Abstract
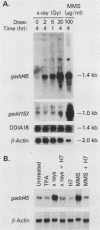
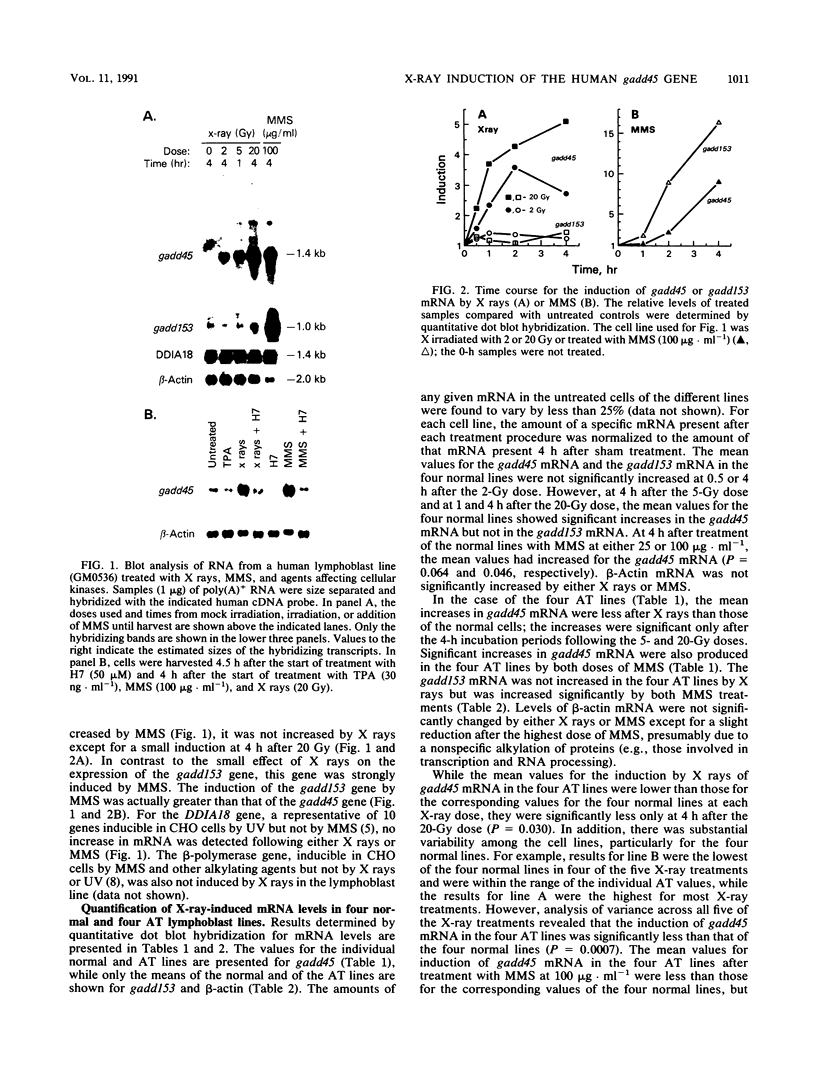
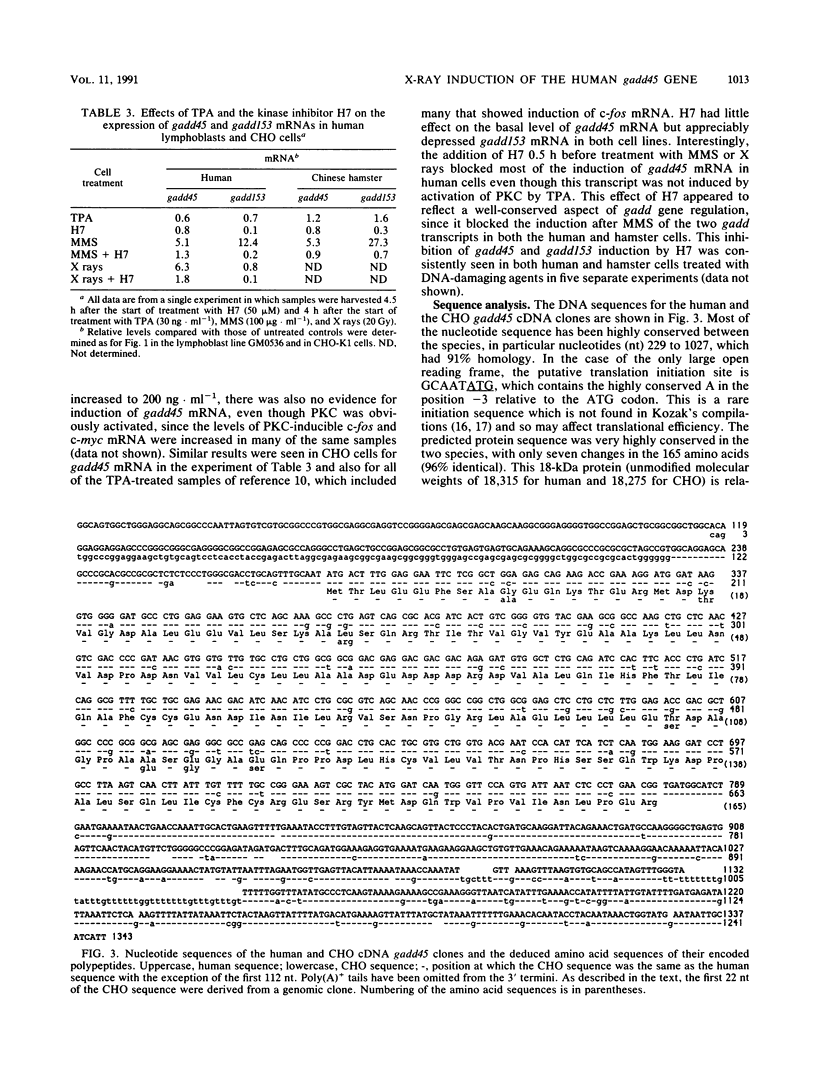
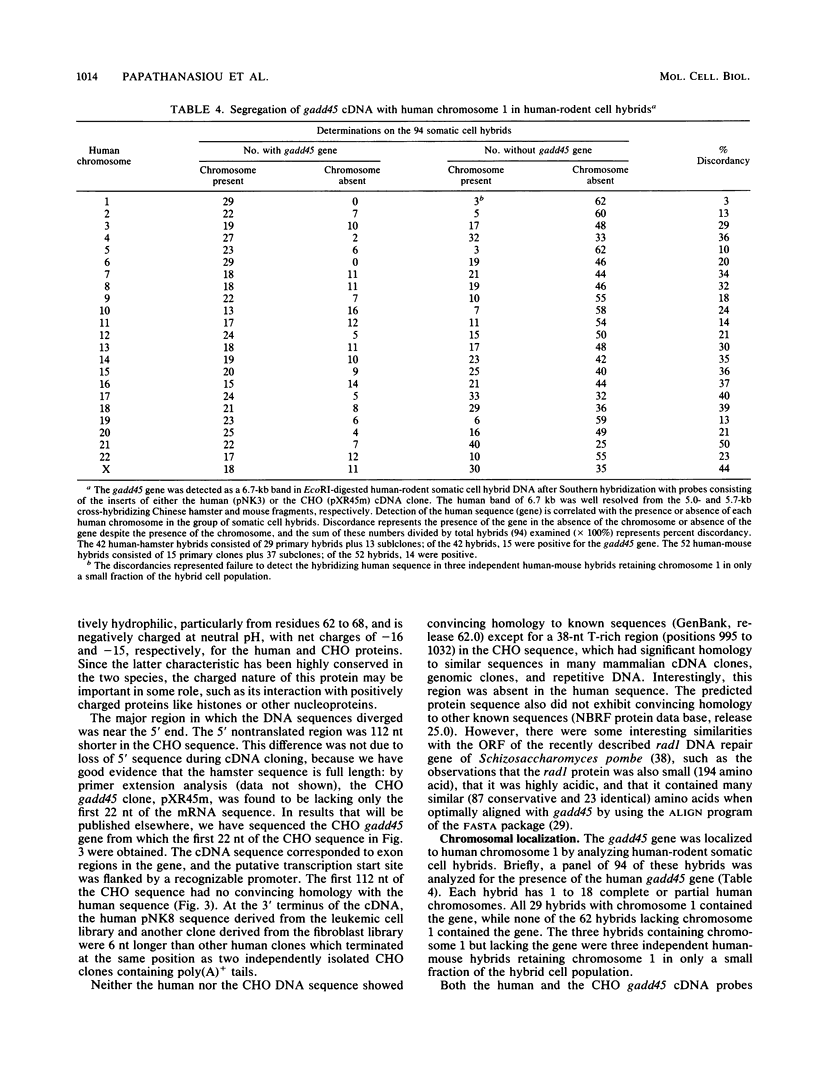
The effect of ionizing radiation on the expression of two DNA-damage-inducible genes, designated gadd45 and gadd153, was examined in cultured human cells. These genes have previously been shown to be strongly and coordinately induced by UV radiation and alkylating agents in human and hamster cells. We found that the gadd45 but not the gadd153 gene is strongly induced by X rays in human cells. The level of gadd45 mRNA increased rapidly after X rays at doses as low as 2 Gy. After 20 Gy of X rays, gadd45 induction, as measured by increased amounts of mRNA, was similar to that produced by the most effective dose of the alkylating agent methyl methanesulfonate. No induction was seen after treatment of either human or hamster cells with 12-O-tetradecanoylphorbol-13-acetate, a known activator of protein kinase C (PKC). Therefore, gadd45 represents the only known mammalian X-ray-responsive gene whose induction is not mediated by PKC. However, induction was blocked by the protein kinase inhibitor H7, indicating that induction is mediated by some other kinase(s). Sequence analysis of human and hamster cDNA clones demonstrated that this gene has been highly conserved and encodes a novel 165-amino-acid polypeptide which is 96% identical in the two species. This gene was localized to the short arm of human chromosome 1 between p12 and p34. When induction in lymphoblast lines from four normal individuals was compared with that in lines from four patients with ataxia telangiectasia, induction by X rays of gadd45 mRNA was less in the cell lines from this cancer-prone radiosensitive disorder. Our results provide evidence for the existence of an X-ray stress response in human cells which is independent of PKC and which is abnormal in taxia telangiectasia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Boothman D. A., Bouvard I., Hughes E. N. Identification and characterization of X-ray-induced proteins in human cells. Cancer Res. 1989 Jun 1;49(11):2871–2878. [PubMed] [Google Scholar]
- Economou J. S., Rhoades K., Essner R., McBride W. H., Gasson J. C., Morton D. L. Genetic analysis of the human tumor necrosis factor alpha/cachectin promoter region in a macrophage cell line. J Exp Med. 1989 Jul 1;170(1):321–326. doi: 10.1084/jem.170.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C., Lamoreaux E. Induction of heat shock protein transcripts and B2 transcripts by various stresses in Chinese hamster cells. Exp Cell Res. 1989 May;182(1):61–74. doi: 10.1016/0014-4827(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr Measurement of M. luteus endonuclease-sensitive lesions by alkaline elution. Mutat Res. 1982 Jun;94(2):263–276. doi: 10.1016/0027-5107(82)90290-1. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan D. E., Spriggs D. R., Beckett M. A., Kufe D. W., Weichselbaum R. R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M. C., Fornace A. J., Jr Estimation of relative mRNA content by filter hybridization to a polythymidylate probe. Biotechniques. 1990 Aug;9(2):174–179. [PubMed] [Google Scholar]
- Hollander M. C., Fornace A. J., Jr Induction of fos RNA by DNA-damaging agents. Cancer Res. 1989 Apr 1;49(7):1687–1692. [PubMed] [Google Scholar]
- Ikushima T. Chromosomal responses to ionizing radiation reminiscent of an adaptive response in cultured Chinese hamster cells. Mutat Res. 1987 Oct;180(2):215–221. doi: 10.1016/0027-5107(87)90217-x. [DOI] [PubMed] [Google Scholar]
- Kinsella T. J., Dobson P. P., Mitchell J. B., Fornace A. J., Jr Enhancement of X ray induced DNA damage by pre-treatment with halogenated pyrimidine analogs. Int J Radiat Oncol Biol Phys. 1987 May;13(5):733–739. doi: 10.1016/0360-3016(87)90292-6. [DOI] [PubMed] [Google Scholar]
- Koval T. M. Enhanced recovery from ionizing radiation damage in a lepidopteran insect cell line. Radiat Res. 1988 Sep;115(3):413–420. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Goldthwait D. A., Samols D. Induction of transcription from the long terminal repeat of Moloney murine sarcoma provirus by UV-irradiation, x-irradiation, and phorbol ester. Proc Natl Acad Sci U S A. 1990 Jan;87(1):36–40. doi: 10.1073/pnas.87.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethy J. D., Fargnoli J., Park J. S., Fornace A. J., Jr, Holbrook N. J. Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J Biol Chem. 1990 Sep 25;265(27):16521–16526. [PubMed] [Google Scholar]
- McBride O. W., Battey J., Hollis G. F., Swan D. C., Siebenlist U., Leder P. Localization of human variable and constant region immunoglobulin heavy chain genes on subtelomeric band q32 of chromosome 14. Nucleic Acids Res. 1982 Dec 20;10(24):8155–8170. doi: 10.1093/nar/10.24.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Hieter P. A., Hollis G. F., Swan D., Otey M. C., Leder P. Chromosomal location of human kappa and lambda immunoglobulin light chain constant region genes. J Exp Med. 1982 May 1;155(5):1480–1490. doi: 10.1084/jem.155.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Swan D. C., Tronick S. R., Gol R., Klimanis D., Moore D. E., Aaronson S. A. Regional chromosomal localization of N-ras, K-ras-1, K-ras-2 and myb oncogenes in human cells. Nucleic Acids Res. 1983 Dec 10;11(23):8221–8236. doi: 10.1093/nar/11.23.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshell A. N., Tarone R. E., Newfield S. A., Andrews A. D., Robbins J. H. A simple and rapid method for evaluating the survival of xeroderma pigmentosum lymphoid lines after irradiation with ultraviolet light. In Vitro. 1981 Apr;17(4):299–307. doi: 10.1007/BF02618141. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri G., Bodycote J., Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984 Feb 10;223(4636):594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- Olson S., Song B. J., Huh T. L., Chi Y. T., Veech R. L., McBride O. W. Three genes for enzymes of the pyruvate dehydrogenase complex map to human chromosomes 3, 7, and X. Am J Hum Genet. 1990 Feb;46(2):340–349. [PMC free article] [PubMed] [Google Scholar]
- Otsuka F., Tarone R. E., Seguin L. R., Robbins J. H. Hypersensitivity to ionizing radiation in cultured cells from Down syndrome patients. J Neurol Sci. 1985 May-Jun;69(1-2):103–112. doi: 10.1016/0022-510x(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7315–7317. doi: 10.1073/pnas.77.12.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuse S., Maenhaut C., Dumont J. E. Regulation of protooncogenes c-fos and c-myc expressions by protein tyrosine kinase, protein kinase C, and cyclic AMP mitogenic pathways in dog primary thyrocytes: a positive and negative control by cyclic AMP on c-myc expression. Exp Cell Res. 1990 Jul;189(1):33–40. doi: 10.1016/0014-4827(90)90253-7. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Otsuka F., Tarone R. E., Polinsky R. J., Brumback R. A., Nee L. E. Parkinson's disease and Alzheimer's disease: hypersensitivity to X rays in cultured cell lines. J Neurol Neurosurg Psychiatry. 1985 Sep;48(9):916–923. doi: 10.1136/jnnp.48.9.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M., Plant J. E., Sturrock J. E., Crathorn A. R. Quantitative aspects of the repair of alkylated DNA in cultured mammalian cells. I. The effect on HeLa and Chinese hamster cell survival of alkylation of cellular macromolecules. Chem Biol Interact. 1971 Feb;3(1):29–47. doi: 10.1016/0009-2797(71)90024-x. [DOI] [PubMed] [Google Scholar]
- Sariban E., Imamura K., Luebbers R., Kufe D. Transcriptional and posttranscriptional regulation of tumor necrosis factor gene expression in human monocytes. J Clin Invest. 1988 May;81(5):1506–1510. doi: 10.1172/JCI113482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta D. N., Zmudzka B. Z., Kumar P., Cobianchi F., Skowronski J., Wilson S. H. Sequence of human DNA polymerase beta mRNA obtained through cDNA cloning. Biochem Biophys Res Commun. 1986 Apr 14;136(1):341–347. doi: 10.1016/0006-291x(86)90916-2. [DOI] [PubMed] [Google Scholar]
- Sherman M. L., Datta R., Hallahan D. E., Weichselbaum R. R., Kufe D. W. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeland E. B., Blomhoff H. K., Funderud S., Shalaby M. R., Espevik T. Interleukin 4 induces selective production of interleukin 6 from normal human B lymphocytes. J Exp Med. 1989 Oct 1;170(4):1463–1468. doi: 10.1084/jem.170.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. F., Willis A. E. Elevation of c-myc protein by DNA strand breakage. Oncogene. 1989 Dec;4(12):1497–1502. [PubMed] [Google Scholar]
- Sunnerhagen P., Seaton B. L., Nasim A., Subramani S. Cloning and analysis of a gene involved in DNA repair and recombination, the rad1 gene of Schizosaccharomyces pombe. Mol Cell Biol. 1990 Jul;10(7):3750–3760. doi: 10.1128/mcb.10.7.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]