Abstract
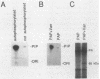
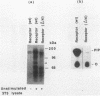
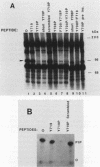
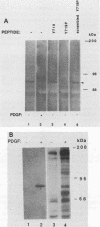
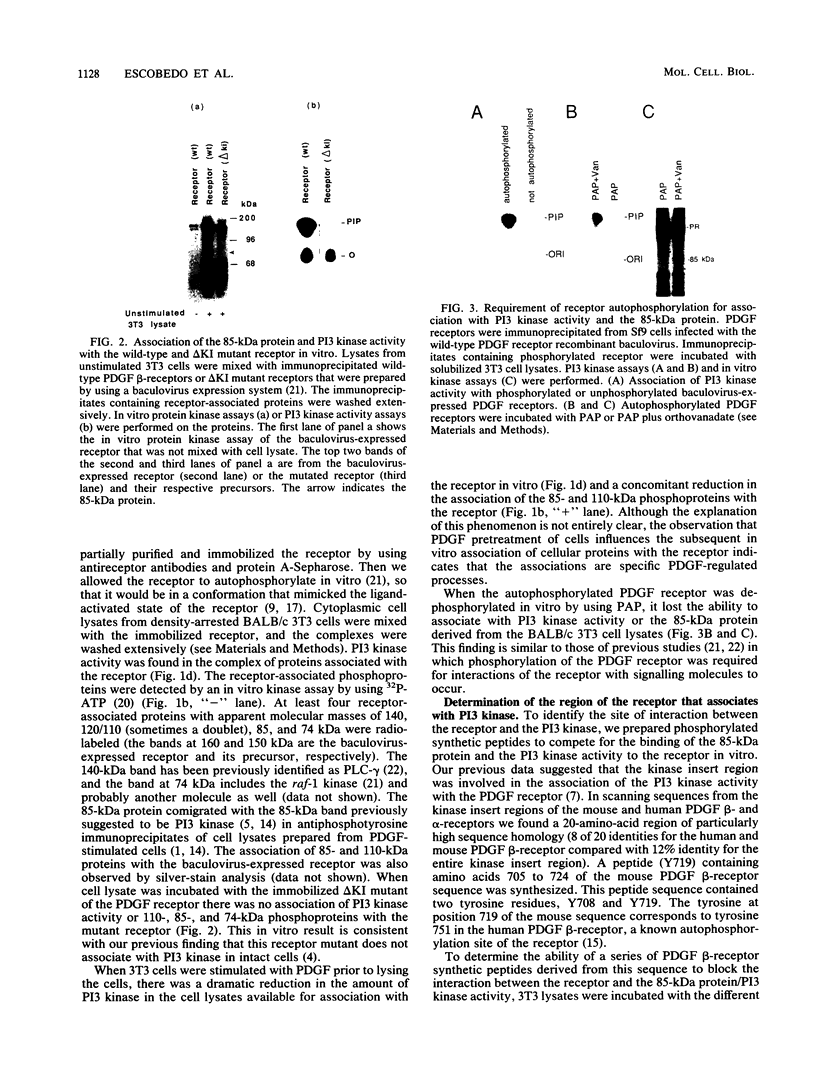
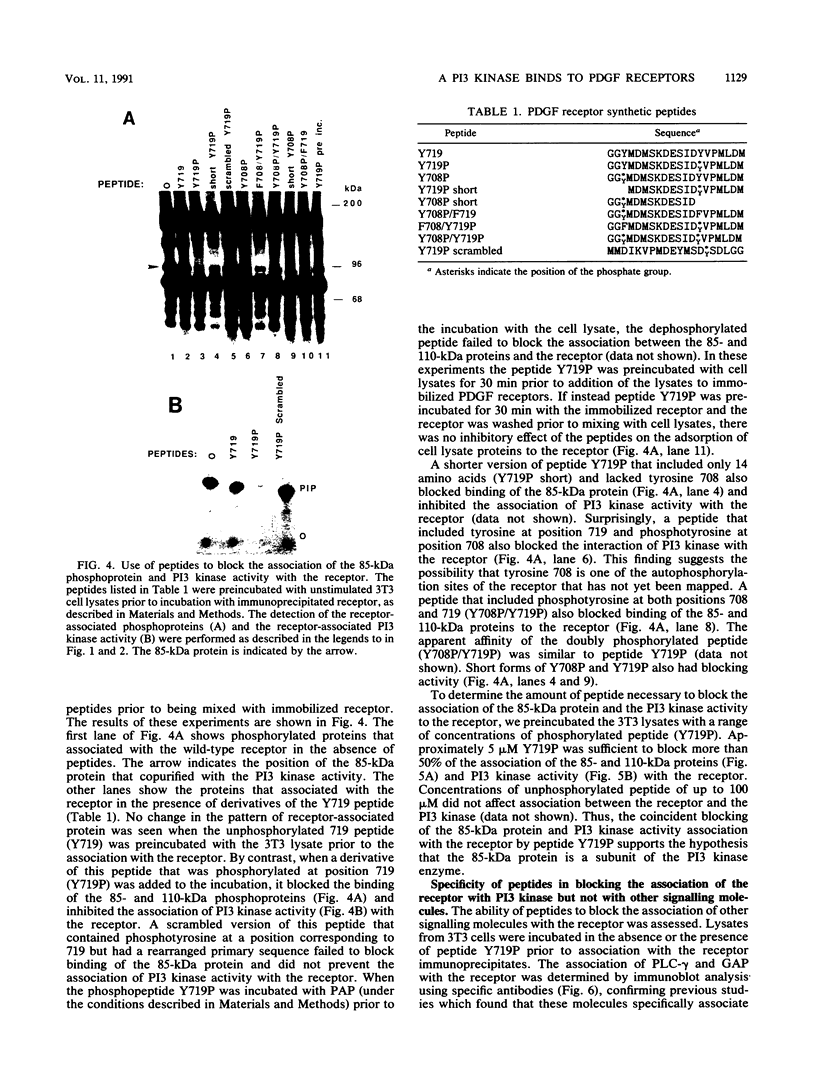
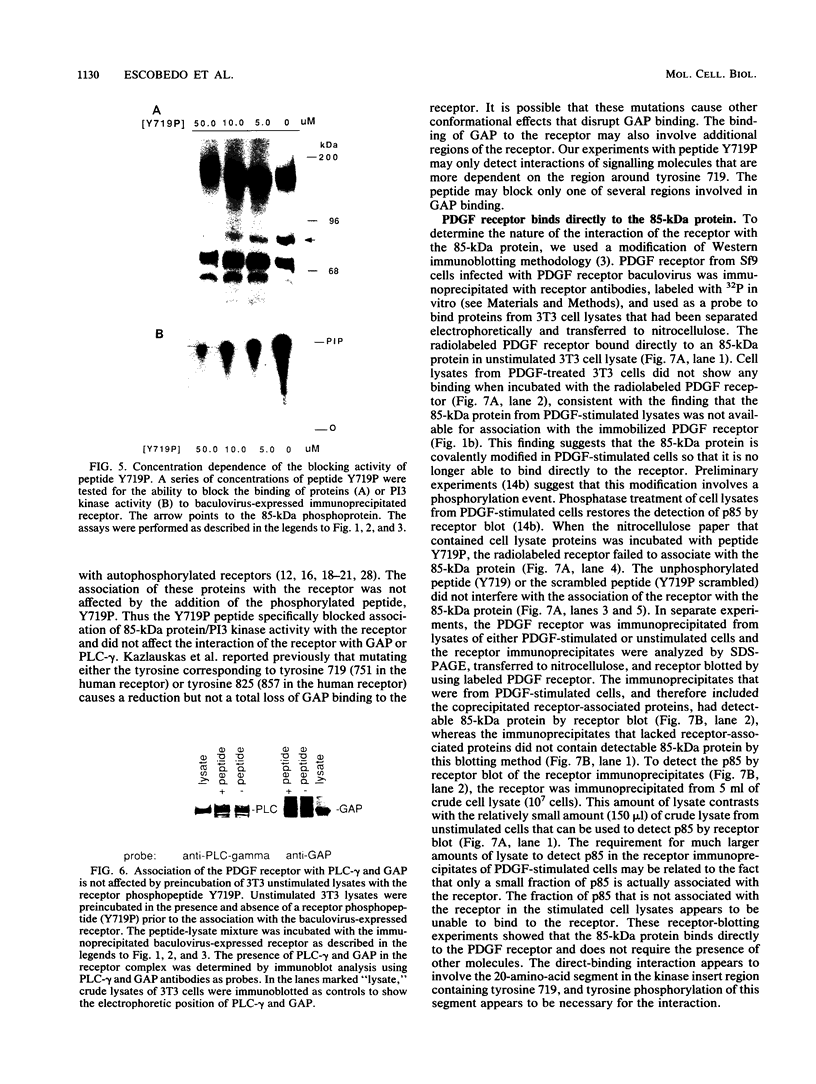
Platelet-derived growth factor (PDGF) stimulates autophosphorylation of the PDGF receptor and association of the receptor with several cytoplasmic molecules, including phosphatidylinositol-3 kinase (PI3 kinase). In this study we examined the association of PI3 kinase with immunoprecipitated autophosphorylated PDGF receptor in vitro. The PI3 kinase from cell lysates bound to the wild-type receptor but not to a mutant receptor that had a deletion of the kinase insert region. A protein of an apparent size of 85 kDa bound to the receptor, consistent with previous observations that a protein of this size is associated with PI3 kinase activity. In addition, 110- and 74-kDa proteins bound to the phosphorylated receptor. Dephosphorylated receptors lost the ability to bind PI3 kinase activity as well as the 85-kDa protein. A 20-amino-acid peptide composed of a sequence in the kinase insert region that included one of the autophosphorylation sites of the receptor (tyrosine 719) as well as a nearby tyrosine (Y708) blocked the binding of PI3 kinase to the receptor, but only when the peptide was phosphorylated on tyrosine residues. A scrambled version of the peptide did not block PI3 kinase binding to the receptor even when it was phosphorylated on tyrosine. These tyrosine-phosphorylated peptides did not block binding of phospholipase C-gamma or GTPase-activating protein to the receptor. In separate experiments (receptor blots), soluble radiolabeled receptor bound specifically to an 85-kDa protein present in sodium dodecyl sulfate-polyacrylamide gel electrophoresis-fractionated 3T3 cell lysates that were transferred to nitrocellulose paper. The binding was blocked by the same tyrosine-phosphorylated peptides that prevented binding of PI3 kinase activity to immobilized receptors. These findings show that the PDGF receptor binds directly to an 85-kDa protein and to a PI3 kinase activity through specific sequences in the kinase insert region. The association of a 110-kDa protein with the receptor also involve these sequences, suggesting that this protein may be a subunit of the PI3 kinase. Phosphotyrosine is an essential structure required for the interactions of these proteins with the PDGF receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Bishayee S., Majumdar S., Khire J., Das M. Ligand-induced dimerization of the platelet-derived growth factor receptor. Monomer-dimer interconversion occurs independent of receptor phosphorylation. J Biol Chem. 1989 Jul 15;264(20):11699–11705. [PubMed] [Google Scholar]
- Cohen B., Liu Y. X., Druker B., Roberts T. M., Schaffhausen B. S. Characterization of pp85, a target of oncogenes and growth factor receptors. Mol Cell Biol. 1990 Jun;10(6):2909–2915. doi: 10.1128/mcb.10.6.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987 Sep 25;50(7):1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Characterization of a tyrosine-specific kinase activity in human fibroblast membranes stimulated by platelet-derived growth factor. J Biol Chem. 1982 Sep 10;257(17):10486–10492. [PubMed] [Google Scholar]
- Escobedo J. A., Williams L. T. A PDGF receptor domain essential for mitogenesis but not for many other responses to PDGF. Nature. 1988 Sep 1;335(6185):85–87. doi: 10.1038/335085a0. [DOI] [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Williams L. T. Mutations of the platelet-derived growth factor receptor that cause a loss of ligand-induced conformational change, subtle changes in kinase activity, and impaired ability to stimulate DNA synthesis. Mol Cell Biol. 1989 Oct;9(10):4473–4478. doi: 10.1128/mcb.9.10.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989 Apr;9(4):1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Ernlund A., Rorsman C., Rönnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989 May 25;264(15):8905–8912. [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Phosphorylation of the PDGF receptor beta subunit creates a tight binding site for phosphatidylinositol 3 kinase. EMBO J. 1990 Oct;9(10):3279–3286. doi: 10.1002/j.1460-2075.1990.tb07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Ellis C., Pawson T., Cooper J. A. Binding of GAP to activated PDGF receptors. Science. 1990 Mar 30;247(4950):1578–1581. doi: 10.1126/science.2157284. [DOI] [PubMed] [Google Scholar]
- Keating M. T., Escobedo J. A., Williams L. T. Ligand activation causes a phosphorylation-dependent change in platelet-derived growth factor receptor conformation. J Biol Chem. 1988 Sep 15;263(26):12805–12808. [PubMed] [Google Scholar]
- Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. Platelet-derived growth factor (PDGF) binding promotes physical association of PDGF receptor with phospholipase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8232–8236. doi: 10.1073/pnas.86.21.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rhee S. G., Williams L. T. Platelet-derived growth factor (PDGF)-dependent association of phospholipase C-gamma with the PDGF receptor signaling complex. Mol Cell Biol. 1990 May;10(5):2359–2366. doi: 10.1128/mcb.10.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley P., Kazlauskas A., Cooper J. A., Rohrschneider L. R. Macrophage colony-stimulating factor-induced tyrosine phosphorylation of c-fms proteins expressed in FDC-P1 and BALB/c 3T3 cells. Mol Cell Biol. 1990 Jun;10(6):2528–2538. doi: 10.1128/mcb.10.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]