Background: PRIP inhibits exocytosis, but the underlying mechanism is unknown.
Results: PRIP interacts with syntaxin-1 and SNAP-25 through its C2 domain and inhibits SNARE complex formation.
Conclusion: Inhibition of exocytosis by PRIP is attributed to the direct binding to SNAREs and the inhibition of SNARE complex formation.
Significance: PRIP is a new member of SNARE-binding proteins bearing C2 domain that are involved in regulating exocytosis.
Keywords: Calcium, Exocytosis, Phosphoinositides, Phospholipase C, Snare Proteins, Synaptotagmin, C2 Domain, SNAP-25, SNARE Complex, Syntaxin
Abstract
Membrane fusion for exocytosis is mediated by SNAREs, forming trans-ternary complexes to bridge vesicle and target membranes. There is an array of accessory proteins that directly interact with and regulate SNARE proteins. PRIP (phospholipase C-related but catalytically inactive protein) is likely one of these proteins; PRIP, consisting of multiple functional modules including pleckstrin homology and C2 domains, inhibited exocytosis, probably via the binding to membrane phosphoinositides through the pleckstrin homology domain. However, the roles of the C2 domain have not yet been investigated. In this study, we found that the C2 domain of PRIP directly interacts with syntaxin 1 and SNAP-25 but not with VAMP2. The C2 domain promoted PRIP to co-localize with syntaxin 1 and SNAP-25 in PC12 cells. The binding profile of the C2 domain to SNAP-25 was comparable with that of synaptotagmin I, and PRIP inhibited synaptotagmin I in binding to SNAP-25 and syntaxin 1. It was also shown that the C2 domain was required for PRIP to suppress SDS-resistant ternary SNARE complex formation and inhibit high K+-induced noradrenalin release from PC12 cells. These results suggest that PRIP inhibits regulated exocytosis through the interaction of its C2 domain with syntaxin 1 and SNAP-25, potentially competing with other SNARE-binding, C2 domain-containing accessory proteins such as synaptotagmin I and by directly inhibiting trans-SNARE complex formation.
Introduction
Exocytosis is one of the fundamental cellular events by which cells secrete neurotransmitters, neuropeptides, and peptide hormones and also distribute membrane proteins such as receptors, channels, and transporters to the cell surface. The final step of exocytosis, membrane fusion, is mediated by heterotrimeric complexes of SNARE proteins (1–3) consisting of members of the vesicle-associated membrane protein (VAMP, also called synaptobrevin)5 family on the vesicular membrane (v-SNARE) and syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) families on the target plasma membrane (t-SNARE). α-Helical SNARE motifs from VAMP and syntaxin and two from SNAP-25 forming parallel coiled-coil bundles are believed to promote fusion of vesicular and target membranes (4, 5). A number of accessory proteins regulating SNARE-mediated membrane fusion have been shown to interact directly with individual SNARE proteins and/or with assembled SNARE protein complexes (6).
PRIP (phospholipase C-related but catalytically inactive protein), consisting of type 1 and type 2, was originally isolated as a novel d-myo-inositol 1,4,5-trisphosphate-binding protein in our laboratory. It was named for its lack of catalytic activity despite structural similarity to phospholipase C (PLC)-δ1 (7, 8), comprising a pleckstrin homology (PH) domain, EF-hand motifs, X and Y motifs, and a C2 domain. In addition to d-myo-inositol 1,4,5-trisphosphate and phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) binding to the PH domain (9, 10), a number of interacting partners have been identified for PRIP including GABARAP (GABAA receptor-associated protein) (11, 12), β subunit of GABAA receptor (13, 14), the catalytic subunit of protein phosphatase 1α and 2A (13, 15, 16), and the phosphorylated (active) form of Akt (17). To explore the biological functions of PRIP in relation to these interacting proteins, we generated PRIP-1 and/or PRIP-2 KO mice whose phenotypes were partly reported (11, 13, 14, 17) and also found that the mice exhibited increased exocytosis of various peptide hormones such as gonadotropins and insulin (18, 19), suggesting that PRIP exerted inhibitory effects on exocytosis. Therefore, female KO mice exhibited impaired reproduction, probably attributed to the hypersecretion of gonadotropins.6 We subsequently investigated the molecular mechanism by which PRIP inhibited dense core vesicle exocytosis. PtdIns(4,5)P2 is required for vesicle exocytosis (20–22), and we found that PtdIns(4,5)P2 binding to its PH domain was required for PRIP to suppress exocytosis. By binding to PtdIns(4,5)P2, PRIP localizes to sites of exocytosis and competes with other molecules such as CAPS (Ca2+-activated protein for secretion) for PtdIns(4,5)P2 binding required for exocytosis.7 In the course of these experiments, however, we noticed that other mechanisms besides PtdIns(4,5)P2 binding of the PH domain are also needed for PRIP to exert full inhibition.
In this study, we investigate the role of the PRIP-C2 domain in the inhibition of exocytosis in light of the many reports that a variety of proteins with C2 domains participate in exocytosis (23, 24). We found that the PRIP-C2 domain showed little interaction with phospholipids but interacted with t-SNARE proteins in a Ca2+-dependent manner. The C2 domain was required for the co-localization of PRIP with t-SNAREs in cells. Moreover, the C2 domain also had direct inhibitory effects on ternary SNARE complex formation and on the participation of synaptotagmin. Thus, we propose that PRIP is a new member of C2 domain-containing proteins that regulate membrane traffic by its negative regulation of exocytosis through a combination of PtdIns(4,5)P2 binding by its PH domain7 and t-SNARE binding by its C2 domain.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
[3H]Noradrenalin (NA) was obtained from GE Healthcare. The Duolink in situ kit for proximity ligation assay (PLA) was from Olink Bioscience (Uppsala, Sweden). The antibodies used were as follows: SNAP-25 (Sigma-Aldrich), VAMP2 (Synaptic Systems, Göttingen, Germany), Munc18 (BD Transduction Laboratories), and syntaxin 1, synaptotagmin I, GFP and GST (Santa Cruz Biotechnology, Santa Cruz, CA). Alexa Fluor 488 anti-rabbit antibody was from Invitrogen. Cy3 anti-mouse antibody, normal rabbit and mouse globulins were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-PRIP-1 mouse monoclonal antibody (2F9) and antigen-purified rabbit polyclonal antibody were prepared in this laboratory as described previously (11, 25).
DNA Constructs
The plasmid to express EGFP-PRIP-1 in mammalian cells and the full-length (PRIP-WT, amino acid residues 1–1096) and deletion mutant (PHXY, amino acid residues 82–704) of His-tagged PRIP-1 for baculovirus expression system were prepared as described previously (10). EGFP-PRIP-1 lacking the C2 domain (PRIPΔC2) was prepared as follows. Both the 5′- and 3′-end regions corresponding to outside the C2 domain of PRIP-1 were amplified by PCR, and the HindIII/SalI fragment of the 5′-end region was first subcloned into HindIII/SalI-digested vector, pEGFP-C3 (Clontech), followed by subcloning the XhoI/SalI fragment of 3′-end region into SalI site of the plasmid prepared as above. The resulting construct was used to express PRIP-1 lacking the residues 714–850 (PRIPΔC2). PRIP, whose Arg at position 134 was replaced with Gln to produce the mutants of R134Q and R134Q/ΔC2 for diminishing PtdIns(4,5)P2 binding, was prepared from the templates, EGFP-PRIP-WT and EGFP-PRIPΔC2, respectively, using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) as described previously (26). Domain organization of PRIP-1 and the related proteins used in this study are depicted in Fig. 1.
FIGURE 1.
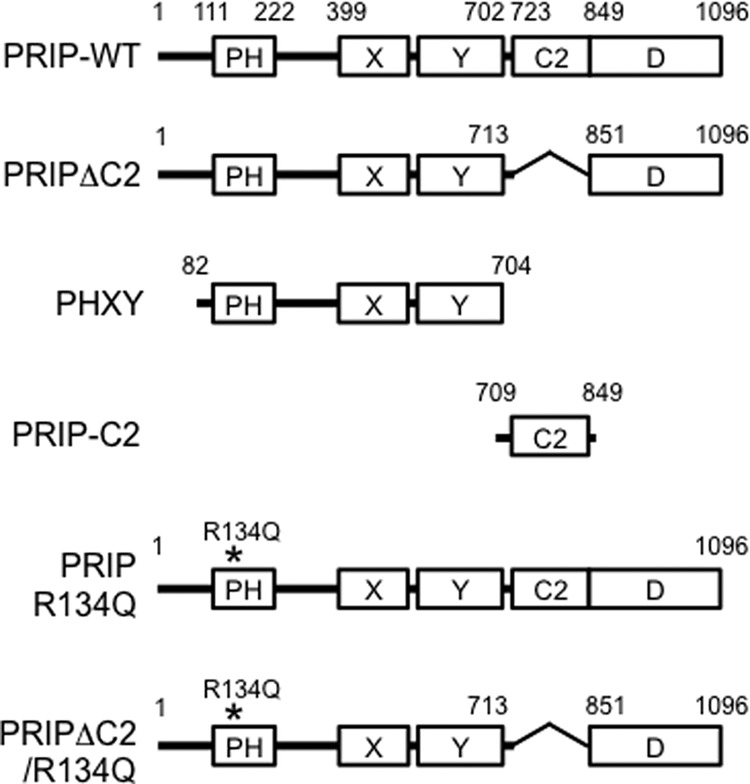
Schematic representation of PRIP-1 used in the study. Domain organization of PRIP-1 and the related proteins used in this study is depicted. EGFP-tagged PRIP-WT, PRIPΔC2, PRIP R134Q, and PRIPΔC2/R134Q were expressed in PC12 cells, whereas His-tagged PRIP-WT, PHXY, PRIP-C2, and GST-tagged PRIP-C2 were expressed in baculovirus or bacterial expression systems and purified for in vitro assays.
Plasmids to express recombinant C2 domain proteins in the bacterial expression system were prepared by subcloning cDNA amplified by PCR from reverse transcripts of rat brain total RNAs into BamHI/SalI site of pGEX (GE Healthcare) or pET-His30 (11) vectors. The primers to amplify each cDNA were as follows: the C2 domains of PRIP-1 (PRIP-C2, amino acid residues 709–849), 5′-TAGGATCCATGGCAAACACAAAGG-3′ and 5′-GGGTCGACGGTTATTGCTATG-3′, PLC-δ1 (PLCδ-C2, amino acid residues 630–755), 5′-GTGGATCCAGGCTCCGTGTCC-3′ and 5′-CGGTCGACGTCCTGGATGGAGATC-3′, rabphilin-3A (Rph-C2B, amino acid residues 529–685), 5′-TAGAATTCCATGGAGCAGGTGGAGCGGATC-3′ and 5′-GCGTCGACCTAGTCGCTCGACACC-3′, synaptotagmin I (Syt-C2A, amino acid residues 142–263), 5′-GCGGATCCCTGGGAAAGCTCCAATATTC-3′ and 5′-GCGTCGACCTGGAGATCACGCCAC-3′ (Syt-C2B, amino acid residues 272–408), 5′-GCGGATCCCTGGGTGACATCTGCTTCTC-3′ and 5′-GCGTCGACCTGCAGAGTGTGCCACTG-3′, (Syt-C2AB, amino acid residues 142–408), 5′-GCGGATCCCTGGGAAAGCTCCAATATTC-3′ and 5′-GCGTCGACCTGCAGAGTGTGCCACTG-3′. The GenBankTM accession numbers of parental proteins are as follows: rat PRIP-1, NP445908; rat PLC-δ1, NP058731; rat synaptotagmin I, NP001028852; and rat rabphilin 3A, NP598202.
The plasmid to express SNARE proteins including the syntaxin 1 (Stx) lacking the C-terminal transmembrane region (StxΔC) has been described elsewhere (27). All of the constructs were fully sequenced to confirm their integrity at the Research Support Center of the Graduate School of Medical Sciences at Kyushu University.
Expression and Purification of Recombinant Proteins
Recombinant full-length SNARE proteins with transmembrane region were prepared by bacterial expression system as described previously in the presence of β-octylglucoside (28). Other recombinant proteins were prepared as described elsewhere by bacterial (16, 29) or baculovirus expression system (10).
Lipid-Protein Overlay Assay
1 nmol of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, 1,2-dipalmitoyl-sn-glycero-3-phosphoserine, and phosphatidylinositol 4,5-bisphosphate were blotted on nitrocellulose membranes. The membranes were air-dried overnight at 4 °C and then were immersed in blocking buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.02% Tween 20, and 3% bovine serum albumin) to reduce background, followed by an incubation with purified GST or GST-fused PRIP-C2, PLCδ-PH, or Syt (synaptotagmin)-C2A at 10 μg/ml in blocking buffer containing free Ca2+ of 10 μm. After extensive washing with the buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.02% Tween 20, and 10 μm CaCl2), the membranes were immunoblotted for bound GST-C2 or PH proteins using anti-GST antibody.
t-SNARE Liposome Binding Assay
Preparation of t-SNARE-incorporated liposomes and the floatation assays were performed as described previously (28), except that GST alone or GST-fused C2 domain was used instead of CAPS. All of the phospholipids used in this study were purchased from Avanti Polar Lipids (Alabaster, AL).
Immunoprecipitation and Western Blotting
The cerebrum of wild type or PRIP-KO mouse was homogenized in the lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, and 1 mm dithiothreitol) containing protease inhibitors (5 μg/ml pepstatin A, 10 μm leupeptin, 1.7 μg/ml aprotinin, and 50 μm 4-amidinophenylmethanesulfonyl fluoride hydrochloride). The lysates were cleared by centrifugation and incubated with antibody of interest or control immunoglobulin at 4 °C overnight, followed by incubation with protein G beads at 4 °C for 1 h. Then the beads were washed with the lysis buffer four times, and the proteins bound to the beads were separated by SDS-PAGE, followed by transfer to polyvinylidene fluoride membranes (Merck-Millipore, Billerica, MA). After blocking, the membrane was blotted with the appropriate antibody, and horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (GE Healthcare), and detected for chemiluminescent signals using an LAS-3000 mini gel documentation system (Fujifilm, Tokyo, Japan). Digital images were analyzed with Image Gauge software (Fujifilm) or National Institutes of Health Image J software to measure the density of each band. The handling of mice and all of the procedures were approved by the Animal Care Committee of Kyushu University, which follows the guidelines of the Japanese Council on Animal Care.
Cell Culture, Transfection, and Stable Cell Lines Expressing PRIP
Rat pheochromocytoma (PC12) cells were maintained and used to establish stable cell lines expressing wild type or several mutants of PRIP as described previously (29). GH3 cells were also maintained routinely.
Immunofluorescence and PLA
The cells were plated onto poly-d-lysine-coated glass coverslips at a density of 4 × 105 cells/well in a 12-well plate and subjected to immunofluorescent observation as described elsewhere (30). In some experiments, the cells were transiently transfected with plasmid DNA using Lipofectamine 2000 (Invitrogen) and OPTI-MEM (Invitrogen) according to the manufacturer's protocol, and 24 h after transfection, the cells were plated onto glass coverslips as described above.
In situ PLA was performed using a Duolink in situ kit following to the manufacturer's protocol, but the cells were prepared, permeabilized, and blocked in the same manner as for immunofluorescent studies. Briefly, two molecules present in permeabilized cells were recognized by respective primary antibody raised in mouse or rabbit, respectively, and secondary antibody to mouse or rabbit Ig conjugated with an unique short DNA strand was then added, followed by ligation of these secondary antibodies and amplification of the ligates. When two molecules are close within 40 nm, successful ligation and amplification are performed for further analysis. The confocal images were obtained using LSM510 META (Carl Zeiss) and analyzed using Duolink Image Tool (Olink Bioscience) to obtain objective quantification of PLA signals.
GST Pulldown Assay
PC12 cell lysate was prepared by homogenizing in the lysis buffer (50 mm Hepes-KOH, pH 7.3, 150 mm NaCl, 1 mm MgCl2, 1 mm dithiothreitol, and 1% Triton X-100) containing protease inhibitors. Cell lysate or recombinant SNARE proteins whose GST tags had been removed by thrombin digestion were mixed with GST alone or GST-fused C2 domain at an equal molar ratio in the binding buffer (10 mm Hepes-KOH, pH 7.3, 100 mm KCl, 3.5 mm MgCl2, 1 mm EGTA, 0.1% Nonidet P-40) and incubated at 4 °C for 2 h. Then glutathione-Sepharose 4B (GE Healthcare) equilibrated with appropriate buffer was added to the mixture and incubated at 4 °C for another 1 h. At the end of the incubation, the beads were washed with the same buffer four times, boiled in a sample buffer for 5 min, and then subjected to SDS-PAGE followed by Western blotting. Similar experiments in a reverse mode using GST-fused SNARE protein and His-tagged PRIP protein were performed in the same condition as described above. The internal standard sample with known protein concentration was included in each blot and used to calculate the amount of protein of interest by fitting to the standard curve. To examine the effect of Ca2+ on the binding of the C2 domains to syntaxin 1 (Stx) or SNAP-25, the pulldown assay was performed in the same binding buffer as above for that in the presence of the calculated amount of Ca2+ to give the free Ca2+ concentration of 10 μm or the buffer containing 1 mm EGTA.
In Vitro SDS-resistant SNARE Complex Formation
The mixture of 10 pmol (equivalent to 0.2 μm) each of the purified GST-SNAP-25, StxΔC-His, and His-VAMP2ΔC were incubated overnight in 48 μl of the binding buffer. At the end of incubation, the mixture was either boiled or incubated in 37 °C for 5 min in a SDS sample buffer and immediately subjected to SDS-PAGE followed by immunoblotting with antibodies indicated in the figures. Additional proteins were included in the mixture of SNAREs during the incubation period. To test the effect of Ca2+ on the SNARE complex formation, the mixture was incubated in the binding buffer containing calculated amounts of CaCl2 to give a free Ca2+ concentration of 10 μm with 1 mm EGTA.
Measurement of [3H]Noradrenalin Release
[3H]NA secretion from the stable PC12 cell lines were measured as previously described (29, 30).
Statistical Analysis
All of the statistical analyses were performed by Student's t test, with a two-tailed value of p < 0.05 considered significant using GraphPad Prism (GraphPad Software).
RESULTS
Interaction of PRIP with t-SNARE Proteins through the C2 Domain
We first examined phospholipid binding of the PRIP-C2 domain in a lipid-protein overlay assay (31). The isolated PRIP-C2 domain fused to GST did not show more binding than GST alone to phospholipids tested in the presence of Ca2+ (Fig. 2). By contrast, strong lipid binding as a positive control was observed as follows: PtdIns(4,5)P2 binding by the PH domain of PLC-δ1 and phosphatidylserine binding by the C2A domain of synaptotagmin I (Syt-C2A), which was abolished in the absence of Ca2+ (Fig. 2).
FIGURE 2.
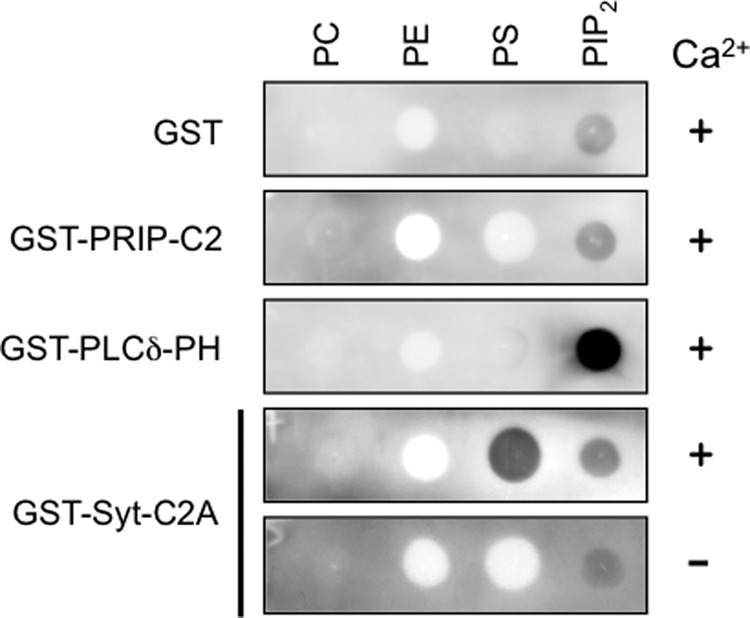
Phospholipid binding of PRIP-C2. Lipid overlay assay was performed using a nitrocellulose membrane on which 1 nmol of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol 4,5-bisphosphate (PIP2) were blotted. Each membrane was probed with GST-fused protein indicated on the left followed by immunodetection with anti-GST antibody. GST-fused PRIP-C2, PLCδ-PH, or Syt-C2A were used. Ca2+ (−) indicates incubation with 1 mm EGTA alone.
Because there are several C2 domain-containing proteins that promote SNARE-mediated membrane fusion by direct SNARE protein interactions (32–36), we examined whether PRIP binds to SNARE proteins via its C2 domain. Anti-PRIP-1 antibody precipitated PRIP-1 along with syntaxin 1 and SNAP-25 from brain lysate of wild type but not from that of PRIP-KO mouse, but no VAMP2 was precipitated (Fig. 3A), indicating that PRIP-1 interacts with syntaxin 1 and SNAP-25 either in a direct or indirect manner. This result was further confirmed, and the region responsible for the binding was identified using the recombinant PRIP-C2 domain protein. The lysate from PC12 cells was applied to GST alone and GST-fused C2 domains of PRIP-1 (PRIP-C2) or GST-C2B of rabphilin 3A (Rph-C2B) as a positive control (known to bind to SNAP-25, Ref. 34) immobilized on glutathione beads, followed by immunoblotting with the indicated antibodies (Fig. 3B). The result clearly showed that both syntaxin 1 and SNAP-25 bound to PRIP-C2, but VAMP2 and Munc18 did not, whereas only SNAP-25 bound to Rph-C2B as previously reported (34, 35). Thus, the C2 domain of PRIP interacts with two t-SNARE proteins, i.e. syntaxin 1 and SNAP-25, but not with the v-SNARE protein, VAMP2. To clarify whether the interactions of PRIP-C2 with syntaxin 1 and SNAP-25 are direct or indirect, because the experiments shown in Fig. 3 (A and B) were done using the lysates from brain or PC12 cells, we next performed GST pulldown assays using recombinant purified protein samples. GST alone or GST-C2 domain proteins immobilized on glutathione beads were incubated with soluble syntaxin 1 lacking the C-terminal transmembrane region (StxΔC) or SNAP-25. Both syntaxin 1 and SNAP-25 bound to PRIP-C2 (Fig. 3C). The binding was comparable with that of the C2B domain of synaptotagmin I (Syt-C2B) as a positive control. The Rph-C2B interacted with SNAP-25, but not with syntaxin 1, agreeing with previous reports (34, 35) and Fig. 3B. The C2 domain of PLC-δ1 (PLCδ-C2), albeit with a high homology to PRIP (8), did not bind to syntaxin 1 or SNAP-25 (Fig. 3C). The GST pulldown assay in a reverse mode was also performed. His-tagged forms of full-length PRIP-1 (PRIP-WT), PRIP-C2, or PHXY (Fig. 1) lacking the C2 domain and the N-terminal extension were assayed using immobilized GST alone, GST-SNAP-25 or GST-StxΔC. PRIP-WT and PRIP-C2, but not PHXY, showed binding to both SNAP-25 and syntaxin 1 (Fig. 3D) confirming that the C2 domain of PRIP directly interacts with t-SNARE proteins.
FIGURE 3.
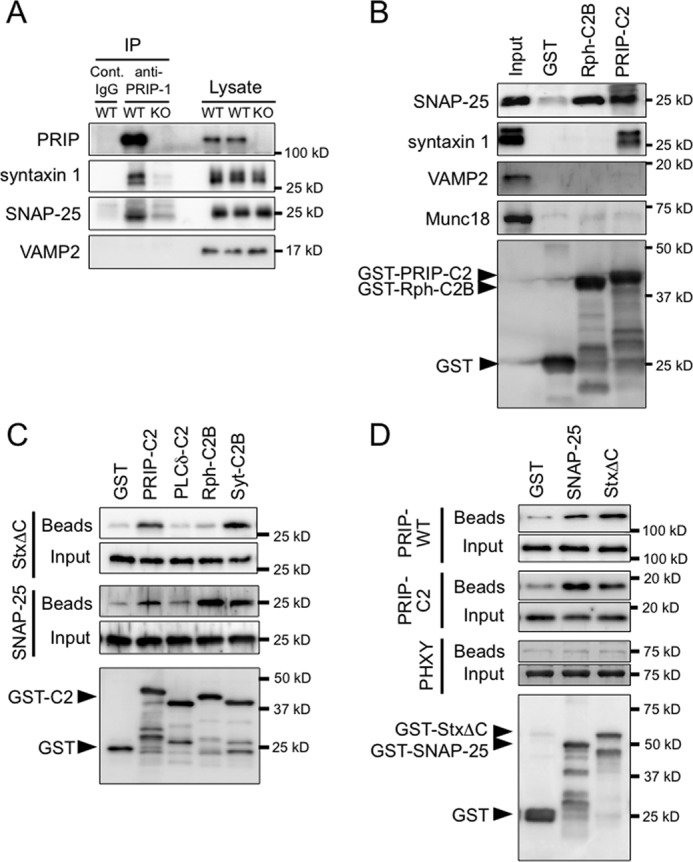
Interaction of PRIP-C2 with SNARE proteins. A, PRIP-1 was immunoprecipitated (IP) using mouse anti-PRIP-1 monoclonal antibody or normal IgG (Cont. IgG) from brain lysates of PRIP-WT and PRIP-KO mice. The lysates and immunoprecipitates were subjected to Western blotting with antibodies against the proteins indicated on the left. 2 or 20% of the total amount of brain lysates or immunoprecipitates, respectively, was applied to SDS-PAGE. Syntaxin 1 was seen in double bands; the lower band would be a degraded product. B, GST or GST-fused C2 domains (PRIP-C2 and Rph-C2B) immobilized on glutathione beads were mixed with PC12 cell lysate, followed by extensive washing, and the bound proteins were analyzed by Western blotting using the antibodies indicated on the left. The bottom panel indicates the blot of the beads, probed with anti-GST antibody. GST-C2 proteins (PRIP-C2 and Rph-C2B) appear as the top band in each lane based on the expected molecular size as indicated by an arrowhead with some degraded proteins below the band. C, the mixture of 100 pmol each of GST or GST-fused C2 domains (PRIP-C2, PLCδ-C2, Rph-C2B, and Syt-C2B) and SNAP-25 or syntaxin 1ΔC (StxΔC) were incubated at 4 °C for 3 h and then applied to glutathione beads. After another hour incubation, the beads were washed extensively followed by Western blotting. SNAP-25 and StxΔC bound to the beads were probed with anti-SNAP-25 and anti-syntaxin 1 antibodies, respectively. The bottom panel indicates the blot of the beads mixed with SNAP-25, which was probed with anti-GST antibody. GST-C2 protein appears as the top band in each lane with some degraded proteins below the band. The identical result was obtained for the amount of immobilized GST proteins from the beads mixed with StxΔC. A band in the lane of PLCδ-C2 detected by anti-SNAP-25 antibody was also detected in the control beads at a similar density, indicating that nonspecific, background interaction. D, GST pulldown assay was performed in the reverse direction shown in C. GST, GST-SNAP-25, or GST-StxΔC was mixed with purified PRIP-WT, PRIP-C2, or PHXY (PRIP-1 lacking the C2 domain; see Fig. 1), and then bound proteins on the beads were analyzed as in B using anti-PRIP-1 polyclonal antibody, which recognizes all PRIP-1 constructs. Three independent experiments were performed with similar results.
Because the binding of syntaxin 1 and SNAP-25 to PRIP-C2 shown in Fig. 3 was assayed by pulldown method using truncated soluble SNARE proteins, we further confirmed the interaction using full-length membrane-integrated SNARE proteins that might exhibit properties different from truncated soluble SNARE proteins (37, 38). Proteoliposomes incorporating full-length syntaxin 1 and SNAP-25 were prepared and incubated with PRIP-WT, PRIP-C2, or GST alone, followed by a gradient centrifugation to detect protein samples in the liposome fraction. Both PRIP-WT and PRIP-C2, but not GST alone, were detected in the floating liposome fraction in the case of proteoliposomes, but not in the protein-free liposome, indicating that PRIP and PRIP-C2 proteins bound to membrane-associated t-SNARE proteins. (Fig. 4).
FIGURE 4.
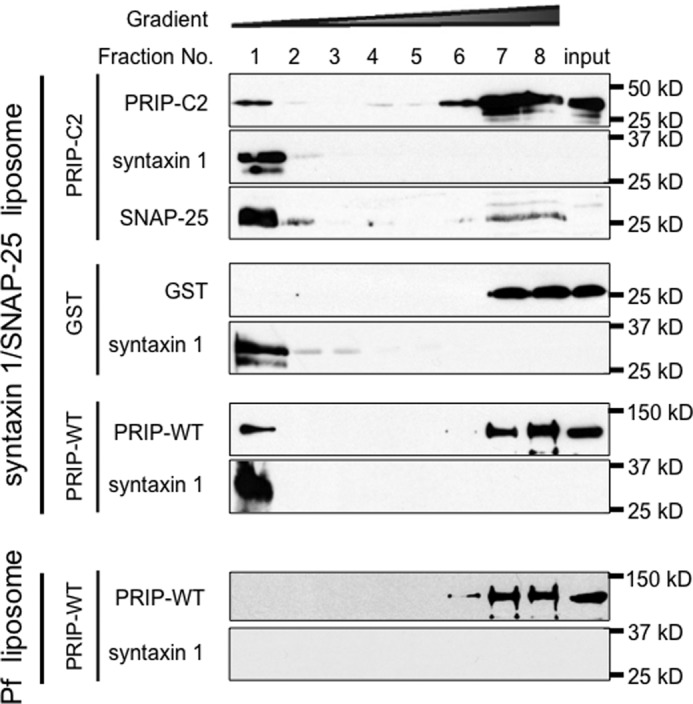
Interaction of PRIP with t-SNAREs incorporated in liposomes. 1 μmol of GST, GST-PRIP-C2 (PRIP-C2), or His-PRIP-WT (PRIP-WT) was incubated with protein-free or t-SNARE containing phosphatidylcholine/phosphatidylserine liposomes, and bound PRIP (fractions 1 and 2) was separated from free PRIP (fractions 6–8) by gradient centrifugation. Fractions were analyzed by immunoblotting for PRIP-1, as well as SNAP-25 and syntaxin 1. Representative results of three experiments are shown.
The C2 domain binding at increasing amounts with t-SNARE proteins were examined by a GST pulldown assay (Fig. 5). The EC50 (effective concentration required for 50% effect) value of 1.1 μm for PRIP-C2 binding to syntaxin 1 was comparable with that of Syt-C2B (2.4 μm), whereas Rph-C2B showed no binding to syntaxin 1 (Fig. 5A). On the other hand, the binding of PRIP-C2 to SNAP-25 (EC50 = 1.9 μm) was lower than that of Rph-C2B (EC50 = 0.4 μm), although it was still comparable with the apparent affinity of Syt-C2B to SNAP-25 (EC50 = 2.1 μm) (Fig. 5B). Molar ratio for the binding was smaller compared with the positive control; to SNAP-25, the Bmax values were 0.18 and 0.18 mol for PRIP-C2 and Syt-C2B, respectively, whereas Bmax = 0.35 mol for Rph-C2B as a positive control. To syntaxin 1, the Bmax value was 0.17 mol for PRIP-C2, whereas it was 0.55 mol for Syt-C2B as a positive control. These results, however, indicate that the binding of PRIP-C2 to both syntaxin 1 and SNAP-25 may be regulatory for SNARE-mediated membrane fusion (27, 34).
FIGURE 5.
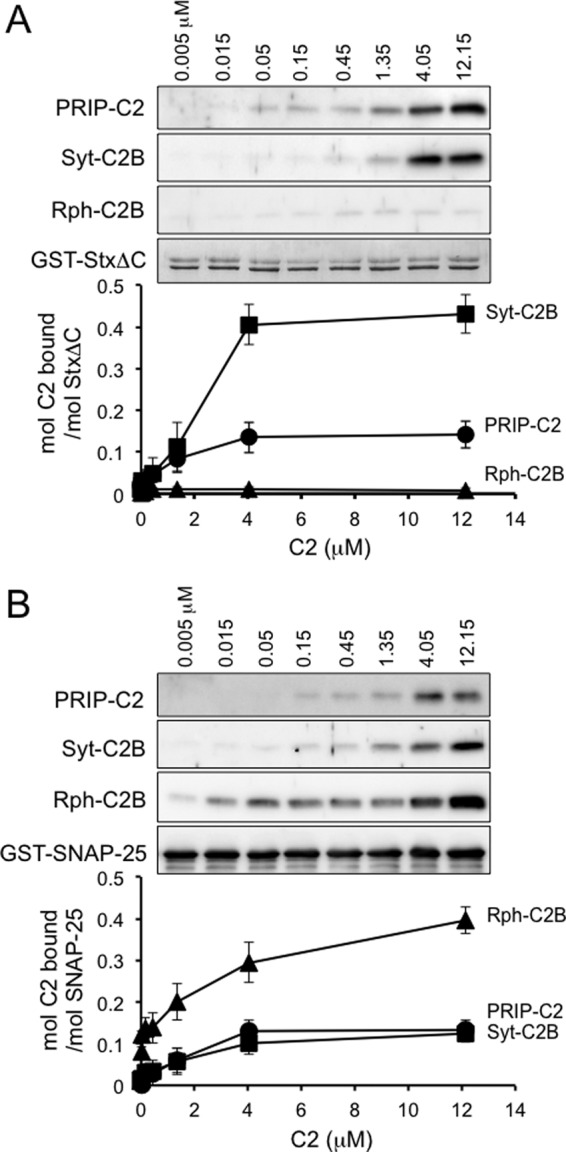
Comparison of binding profile of C2 domains to t-SNARE proteins. Purified C2 domains were incubated with GST-StxΔC (A) or GST-SNAP-25 (B) immobilized on glutathione beads at the indicated concentrations, and the C2 domains bound to the beads were determined by Western blotting. The proteins bound on the beads were quantified as described under “Experimental Procedures” and expressed as molar ratio of the C2 domain of PRIP-1 (PRIP-C2; circle), synaptotagmin I (Syt-C2B; square), or rabphilin 3A (Rph-C2B; triangle) to StxΔC (A) or SNAP-25 (B) bound on the beads. The EC50 and Bmax values described under “Results” were obtained by nonlinear regression curve fits to the data (means ± S.D., n = 3).
Co-localization of PRIP with t-SNARE Proteins in Cells
We next examined the subcellular localization of PRIP with SNAP-25 and syntaxin 1 in rat pheochromocytoma, PC12 cells where we observed PRIP-mediated down-regulation of NA release (29). Because PC12 cells do not express detectable levels of endogenous PRIP (Fig. 6F), we established stable PC12 cell lines expressing wild type or the mutant version of PRIP-1 fused to EGFP (Fig. 6A). The cells were processed for immunofluorescence of EGFP-PRIP-1 and endogenous SNAP-25 or syntaxin 1 with respective antibodies. PRIP-WT appeared abundant because of exogenous expression but localized to both cytosol and plasma membrane, whereas both syntaxin 1 (Fig. 6B) and SNAP-25 (Fig. 6C) were mainly present at the plasma membrane. Both syntaxin 1 and SNAP-25 showed co-localization with PRIP-1 at the plasma membrane as observed in the merged images (Fig. 6, B and C, upper panels). By contrast, PRIP-1 lacking its C2 domain (PRIPΔC2) showed less localization at the plasma membrane with a diffuse distribution in the cytosol, resulting in less co-localization with either syntaxin 1 or SNAP-25 (Fig. 6, B and C, lower panels). These results indicate that PRIP-C2 partly contributes to the co-localization of PRIP with t-SNAREs in the cells.
FIGURE 6.
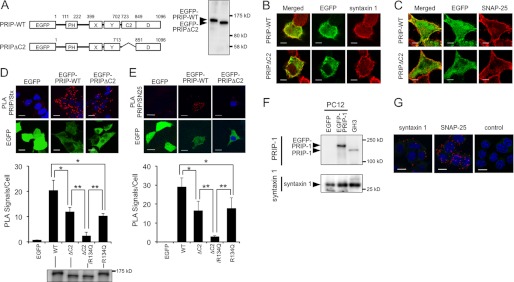
Co-localization of PRIP with t-SNARE proteins in intact cells. A, proper expression with the expected molecular mass of each construct in PC12 cells was confirmed by Western blotting with anti-GFP antibody. B and C, PC12 cells expressing EGFP-PRIP-WT or EGFP-PRIPΔC2 were cultured on coverslips and visualized in green for EGFP and red for intrinsic syntaxin 1 (B) or SNAP-25 (C) by indirect immunofluorescence using a combination of rabbit antibody against GFP and Alexa 488-conjugated rabbit IgG and mouse antibodies against syntaxin 1 or SNAP-25 and Cy3-conjugated mouse IgG, respectively. The yellowish staining in the merged picture indicates co-localization of EGFP-PRIP-WT or EGFP-PRIPΔC2 with the SNARE proteins. Scale bars, 5 μm. D and E, PC12 cells expressing EGFP-fused PRIP-WT or the mutant constructs indicated in the graphs were cultured on coverslips and subjected to PLA assay using the combination of the antibodies against PRIP-1 and syntaxin 1 (Stx) or SNAP-25 (SN25) and visualized in red for PLA signals, green for EGFP signals, and blue for counter stained nucleus. Scale bars, 10 μm. Typical images for EGFP, WT, and ΔC2 constructs of PRIP expressing cells are shown. In each image, the number of PLA signals/cell was counted using Duolink Image Tool with manual corrections and presented as bar graphs. More than 30 cells for each experiment were counted, and the data are the means ± S.D. of three experiments. Significance by Student's t tests is represented by * or ** for p < 0.05 or p < 0.01, respectively. Comparable expression of each construct in PC12 cells was confirmed by Western blotting with anti-GFP antibody in D. F, endogenous expression of PRIP-1 in GH3 cells but not in PC12 cells were confirmed. The same numbers of GH3 cells and PC12 cells expressing EGFP alone or EGFP-PRIP-WT were subjected to SDS-PAGE, followed by Western blotting by anti-PRIP-1 antibody. The presence of endogenous syntaxin 1 in GH3 and PC12 cells was also analyzed with anti-syntaxin 1 antibody. G, GH3 cells were cultured on coverslips and subjected to PLA assay using the combination of the antibodies against PRIP-1 and syntaxin 1, SNAP-25, or mouse control IgG and visualized in red for PLA signals and blue for counter stained nucleus. Scale bars, 10 μm. Typical images among more than four observations were shown. Green are background signals that were visualized by hyper enhancement of the green channel to help cell shape recognition.
We further confirmed the importance of PRIP-C2 in co-localization with t-SNARE proteins by using in situ PLA technology, which visualizes protein-protein interactions quantitatively as fluorescent spots by rolling circle amplification reactions dependent on the close proximity (<40 nm) of the target proteins (39). PC12 cells were probed with a combination of mouse antibody against SNAP-25 or syntaxin 1 and rabbit antibody against PRIP-1 as primary antibodies, followed by further probing with PLA probes for mouse and rabbit primary antibodies, ligation, and amplification reactions. As shown in Fig. 6D, red fluorescent spots indicating co-localization of PRIP and syntaxin 1 were observed in the PC12 cells expressing EGFP-PRIP-WT and, to a smaller extent EGFP-PRIPΔC2, with none in the cells expressing EGFP alone (upper panels). Similar results were obtained for SNAP-25 proximity with PRIP or PRIPΔC2 (Fig. 6E). Consistent with the results in Fig. 6 (B and C), the number of signals per cell were significantly decreased by the lack of the C2 domain of PRIP, suggesting that the C2 domain contributes to the co-localization of PRIP with syntaxin 1 and SNAP-25 (Fig. 6, D and E, bar graphs). Because PC12 cells expressing similar amounts of exogenous PRIP-WT or the related proteins were used for PLA experiments (see the blot in Fig. 6D), the signals were comparable. However, the results using PC12 cells were observed by exogenous expression. Thus, GH3 endocrine cells that express endogenous PRIP were analyzed by PLA (Fig. 6F); positive signals indicating proximal presence of endogenous PRIP with SNAP-25 and syntaxin 1 were observed (Fig. 6G).
We found that PtdIns(4,5)P2 binding to the PH domain is required for PRIP to inhibit exocytosis.7 Therefore, further experiments using the mutation (R134Q) in the PH domain, which lacks binding to PtdIns(4,5)P2, were performed. Double mutation of ΔC2 and R134Q showed an almost complete loss of the co-localization of PRIP with t-SNARE proteins in PC12 cells (Fig. 6, D and E, graphs).
PRIP Effect on the Binding of Synaptotagmin I to t-SNARE Proteins
Binding parameters of the C2 domain of PRIP to t-SNAREs were comparable with those of synaptotagmin I, suggesting the possibility that PRIP competes with synaptotagmin for binding to t-SNARE proteins. To test this possibility, brain lysates prepared from WT and PRIP-KO mice were immunoprecipitated by anti-SNAP-25 antibody, followed by immunoblotting with antibodies of interest (Fig. 7A). From the band density in the precipitates compared with that in the lysates, we estimated that ∼60% of the SNAP-25 present in the lysates was immunoprecipitated by anti-SNAP-25 antibody, and the value was similar for both WT and PRIP-KO mice. The amount of synaptotagmin I (SytI) precipitated along with SNAP-25 was increased in PRIP-KO mouse by 2-fold (WT, 4.8 ± 0.8%; KO, 10.2 ± 1.9%), whereas the amount of syntaxin 1 (WT, 24.1 ± 2.5%; KO, 24.6 ± 1.8%) was not affected by the absence of PRIP (Fig. 7B). A similar effect of the absence of PRIP in increasing Munc18 in SNAP-25 immunoprecipitates was observed (Fig. 7A). It should also be noted that PRIP (5.1 ± 1.3%) was found in the immunocomplex among syntaxin 1, SytI, and SNAP-25 in WT mice. The results suggest that PRIP inhibits the binding of synaptotagmin I to the t-SNARE complex of syntaxin 1 and SNAP-25, leading to the inhibition of regulated exocytosis. In vitro pulldown assays were also performed first using the isolated C2 domains from PRIP (PRIP-C2) and synaptotagmin I (Syt-C2B); GST-SNAP-25 or GST-StxΔC was incubated with increasing amounts of PRIP-C2 at a fixed amount of Syt-C2B. The results show that the addition of increasing PRIP-C2 caused increased binding of PRIP-C2 along with decreased binding of Syt-C2B, indicating competition (Fig. 7, C and D). PRIP-C2 appeared to bind to StxΔC well, particularly in the presence of Ca2+. Similar results were observed in the assay with t-SNARE complexes of GST-SNAP-25 plus StxΔC (Fig. 7E). When full-length molecules of PRIP and synaptotagmin I (without membrane spanning region) were used in the pulldown assay using GST-SNAP-25 and t-SNARE (GST-SNAP-25 plus StxΔC), similar results indicating competition were observed (data not shown). The figure also includes the results obtained in the presence or absence of Ca2+, which will be described later.
FIGURE 7.
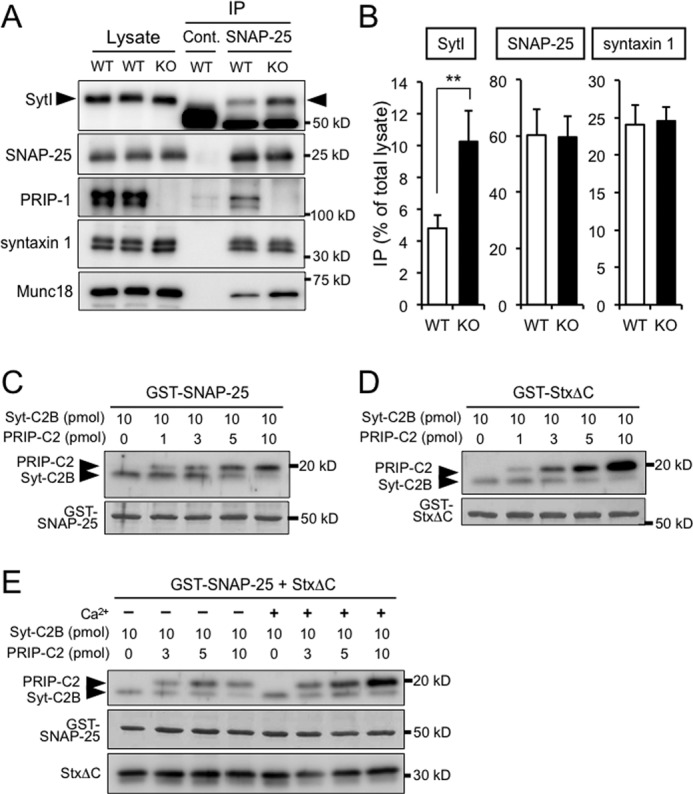
Effect of PRIP on the binding of synaptotagmin I to t-SNARE proteins. A, immunoprecipitates of brain lysates prepared from WT and PRIP-KO mice with anti-SNAP-25 antibody were immunoblotted with antibodies indicated on the left. The amounts of brain lysates and the immunoprecipitates applied to each lane of SDS-PAGE were as follows: SNAP-25 and syntaxin 1, 4 and 10%; synaptotagmin I, PRIP-1, and Munc18, 2 and 20%, respectively. The arrowheads in the top panel indicate the bands of SytI, whereas the bands at 50 kDa are the heavy chain of the IgG used for immunoprecipitation (IP). B, graph was shown as follows; the amount of each protein in the immunocomplex by anti-SNAP-25 antibody was measured from the band intensities and expressed as a percentage of total amount in the lysate. The data are the means ± S.D. of three experiments. Significance by Student's t tests is represented by ** for p < 0.01. C and D, increasing amounts of PRIP-C2 (1–10 pmol) were applied to GST-SNAP-25 (C) or GST-syntaxin 1ΔC (StxΔC) (D) immobilized on glutathione beads (50 pmol each) in the presence of the fixed amount of Syt-C2B (10 pmol) and Ca2+, and the C2 domains bound to the beads were analyzed by Western blotting using anti-His tag antibody to detect both PRIP-C2 and Syt-C2B simultaneously. E, GST pulldown assay with t-SNARE complexes was performed; GST-SNAP-25 plus StxΔC immobilized on glutathione beads (50 pmol each) was incubated with increasing amounts of PRIP-C2 (3–10 pmol) and a fixed amount of Syt-C2B (10 pmol) in the presence or absence of Ca2+. Cont., control.
Effect of PRIP on Ternary SNARE Complex Formation
The formation of SDS-resistant heterotrimeric SNARE complexes consisting of VAMP2, SNAP-25, and syntaxin 1 was assayed because there is growing evidence to indicate that the amount of the SNARE complex correlates well with the extent of exocytosis (6, 40–42). To examine the effect of PRIP on SNARE complex formation, we used recombinant SNAP-25, soluble syntaxin 1ΔC, and soluble VAMP2ΔC prepared by bacterial expression. An equimolar mixture of these three SNARE proteins were incubated and treated with SDS sample buffer with or without boiling, followed by SDS-PAGE analysis and Western blotting. Two major high molecular mass bands (∼110 and 220 kDa) were detected with antibodies against GST-SNAP-25, syntaxin 1, and VAMP2, which were lacking in boiled samples (Fig. 8A). The band densities of individual SNARE proteins were more intense following boiling, indicating that high molecular mass bands prior to boiling represented SNARE complexes as previously reported (4, 40). We then examined the effect of PRIP and the related proteins on complex formation. PRIP-WT inhibited SNARE complex formation in a dose-dependent manner, whereas PHXY showed no effect (Fig. 8, B and C). Isolated PRIP-C2 at higher concentrations showed inhibitory effects, but PLC-δ1 even at high concentrations had no effect. The results indicate that PRIP inhibits the SDS-resistant SNARE complex formation in a dose-dependent manner, likely by binding to SNARE proteins through its C2 domain. By contrast, Syt-C2B was ineffective in the inhibition of SNARE complex formation (Fig. 8, B and C). Studies of the time-dependent formation of SDS-resistant SNARE complexes revealed that PRIP delayed complex formation (data not shown).
FIGURE 8.
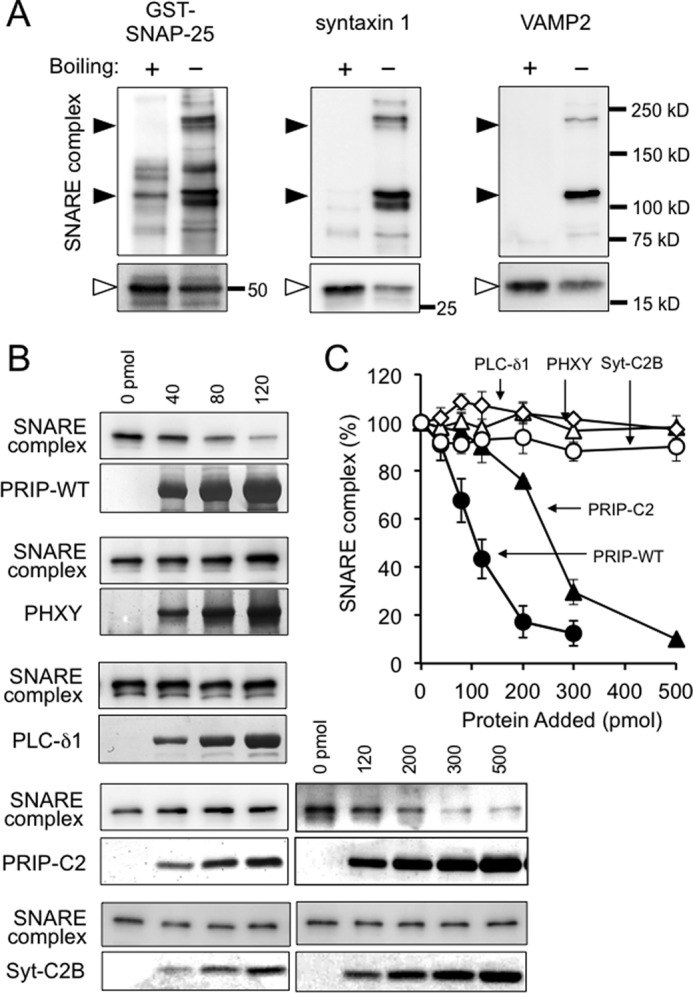
Effect of PRIP on SDS-resistant SNARE complex formation. A, mixture of the purified recombinant proteins of His-VAMP2ΔC, syntaxin 1ΔC, and GST-SNAP-25 was incubated overnight at 4 °C and then subjected to SDS-PAGE with or without boiling after the addition of sample buffer, followed by Western blotting using the indicated antibodies. The black arrowheads indicate high molecular mass bands observed only in the unboiled sample, whereas open arrowheads indicate the bands with the expected molecular masses of monomeric proteins. B, mixture of the three SNARE proteins were incubated for 3 h at 4 °C in the presence or absence of the indicated concentrations of purified wild type PRIP-1 (PRIP-WT), deleted PRIP-1 mutants (PHXY and PRIP-C2), PLC-δ1, or deleted synaptotagmin I mutant (Syt-C2B), followed by Western blotting without boiling. Only the bands of 220 kDa detected with anti-VAMP2 antibody are shown in the figure, but the bands of 110 kDa behaved similarly with 220-kDa bands detected with either antibody. C, the amount of SNARE complex formed shown in B was expressed as relative to that in the absence of PRIP, PLC-δ1, or Syt-C2B. All of the data are the means ± S.E. of four experiments. Closed circle, PRIP-WT; closed triangle, PRIP-C2; open triangle, PHXY; open diamond, PLC-δ1; open circle, Syt-C2B.
Ca2+ Dependence of the Binding to t-SNAREs and the Inhibitory Effect on SNARE Complex Formation by PRIP
Because the binding of the C2 domain of synaptotagmin I to t-SNARE proteins is Ca2+-dependent (32), and the aspartate residues required for Ca2+ binding are relatively well conserved in PRIP-C2, we tested whether the binding of PRIP-C2 to t-SNARE proteins is Ca2+-dependent. The GST pulldown assay was performed in the presence or absence of 10 μm Ca2+. Both the binding of PRIP-C2 to syntaxin 1 and to SNAP-25 exhibited stimulation by Ca2+ with an overall Ca2+ dependence less than that observed for the positive control, Syt-C2B (Fig. 9A). The Ca2+ effect on the competition of PRIP-C2 with Syt-C2B on t-SNARE complexes was also assayed by pulldown assay, whose results were shown in Fig. 7E. GST-SNAP-25 plus syntaxin 1 (StxΔC) was incubated with increasing amount of PRIP-C2 at fixed Syt-C2B in the presence or absence of Ca2+. The results showed similar competition independent of Ca2+ but more binding of PRIP-C2 in the presence of Ca2+.
FIGURE 9.
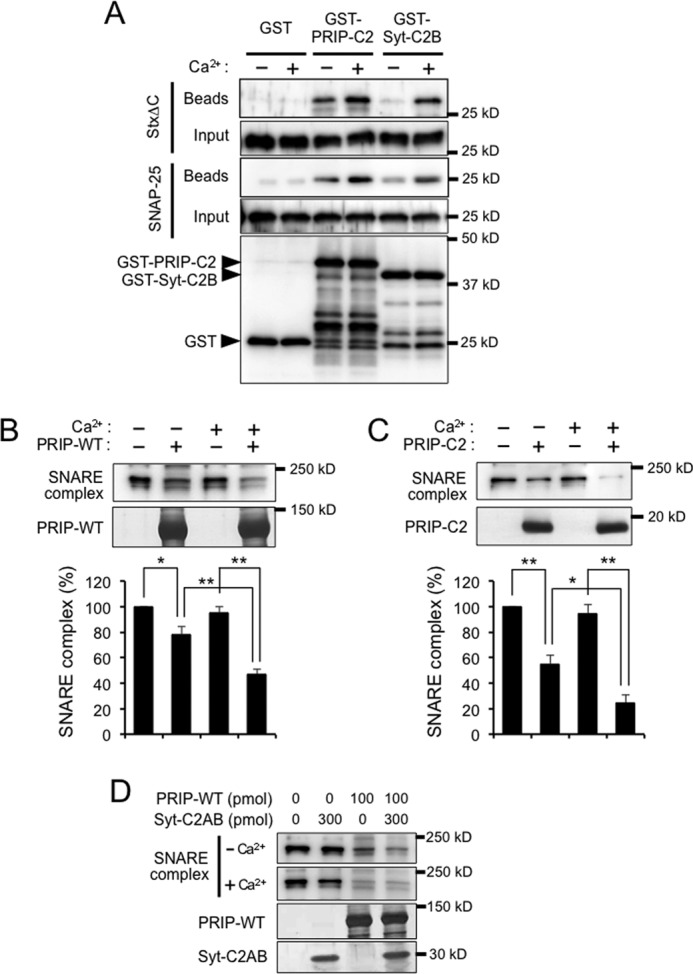
Effect of Ca2+ on the binding of PRIP-C2 to SNARE proteins and SNARE complex formation. A, PRIP-C2 or Syt-C2B immobilized on the glutathione beads was incubated with StxΔC or SNAP-25 in the presence or absence of Ca2+. After extensive washing, the beads were probed with anti-SNAP-25 or syntaxin 1 antibody. The blot with anti-GST antibody shows GST or GST-C2 proteins on the beads, and the bands corresponding to GST or GST-C2 are indicated by arrowheads, whereas the antibody detected degraded products of GST-C2 proteins with lower molecular mass. B and C, a mixture of SNARE proteins was incubated in the presence of 100 pmol of PRIP-WT (B) or 300 pmol of PRIP-C2 (C) as in Fig. 8, in the presence or absence of Ca2+. Formation of the SDS-resistant SNARE complex was expressed as in Fig. 8. D, SNARE complex formation was analyzed in the presence of Syt-C2AB and/or PRIP-WT at the indicated concentrations in the presence or absence of Ca2+. All of the data are the means ± S.E. of four experiments. Significance by Student's t tests is represented by * or ** for p < 0.05 or p < 0.01, respectively.
The Ca2+ dependence of the effect of PRIP and PRIP-C2 on the SDS-resistant SNARE complex formation was assayed. The inhibition of SNARE complex formation by full-length PRIP and PRIP-C2 shown in Fig. 8 (B and C) was stronger in the presence of 10 μm Ca2+ (Fig. 9, B and C). These results suggest that PRIP or PRIP-C2 might inhibit exocytosis in both Ca2+-independent and Ca2+-dependent manners but more strongly in the presence of Ca2+. Because PRIP competed with synaptotagmin I for SNAP-25 in brain (Fig. 7A), we also examined the effect of PRIP on SDS-resistant SNARE complex formation in the presence of synaptotagmin I. Syt-C2AB by itself showed little effect on SNARE complex formation, as already shown with Syt-C2B in Fig. 8B, and also did not affect the inhibition by PRIP (Fig. 9D). However, when the isolated PRIP-C2 was used, the inhibition was rescued by Syt-C2B only in the presence of Ca2+ (data not shown).
Effect of PRIP on NA Release from PC12
Lastly, we examined the role of full-length PRIP-1 and PRIP-C2 in [3H]NA release using PC12 cell lines stably expressing EGFP, EGFP-PRIP-WT, or EGFP-PRIPΔC2 (Fig. 10, upper panel). [3H]NA release without stimulation for 2 min was slightly decreased in cells expressing PRIP-WT or PRIPΔC2 but did not achieve statistical significance. High K+-induced secretion of [3H]NA was almost completely inhibited in the cells expressing wild type PRIP-1, whereas the inhibitory effect of PRIPΔC2 was partial but still significant (Fig. 10, graph). The results indicate that full-length PRIP-1 inhibits regulated exocytosis, and the C2 domain is involved in the inhibition. The inhibition may result from the direct binding of the C2 domain to t-SNAREs, leading to the inhibition of SNARE complex formation. The rest of the inhibition observed with PRIPΔC2 may involve other regions of PRIP-1 such as the PH domain.7
FIGURE 10.
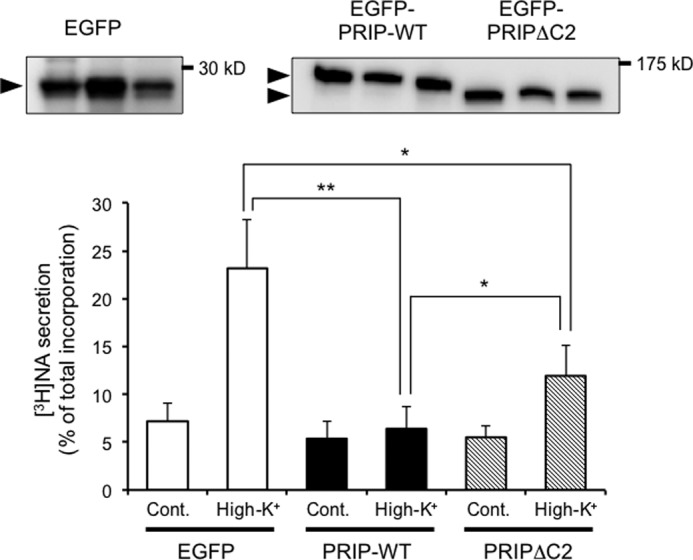
Involvement of the C2 domain in inhibition of [3H]NA secretion by PRIP. PC12 cells expressing EGFP, EGFP-PRIP-WT, or EGFP-PRIPΔC2 were incorporated with [3H]NA and then subjected to [3H]NA secretion assay induced by high K+ solution (56 mm KCl) for 2 min. The upper panels show the expression levels of EGFP constructs in three independent cell lines, detected by anti-GFP antibody. There was no apparent difference in exocytosis among these cell lines. All of the data are the means ± S.D. of five experiments using three independent cell lines for each construct. Significance by Student's t tests is represented by * or ** for p < 0.05 or p < 0.01, respectively. Cont., control.
DISCUSSION
In this study, we identified PRIP as a new C2 domain-containing protein involved in the regulation of exocytosis. The study was initiated by the finding that PRIP-KO mice exhibited up-regulation of dense core vesicle exocytosis (18, 19). To explore the molecular mechanisms by which PRIP negatively regulates exocytosis, studies were first undertaken to show the involvement of the PH domain in binding to PtdIns(4,5)P2. However, the role of the PH domain could not fully explain the inhibition of exocytosis by PRIP.7 The involvement of the C2 domain of PRIP in the inhibitory process was investigated in the present study, and the results show that PRIP directly interacts with both syntaxin 1 and SNAP-25. Despite structural similarity, the C2 domain of PLC-δ1 bound to neither syntaxin 1 nor SNAP-25, indicating the specificity of the PRIP C2 domain. The majority of the cellular experiments in this study were performed using PC12 cells with exogenously expressed PRIP, because PC12 cells contain no detectable level of PRIP by the antibody. However, the inhibitory effect of PRIP is not limited to overexpression, because PRIP-KO mice exhibit increased secretion of gonadotropins and insulin, indicating that physiologically relevant levels of PRIP are inhibitory.
There is a group of C2 domain-containing proteins with enzymatic activity regulating membrane traffic through the regulation of small G-proteins or the synthesis of membrane phospholipids (43–45). On the other hand, there is another group of C2 domain-containing proteins with no enzymatic activity that play important roles by physical interaction with proteins involved in exocytosis. The latter group, which includes synaptotagmin, Munc13, rabphilin, DOC2, and CAPS, binds directly to SNARE proteins (3, 28, 32–35, 46, 47). They play important roles as regulatory proteins specific to distinct vesicular populations and/or specific to different phases of exocytosis, depending on their affinities, specificities, or Ca2+ dependence. The C2 domain of PRIP is in this latter group and thus might be involved in inhibiting exocytosis by competing for SNARE complex assembly with proteins that promote membrane fusion. The present work also suggested that PRIP interferes with synaptotagmin function; thus competition at multiple levels may be responsible for the inhibition of exocytosis.
PRIP or its C2 domain showed direct effects on the formation of SDS-resistant SNARE complexes in vitro. Although the precise role of SDS-resistant SNARE complexes in vivo have not yet been revealed, it is generally accepted that ternary SNARE complex formation is required for vesicles to fuse or become ready to fuse with target membranes (42). In this study, we showed that PRIP inhibited the formation of SDS-resistant ternary SNARE complexes in a dose-dependent manner through the direct binding of the PRIP-C2 domain to SNARE proteins. Although the isolated C2 domain was less potent than the full-length PRIP-1, which is often observed (9), it could be concluded that the C2 domain of PRIP participates in the inhibition. In this context, PRIP may be involved in the inhibition of exocytosis not only by competing for SNAREs with other SNARE-binding proteins that promote exocytosis, but also by inhibiting SNARE complex formation directly.
The binding of PRIP to SNARE proteins was enhanced by Ca2+, which also correlated with the inhibitory effect of PRIP on SDS-resistant SNARE complex formation. Structural analyses on multiple C2 domains revealed that Ca2+-binding sites reside in loops at the top of a β-barrel structure with conserved aspartate residues in loops 1 and 3 primarily responsible for direct recognition of Ca2+ (43, 48). In PRIP-C2, only one aspartate in the loop 1 is conserved, but it is the same with the C2 domain of PLC-δ1, which was shown to bind Ca2+ (49). In calcium-binding loop 3 of PRIP-C2, two of three aspartate residues are conserved, and the other is replaced by glutamate. However, the structural analysis of Rph-C2A, in which an aspartate residue is also replaced by glutamate at the same position as PRIP, revealed that the glutamate could be directly involved in the recognition of Ca2+ (50). Thus, the results regarding promotion of PRIP binding with SNARE protein by Ca2+ is probably attributed to the conserved acidic residues for Ca2+ binding in PRIP-C2.
There are several C2 domains that directly interact with SNARE proteins (32–36, 46, 47) with differing specificity. The C2 domains of rabphilin and Rab3-interacting molecule bind only to SNAP-25 (34, 35, 46), and the C2 domains of synaptotagmin, DOC2, and otoferlin bind to both SNAP-25 and syntaxins, although the Ca2+ dependence and preference for distinct syntaxin isoforms vary (32, 33, 36, 47). PRIP-C2 interacted with both syntaxin 1 and SNAP-25, but the C2 domain of PLC-δ1, despite its structural similarity, did not bind to either syntaxin 1 or SNAP-25. CAPS and Munc13 contain C2 domains, but interactions with t-SNARE proteins are not mediated through the C2 domains (3, 28, 51). It remains to be clarified how the SNARE binding specificity of the C2 domains are accomplished because structures of any C2 domains as complexes with SNARE proteins are not available. The results from mutational analyses of synaptotagmin I (52, 53) and NMR analysis of rabphilin-C2B (35) suggested that the surface structures for SNAP-25 binding differ. A structural analysis of the PRIP-C2 is underway to clarify the binding mode of PRIP-C2 with the SNARE component proteins.
In conclusion, the current results suggest that PRIP is a C2 domain-containing protein that regulates vesicular transport through C2 domain interactions with both SNAP-25 and syntaxin 1. SNARE binding by the C2 domain was required for PRIP to co-localize with t-SNARE proteins in cells and to inhibit SDS-resistant SNARE complex formation in vitro. Considering that membrane microdomains for exocytosis contain PtdIns(4,5)P2 and the t-SNARE component proteins, syntaxin 1 and SNAP-25, PRIP would exert its inhibitory role by the combinatorial function of its PH and C2 domain, binding to PtdIns(4,5)P2 and t-SNAREs, respectively.
Acknowledgments
We appreciate the Research Support Center of the Graduate School of Medical Sciences at Kyushu University for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant DK025861 (to T. F. J. M). This work was also supported by Japan Society for the Promotion of Science KAKENHI Grants 24592805 (to H. T.), 23792127 (to J. G.), and 21249089, 23659782, and 24229009 (to M. H.).
M. Matsuda, M. Kotani, and M. Hirata, manuscript in preparation.
H. Takeuchi, J. Gao, Z. Zhang, D. James, T. Martin, and M. Hirata, manuscript in preparation.
- VAMP
- vesicle-associated membrane protein
- NA
- noradrenalin
- PLC
- phospholipase C
- PtdIns(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- SNAP-25
- synaptosome-associated protein of 25 kDa
- PH
- pleckstrin homology
- PLA
- proximity ligation assay
- Stx
- syntaxin
- Syt
- synaptotagmin.
REFERENCES
- 1. Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) SNAREpins. Minimal machinery for membrane fusion. Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 2. Hong W. (2005) SNAREs and traffic. Biochim. Biophys. Acta 1744, 493–517 [PubMed] [Google Scholar]
- 3. Malsam J., Kreye S., Söllner T. H. (2008) Membrane fusion. SNAREs and regulation. Cell Mol. Life Sci. 65, 2814–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sutton R. B., Fasshauer D., Jahn R., Brunger A. T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 [DOI] [PubMed] [Google Scholar]
- 5. Sugita S. (2008) Mechanisms of exocytosis. Acta Physiol. (Oxf.) 192, 185–193 [DOI] [PubMed] [Google Scholar]
- 6. McNew J. A., Parlati F., Fukuda R., Johnston R. J., Paz K., Paumet F., Söllner T. H., Rothman J. E. (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153–159 [DOI] [PubMed] [Google Scholar]
- 7. Kanematsu T., Takeya H., Watanabe Y., Ozaki S., Yoshida M., Koga T., Iwanaga S., Hirata M. (1992) Putative inositol 1,4,5-trisphosphate binding proteins in rat brain cytosol. J. Biol. Chem. 267, 6518–6525 [PubMed] [Google Scholar]
- 8. Kanematsu T., Misumi Y., Watanabe Y., Ozaki S., Koga T., Iwanaga S., Ikehara Y., Hirata M. (1996) A new inositol 1,4,5-trisphosphate binding protein similar to phospholipase C-δ1. Biochem. J. 313, 319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeuchi H., Kanematsu T., Misumi Y., Yaakob H. B., Yagisawa H., Ikehara Y., Watanabe Y., Tan Z., Shears S. B., Hirata M. (1996) Localization of a high-affinity inositol 1,4,5-trisphosphate/inositol 1,4,5,6-tetrakisphosphate binding domain to the pleckstrin homology module of a new 130 kDa protein. Characterization of the determinants of structural specificity. Biochem. J. 318, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeuchi H., Oike M., Paterson H. F., Allen V., Kanematsu T., Ito Y., Erneux C., Katan M., Hirata M. (2000) Inhibition of Ca2+ signalling by p130, a phospholipase-C-related catalytically inactive protein. Critical role of the p130 pleckstrin homology domain. Biochem. J. 349, 357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanematsu T., Jang I. S., Yamaguchi T., Nagahama H., Yoshimura K., Hidaka K., Matsuda M., Takeuchi H., Misumi Y., Nakayama K., Yamamoto T., Akaike N., Hirata M., Nakayama K. (2002) Role of the PLC-related, catalytically inactive protein p130 in GABAA receptor function. EMBO J. 21, 1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uji A., Matsuda M., Kukita T., Maeda K., Kanematsu T., Hirata M. (2002) Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2. Comparison with PRIP-1. Life Sci. 72, 443–453 [DOI] [PubMed] [Google Scholar]
- 13. Kanematsu T., Yasunaga A., Mizoguchi Y., Kuratani A., Kittler J. T., Jovanovic J. N., Takenaka K., Nakayama K. I., Fukami K., Takenawa T., Moss S. J., Nabekura J., Hirata M. (2006) Modulation of GABAA receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J. Biol. Chem. 281, 22180–22189 [DOI] [PubMed] [Google Scholar]
- 14. Kanematsu T., Fujii M., Mizokami A., Kittler J. T., Nabekura J., Moss S. J., Hirata M. (2007) Phospholipase C-related inactive protein is implicated in the constitutive internalization of GABAA receptors mediated by clathrin and AP2 adaptor complex. J. Neurochem. 101, 898–905 [DOI] [PubMed] [Google Scholar]
- 15. Yoshimura K., Takeuchi H., Sato O., Hidaka K., Doira N., Terunuma M., Harada K., Ogawa Y., Ito Y., Kanematsu T., Hirata M. (2001) Interaction of p130 with, and consequent inhibition of, the catalytic subunit of protein phosphatase 1α. J. Biol. Chem. 276, 17908–17913 [DOI] [PubMed] [Google Scholar]
- 16. Sugiyama G., Takeuchi H., Nagano K., Gao J., Ohyama Y., Mori Y., Hirata M. (2012) Regulated interaction of protein phosphatase 1 and protein phosphatase 2A with phospholipase C-related but catalytically inactive protein. Biochemistry 51, 3394–3403 [DOI] [PubMed] [Google Scholar]
- 17. Fujii M., Kanematsu T., Ishibashi H., Fukami K., Takenawa T., Nakayama K. I., Moss S. J., Nabekura J., Hirata M. (2010) Phospholipase C-related but catalytically inactive protein is required for insulin-induced cell surface expression of γ-aminobutyric acid type A receptors. J. Biol. Chem. 285, 4837–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuda M., Tsutsumi K., Kanematsu T., Fukami K., Terada Y., Takenawa T., Nakayama K. I., Hirata M. (2009) Involvement of phospholipase C-related inactive protein in the mouse reproductive system through the regulation of gonadotropin levels. Biol. Reprod. 81, 681–689 [DOI] [PubMed] [Google Scholar]
- 19. Doira N., Kanematsu T., Matsuda M., Takeuchi H., Nakano H., Ito Y., Nakayama K., Nakayama K., Hirata M. (2001) Hyperinsulinemia in PRIP-1 gene deleted mice. Biomed. Res. 22, 157–165 [Google Scholar]
- 20. Hay J. C., Martin T. F. (1993) Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature 366, 572–575 [DOI] [PubMed] [Google Scholar]
- 21. Holz R. W., Hlubek M. D., Sorensen S. D., Fisher S. K., Balla T., Ozaki S., Prestwich G. D., Stuenkel E. L., Bittner M. A. (2000) A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 275, 17878–17885 [DOI] [PubMed] [Google Scholar]
- 22. James D. J., Khodthong C., Kowalchyk J. A., Martin T. F. (2008) Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J. Cell Biol. 182, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walter A. M., Groffen A. J., Sørensen J. B., Verhage M. (2011) Multiple Ca2+ sensors in secretion. Teammates, competitors or autocrats? Trends Neurosci. 34, 487–497 [DOI] [PubMed] [Google Scholar]
- 24. Chapman E. R. (2008) How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77, 615–641 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida M., Kanematsu T., Watanabe Y., Koga T., Ozaki S., Iwanaga S., Hirata M. (1994) d-myo-Inositol 1,4,5-trisphosphate-binding proteins in rat brain membranes. J. Biochem. 115, 973–980 [DOI] [PubMed] [Google Scholar]
- 26. Gao J., Takeuchi H., Zhang Z., Fujii M., Kanematsu T., Hirata M. (2009) Binding of phospholipase C-related but catalytically inactive protein to phosphatidylinositol 4,5-bisphosphate via the PH domain. Cell Signal. 21, 1180–1186 [DOI] [PubMed] [Google Scholar]
- 27. Daily N. J., Boswell K. L., James D. J., Martin T. F. (2010) Novel interactions of CAPS (Ca2+-dependent activator protein for secretion) with the three neuronal SNARE proteins required for vesicle fusion. J. Biol. Chem. 285, 35320–35329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. James D. J., Kowalchyk J., Daily N., Petrie M., Martin T. F. (2009) CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc. Natl. Acad. Sci. U.S.A. 106, 17308–17313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao J., Takeuchi H., Zhang Z., Fukuda M., Hirata M. (2012) Phospholipase C-related but catalytically inactive protein (PRIP) modulates synaptosomal-associated protein 25 (SNAP-25) phosphorylation and exocytosis. J. Biol. Chem. 287, 10565–10578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao J., Takeuchi H., Umebayashi H., Zhang Z., Matsuda M., Hirata M. (2010) Assay of dense-core vesicle exocytosis using permeabilized PC12 cells. Adv. Enzyme Regul. 50, 237–246 [DOI] [PubMed] [Google Scholar]
- 31. Takeuchi H., Takeuchi T., Gao J., Cantley L. C., Hirata M. (2010) Characterization of PXK as a protein involved in epidermal growth factor receptor trafficking. Mol. Cell Biol. 30, 1689–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bai J., Wang C. T., Richards D. A., Jackson M. B., Chapman E. R. (2004) Fusion pore dynamics are regulated by synaptotagmin·t-SNARE interactions. Neuron 41, 929–942 [DOI] [PubMed] [Google Scholar]
- 33. Ramakrishnan N. A., Drescher M. J., Drescher D. G. (2009) Direct interaction of otoferlin with syntaxin 1A, SNAP-25, and the L-type voltage-gated calcium channel Cav1.3. J. Biol. Chem. 284, 1364–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsuboi T., Fukuda M. (2005) The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense core vesicle exocytosis in PC12 cells. J. Biol. Chem. 280, 39253–39259 [DOI] [PubMed] [Google Scholar]
- 35. Deák F., Shin O. H., Tang J., Hanson P., Ubach J., Jahn R., Rizo J., Kavalali E. T., Südhof T. C. (2006) Rabphilin regulates SNARE-dependent re-priming of synaptic vesicles for fusion. EMBO J. 25, 2856–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masumoto T., Suzuki K., Ohmori I., Michiue H., Tomizawa K., Fujimura A., Nishiki T., Matsui H. (2012) Ca2+-independent syntaxin binding to the C2B effector region of synaptotagmin. Mol. Cell Neurosci. 49, 1–8 [DOI] [PubMed] [Google Scholar]
- 37. Lang T., Margittai M., Hölzler H., Jahn R. (2002) SNAREs in native plasma membranes are active and readily form core complexes with endogenous and exogenous SNAREs. J. Cell Biol. 158, 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ellena J. F., Liang B., Wiktor M., Stein A., Cafiso D. S., Jahn R., Tamm L. K. (2009) Dynamic structure of lipid-bound synaptobrevin suggests a nucleation-propagation mechanism for trans-SNARE complex formation. Proc. Natl. Acad. Sci. U.S.A. 106, 20306–20311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 40. Hayashi T., McMahon H., Yamasaki S., Binz T., Hata Y., Südhof T. C., Niemann H. (1994) Synaptic vesicle membrane fusion complex. Action of clostridial neurotoxins on assembly. EMBO J. 13, 5051–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kubista H., Edelbauer H., Boehm S. (2004) Evidence for structural and functional diversity among SDS-resistant SNARE complexes in neuroendocrine cells. J. Cell Sci. 117, 955–966 [DOI] [PubMed] [Google Scholar]
- 42. Xu T., Rammner B., Margittai M., Artalejo A. R., Neher E., Jahn R. (1999) Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell 99, 713–722 [DOI] [PubMed] [Google Scholar]
- 43. Nalefski E. A., Falke J. J. (1996) The C2 domain calcium-binding motif. Structural and functional diversity. Protein Sci. 5, 2375–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Czech M. P. (2003) Dynamics of phosphoinositides in membrane retrieval and insertion. Annu. Rev. Physiol. 65, 791–815 [DOI] [PubMed] [Google Scholar]
- 45. Kwiatkowska K. (2010) One lipid, multiple functions. How various pools of PI(4,5)P2 are created in the plasma membrane. Cell Mol. Life Sci. 67, 3927–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coppola T., Magnin-Luthi S., Perret-Menoud V., Gattesco S., Schiavo G., Regazzi R. (2001) Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J. Biol. Chem. 276, 32756–32762 [DOI] [PubMed] [Google Scholar]
- 47. Friedrich R., Groffen A. J., Connell E., van Weering J. R., Gutman O., Henis Y. I., Davletov B., Ashery U. (2008) DOC2B acts as a calcium switch and enhances vesicle fusion. J. Neurosci. 28, 6794–6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pang Z. P., Südhof T. C. (2010) Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 22, 496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Essen L. O., Perisic O., Lynch D. E., Katan M., Williams R. L. (1997) A ternary metal binding site in the C2 domain of phosphoinositide-specific phospholipase C-δ1. Biochemistry 36, 2753–2762 [DOI] [PubMed] [Google Scholar]
- 50. Coudevylle N., Montaville P., Leonov A., Zweckstetter M., Becker S. (2008) Structural determinants for Ca2+ and phosphatidylinositol 4,5-bisphosphate binding by the C2A domain of rabphilin-3A. J. Biol. Chem. 283, 35918–35928 [DOI] [PubMed] [Google Scholar]
- 51. Carr C. M., Rizo J. (2010) At the junction of SNARE and SM protein function. Curr. Opin. Cell Biol. 22, 488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernández-Chacón R., Königstorfer A., Gerber S. H., García J., Matos M. F., Stevens C. F., Brose N., Rizo J., Rosenmund C., Südhof T. C. (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 [DOI] [PubMed] [Google Scholar]
- 53. Rickman C., Archer D. A., Meunier F. A., Craxton M., Fukuda M., Burgoyne R. D., Davletov B. (2004) Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279, 12574–12579 [DOI] [PubMed] [Google Scholar]


