Abstract
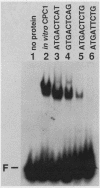
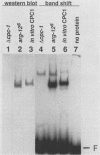
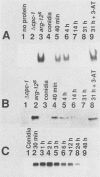
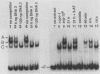
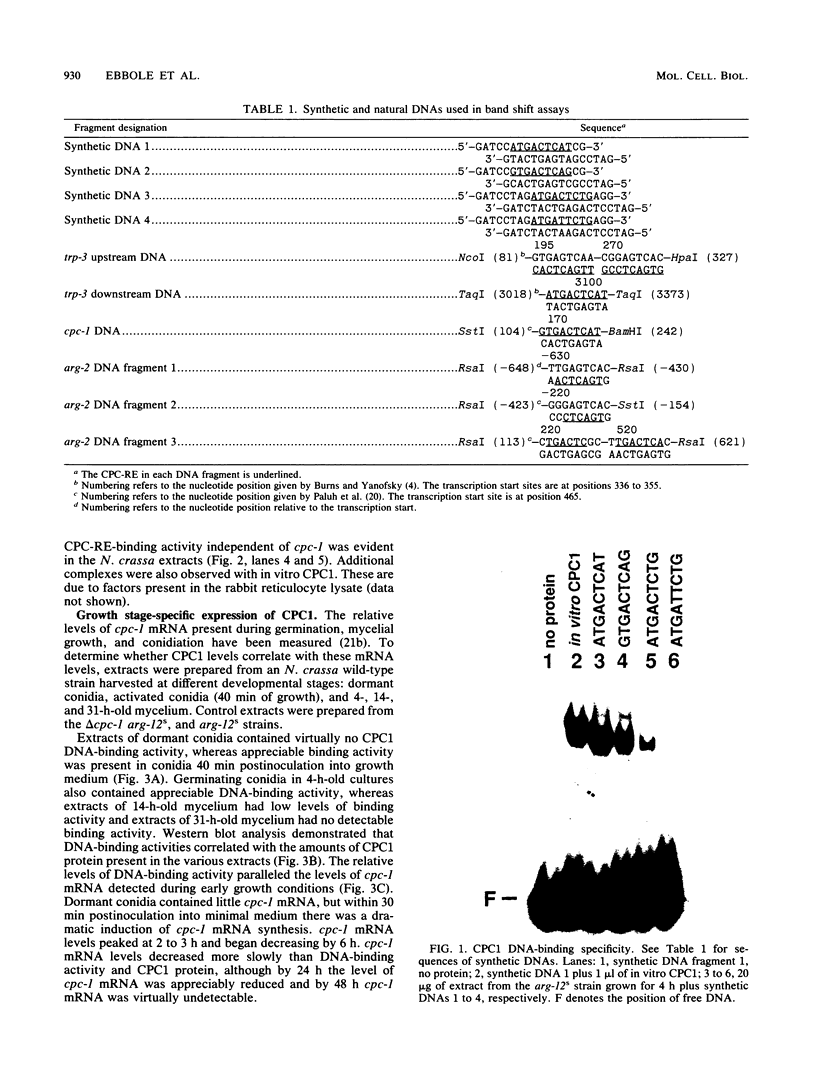
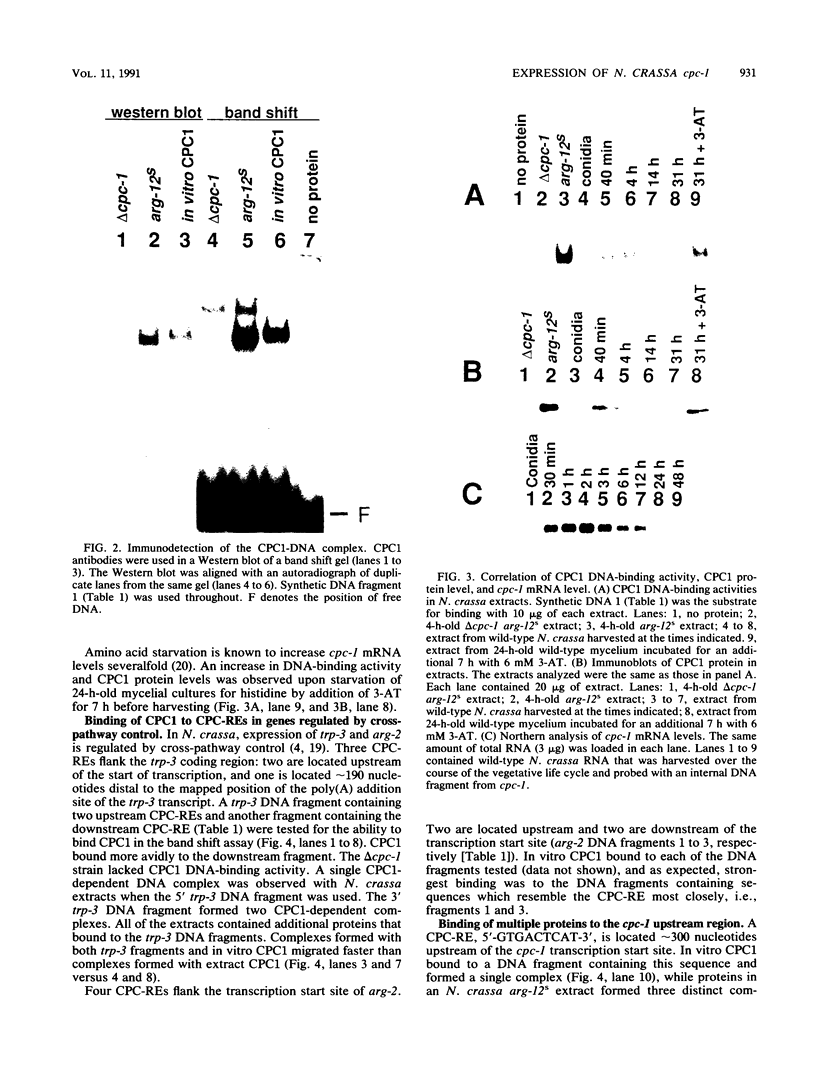
CPCI, the principal regulatory protein required for cross-pathway control of amino acid biosynthetic genes in Neurospora crassa, contains a domain similar to the DNA-binding domain of GCN4, the corresponding general regulator in Saccharomyces cerevisiae. We examined binding by CPC1 synthesized in vitro and by CPC1 present in N. crassa whole-cell extracts. CPCI from both sources was shown to bind to the DNA sequence 5'-ATGACTCAT-3', which is also the preferred recognition sequence of GCN4, CPC1 was confirmed as the source of DNA-binding activity in extracts by immunoblotting. Slightly mobility differences between DNA complexes containing CPCI synthesized in vitro and CPC1 in mycelial extracts were observed. Analyses of N. crassa extracts from different stages of asexual development revealed that CPC1 was abundant immediately following spore germination and through early mycelial growth but was scarce subsequently. CPC1 levels could be increased at any time by imposing amino acid starvation. Copies of the CPC1 response element are located upstream of several genes regulated by cross-pathway control, including cpc-1 itself.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt K., Fink G. R. GCN4 protein, a positive transcription factor in yeast, binds general control promoters at all 5' TGACTC 3' sequences. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8516–8520. doi: 10.1073/pnas.83.22.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelmess I. B. Mutants affecting amino acid cross-pathway control in Neurospora crassa. Genet Res. 1982 Apr;39(2):169–185. doi: 10.1017/s0016672300020863. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Yanofsky C. Nucleotide sequence of the Neurospora crassa trp-3 gene encoding tryptophan synthetase and comparison of the trp-3 polypeptide with its homologs in Saccharomyces cerevisiae and Escherichia coli. J Biol Chem. 1989 Mar 5;264(7):3840–3848. [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Ebbole D. J., Zalkin H. Interaction of a putative repressor protein with an extended control region of the Bacillus subtilis pur operon. J Biol Chem. 1989 Feb 25;264(6):3553–3561. [PubMed] [Google Scholar]
- Flint H. J. Changes in gene expression elicited by amino acid limitation in Neurospora crassa strains having normal or mutant cross-pathway amino acid control. Mol Gen Genet. 1985;200(2):283–290. doi: 10.1007/BF00425437. [DOI] [PubMed] [Google Scholar]
- Hendrickson W., Schleif R. A dimer of AraC protein contacts three adjacent major groove regions of the araI DNA site. Proc Natl Acad Sci U S A. 1985 May;82(10):3129–3133. doi: 10.1073/pnas.82.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988 Jun;52(2):248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer K. P., Hammes G. G. Cloning and expression of the yeast plasma membrane ATPase in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):14389–14395. [PubMed] [Google Scholar]
- Hope I. A., Struhl K. GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell. 1985 Nov;43(1):177–188. doi: 10.1016/0092-8674(85)90022-4. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Moye-Rowley W. S., Harshman K. D., Parker C. S. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989 Mar;3(3):283–292. doi: 10.1101/gad.3.3.283. [DOI] [PubMed] [Google Scholar]
- Oliphant A. R., Brandl C. J., Struhl K. Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random-sequence oligonucleotides: analysis of yeast GCN4 protein. Mol Cell Biol. 1989 Jul;9(7):2944–2949. doi: 10.1128/mcb.9.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Orbach M. J., Sachs M. S., Yanofsky C. The Neurospora crassa arg-2 locus. Structure and expression of the gene encoding the small subunit of arginine-specific carbamoyl phosphate synthetase. J Biol Chem. 1990 Jul 5;265(19):10981–10987. [PubMed] [Google Scholar]
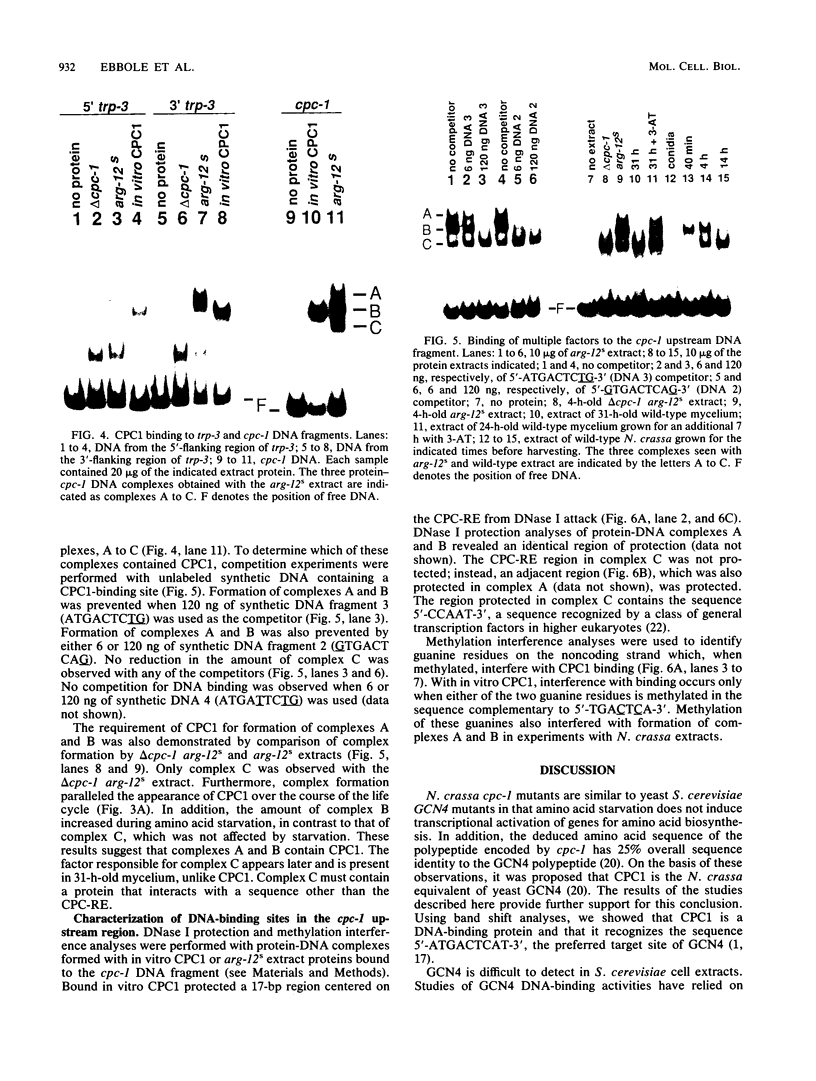
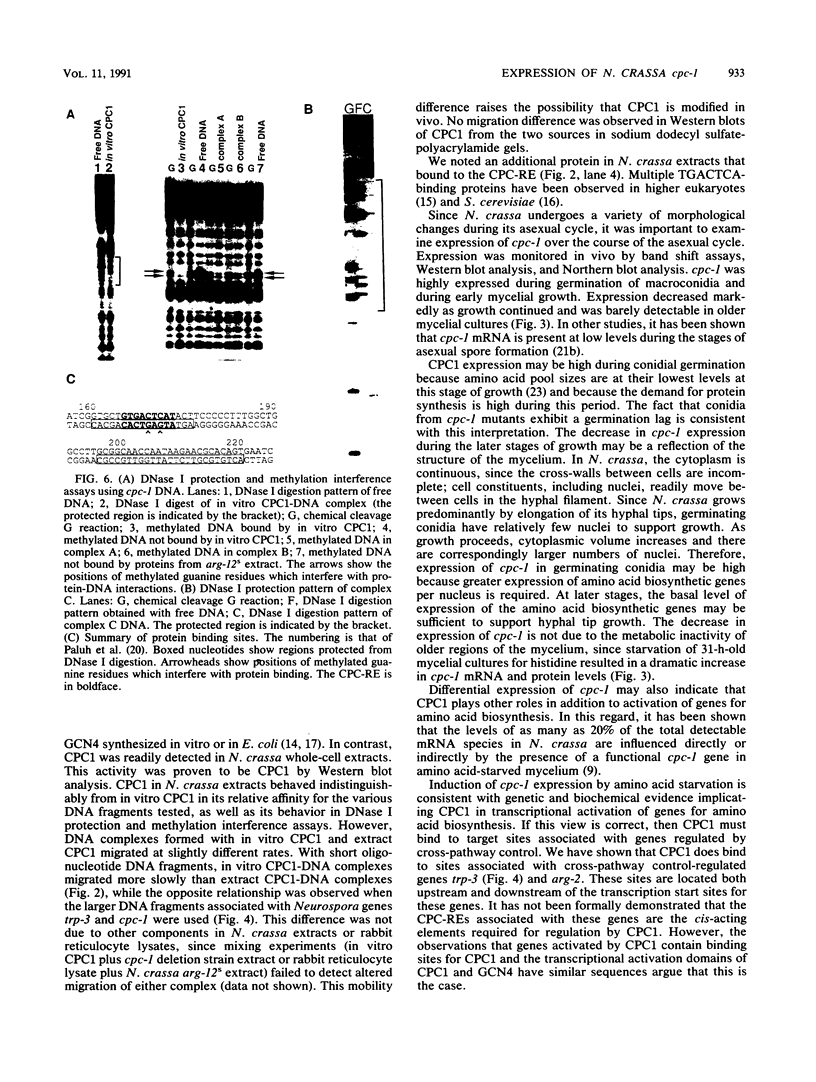
- Paluh J. L., Orbach M. J., Legerton T. L., Yanofsky C. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3728–3732. doi: 10.1073/pnas.85.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J. L., Plamann M., Krüger D., Barthelmess I. B., Yanofsky C., Perkins D. D. Determination of the inactivating alterations in two mutant alleles of the Neurospora crassa cross-pathway control gene cpc-1. Genetics. 1990 Mar;124(3):599–606. doi: 10.1093/genetics/124.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev. 1976 Mar;40(1):1–41. doi: 10.1128/br.40.1.1-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling E. Autoregulation--a common property of eukaryotic transcription factors? Trends Genet. 1989 May;5(5):131–133. doi: 10.1016/0168-9525(89)90049-8. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]