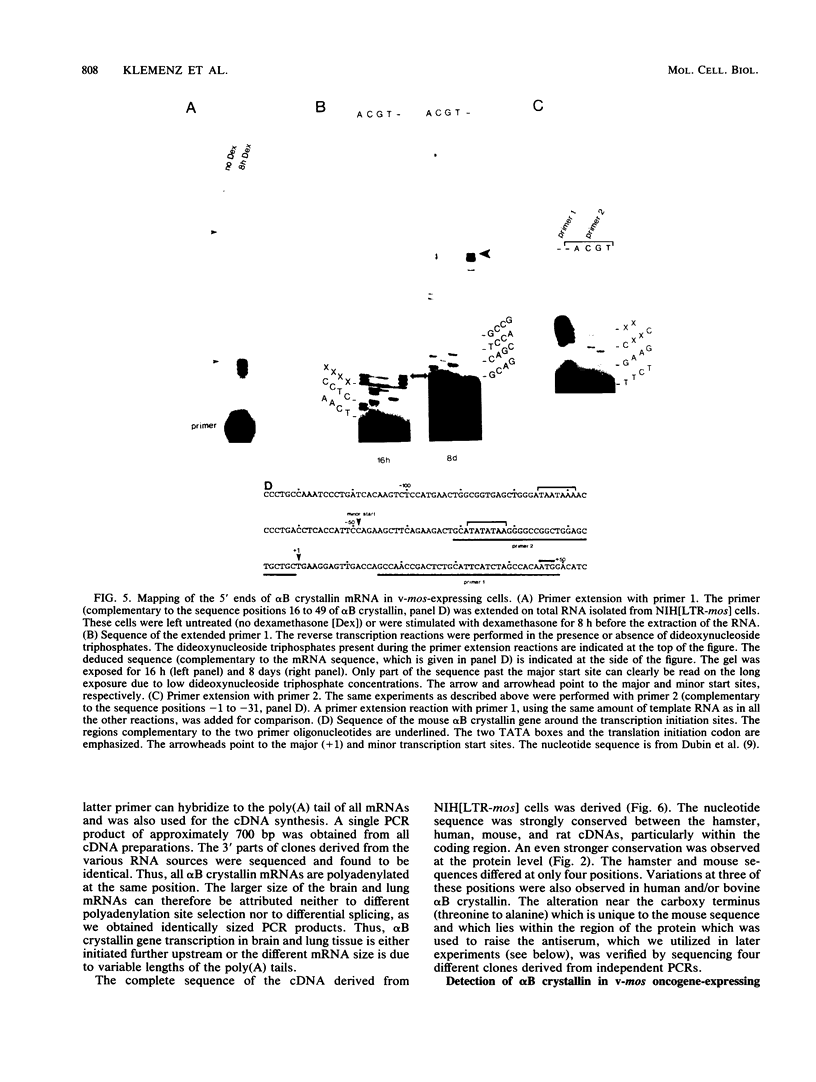
Abstract
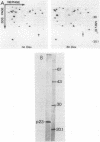
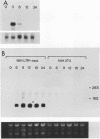
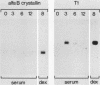
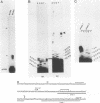
The conditional expression of the v-mos and Ha-ras(EJ) oncogenes in NIH 3T3 cells leads to the accumulation of a 23-kDa protein (p23) (R. Klemenz, S. Hoffmann, R. Jaggi, and A.-K. Werenskiold, Oncogene 4:799-803, 1989). We purified p23 to homogeneity and determined part of the amino acid sequence. The obtained sequence is identical with that of the eye lens protein alpha B crystallin. Northern (RNA) blot and Western immunoblot experiments were performed to demonstrate that alpha B crystallin mRNA and protein do indeed accumulate as a consequence of v-mos and Ha-ras oncogene expression. Comparison of cDNA clones obtained from the mRNA of eye lenses and of oncogene-expressing fibroblasts revealed identity between them. The major transcription initiation site of the alpha B crystallin gene in our experimental system was shown by primer extension experiments to be identical with the one used in eye epithelial cells. In addition, we identified a second minor initiation site 49 nucleotides further upstream. Serum growth factors did not stimulate alpha B crystallin expression in growth-arrested cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Suhan J. P., Welch W. J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988 Dec;8(12):5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Welch W. J. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987 Nov 15;262(32):15359–15369. [PubMed] [Google Scholar]
- Bhat S. P., Nagineni C. N. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989 Jan 16;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Lens proteins. More molecular opportunism. Nature. 1988 Nov 3;336(6194):18–18. doi: 10.1038/336018a0. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2767–2771. doi: 10.1073/pnas.75.6.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R. Extracellular signals, transcriptional responses and cellular specificity. Trends Biochem Sci. 1989 Nov;14(11):455–458. doi: 10.1016/0968-0004(89)90105-9. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Jaggi R., Salmons B., Muellener D., Groner B. The v-mos and H-ras oncogene expression represses glucocorticoid hormone-dependent transcription from the mouse mammary tumor virus LTR. EMBO J. 1986 Oct;5(10):2609–2616. doi: 10.1002/j.1460-2075.1986.tb04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Jr, Sharp P. A. Regulation of heat shock protein 70 gene expression by c-myc. Nature. 1984 Nov 15;312(5991):280–282. doi: 10.1038/312280a0. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Hoffmann S., Jaggi R., Werenskiold A. K. The v-mos and c-Ha-ras oncoproteins exert similar effects on the pattern of protein synthesis. Oncogene. 1989 Jun;4(6):799–803. [PubMed] [Google Scholar]
- Klemenz R., Hoffmann S., Werenskiold A. K. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5708–5712. doi: 10.1073/pnas.86.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps J. A., de Man B. M., de Jong W. W. The primary structure of the B2 chain of human alpha-crystallin. FEBS Lett. 1977 Feb 15;74(1):82–84. doi: 10.1016/0014-5793(77)80757-6. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Latchman D. S. A cellular protein related to heat-shock protein 90 accumulates during herpes simplex virus infection and is overexpressed in transformed cells. Exp Cell Res. 1988 Sep;178(1):169–179. doi: 10.1016/0014-4827(88)90388-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Longoni S., Lattonen S., Bullock G., Chiesi M. Cardiac alpha-crystallin. II. Intracellular localization. Mol Cell Biochem. 1990 Sep 21;97(2):121–128. doi: 10.1007/BF00221053. [DOI] [PubMed] [Google Scholar]
- Mahon K. A., Chepelinsky A. B., Khillan J. S., Overbeek P. A., Piatigorsky J., Westphal H. Oncogenesis of the lens in transgenic mice. Science. 1987 Mar 27;235(4796):1622–1628. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nagineni C. N., Bhat S. P. Maintenance of the synthesis of alpha B-crystallin and progressive expression of beta Bp-crystallin in human fetal lens epithelial cells in culture. Dev Biol. 1988 Nov;130(1):402–405. doi: 10.1016/0012-1606(88)90446-0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989 Mar;9(3):1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Omar R. A., Lanks K. W. Heat shock protein synthesis and cell survival in clones of normal and simian virus 40-transformed mouse embryo cells. Cancer Res. 1984 Sep;44(9):3976–3982. [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. D., Ostrowski M. C. Rapid and selective alterations in the expression of cellular genes accompany conditional transcription of Ha-v-ras in NIH 3T3 cells. Mol Cell Biol. 1987 Jul;7(7):2512–2520. doi: 10.1128/mcb.7.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins and their genes: diversity and tissue-specific expression. FASEB J. 1989 Jun;3(8):1933–1940. doi: 10.1096/fasebj.3.8.2656357. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. J. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989 Apr 21;57(2):197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Quax-Jeuken Y., Quax W., van Rens G., Khan P. M., Bloemendal H. Complete structure of the alpha B-crystallin gene: conservation of the exon-intron distribution in the two nonlinked alpha-crystallin genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5819–5823. doi: 10.1073/pnas.82.17.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Stiles C. D. Serum-inducible genes. Adv Cancer Res. 1989;53:1–32. doi: 10.1016/s0065-230x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Moritz R. L., Nice E. E., Grego B. A high-performance liquid chromatography procedure for recovering subnanomole amounts of protein from SDS-gel electroeluates for gas-phase sequence analysis. Eur J Biochem. 1987 May 15;165(1):21–29. doi: 10.1111/j.1432-1033.1987.tb11189.x. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Moritz R. L., Rubira M. R., Gorman J. J., Van Snick J. Complete amino acid sequence of a new murine T-cell growth factor P40. Eur J Biochem. 1989 Aug 15;183(3):715–722. doi: 10.1111/j.1432-1033.1989.tb21103.x. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Nice E. C. In situ cyanogen bromide cleavage of N-terminally blocked proteins in a gas-phase sequencer. Biochem Int. 1984 Jun;8(6):787–791. [PubMed] [Google Scholar]
- Sistonen L., Hölttä E., Mäkelä T. P., Keski-Oja J., Alitalo K. The cellular response to induction of the p21 c-Ha-ras oncoprotein includes stimulation of jun gene expression. EMBO J. 1989 Mar;8(3):815–822. doi: 10.1002/j.1460-2075.1989.tb03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Watson T., Kung H. F., Curran T. Microinjection of transforming ras protein induces c-fos expression. Mol Cell Biol. 1987 Jan;7(1):523–527. doi: 10.1128/mcb.7.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano S., Stoeckle M. Y., Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1987 May 8;49(3):321–328. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. L. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989 Nov;9(11):5265–5271. doi: 10.1128/mcb.9.11.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarev S. I., Zinovieva R. D. Squid major lens polypeptides are homologous to glutathione S-transferases subunits. Nature. 1988 Nov 3;336(6194):86–88. doi: 10.1038/336086a0. [DOI] [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., De Jong W. W., Hilderink A., Bloemendal H. The amino-acids sequence of the alphaB2 chain of bovine alpha-crystallin. Eur J Biochem. 1974 Nov 1;49(1):157–168. doi: 10.1111/j.1432-1033.1974.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Perez-Mutul J., Wasylyk B. The c-Ha-ras oncogene and a tumor promoter activate the polyoma virus enhancer. Cell. 1987 Feb 13;48(3):525–534. doi: 10.1016/0092-8674(87)90203-0. [DOI] [PubMed] [Google Scholar]
- Werenskiold A. K., Hoffmann S., Klemenz R. Induction of a mitogen-responsive gene after expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Mol Cell Biol. 1989 Nov;9(11):5207–5214. doi: 10.1128/mcb.9.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G. Domain structure and evolution in alpha-crystallins and small heat-shock proteins. FEBS Lett. 1985 Feb 11;181(1):1–6. doi: 10.1016/0014-5793(85)81102-9. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hendriks W., Mulders J. W., Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989 Sep;14(9):365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]