Abstract
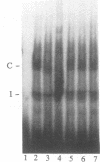
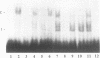
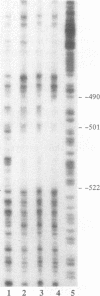
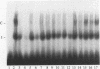
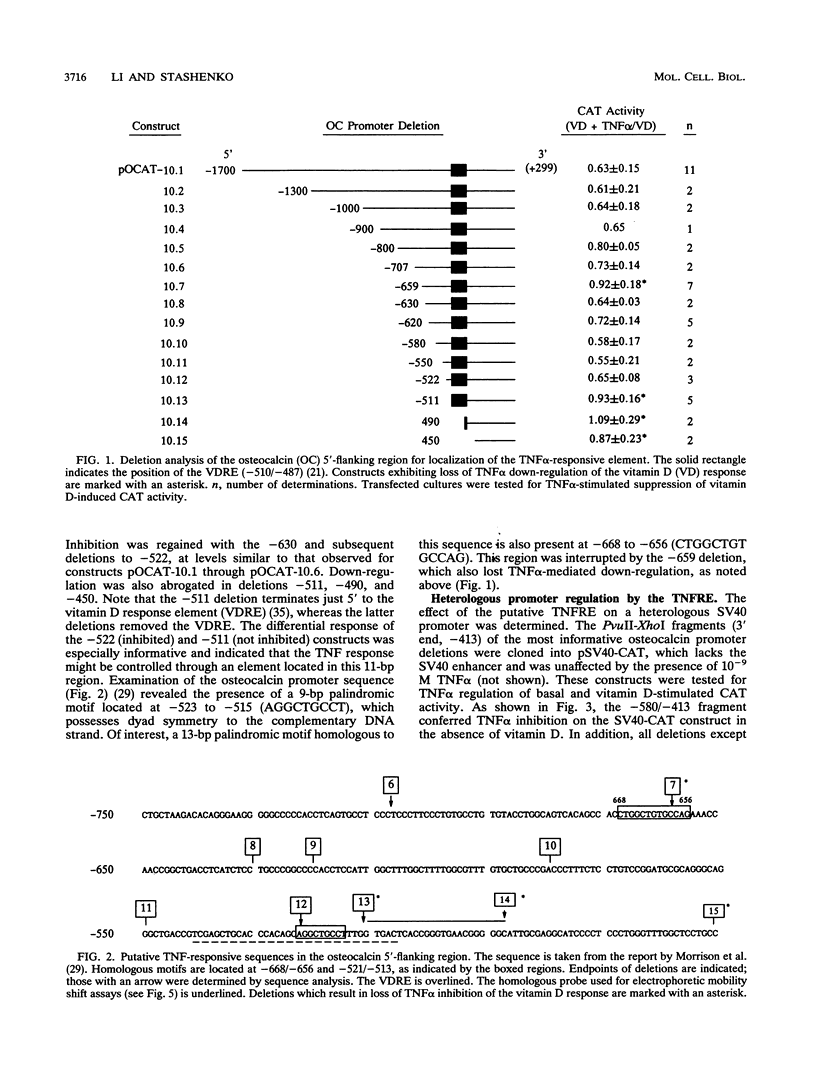
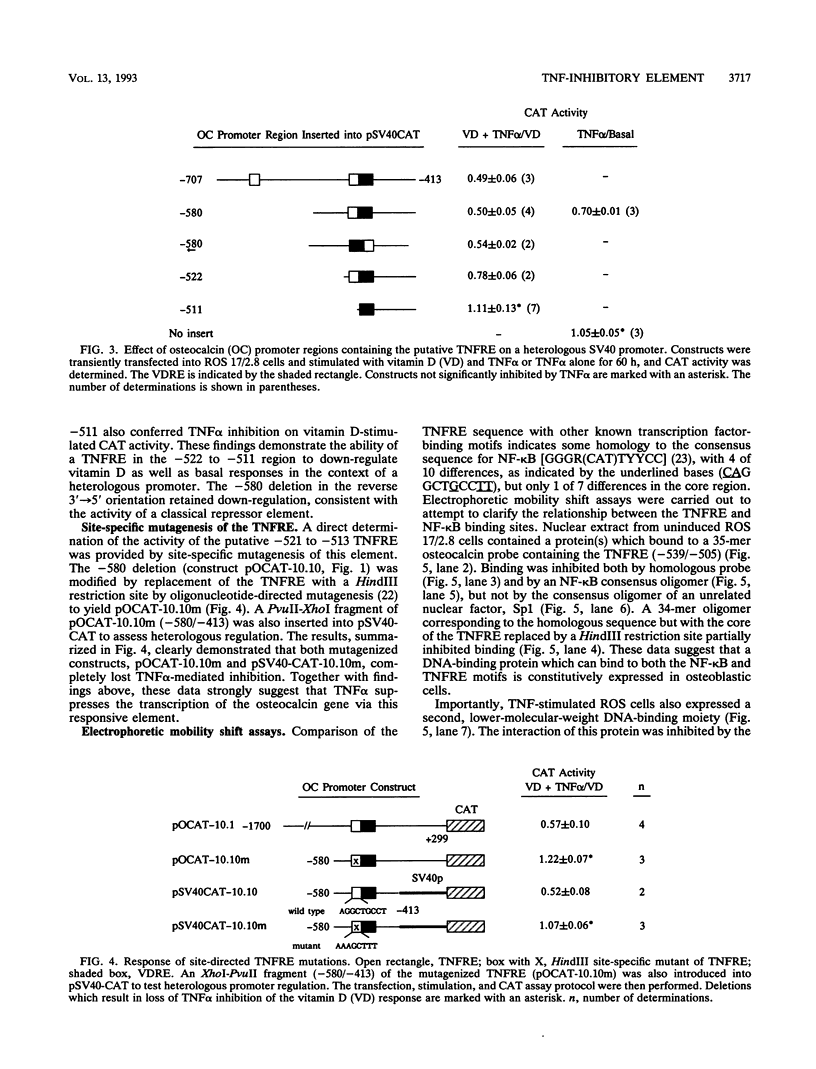
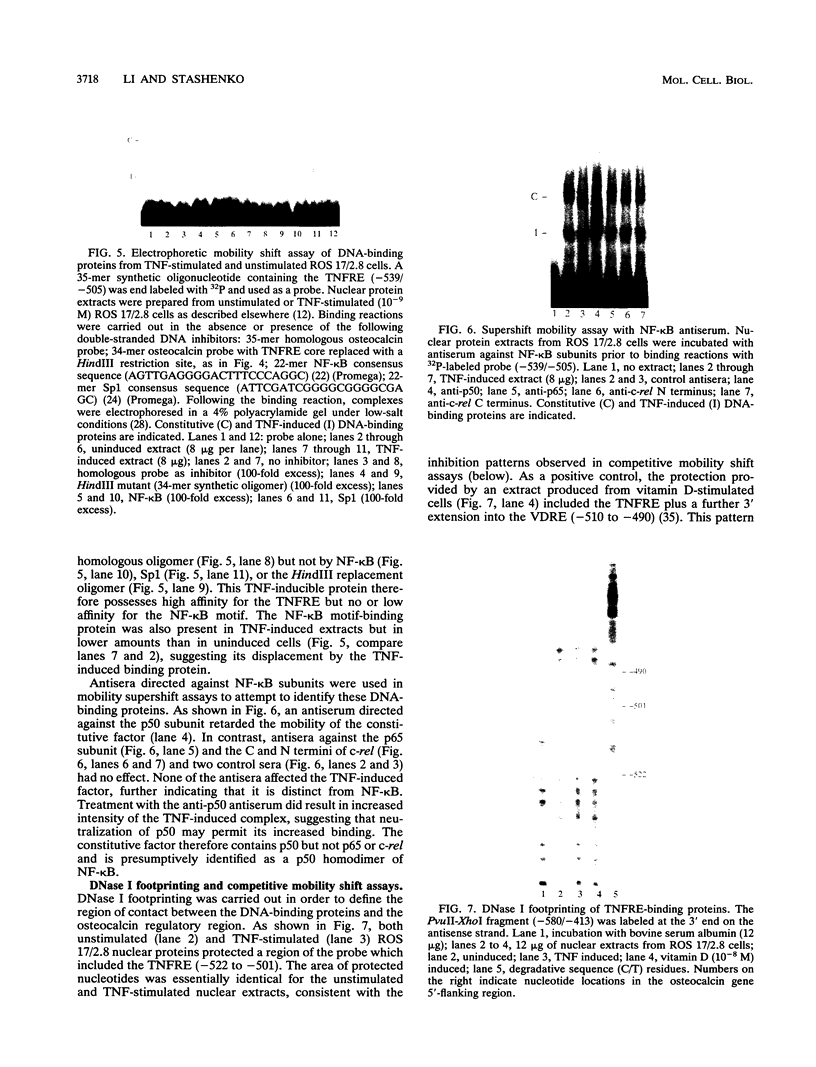
Tumor necrosis factor (TNF) down-regulates the production of bone matrix proteins by osteoblasts, thereby inhibiting bone formation. Osteocalcin, the major noncollagenous protein in bone, is inhibited by TNF at the transcriptional level. Mapping studies were undertaken to characterize the TNF-responsive element (TNFRE) in the osteocalcin promoter. Deletion analysis localized the TNFRE to the -522/-511 region, which contains a 9-bp palindromic motif (AGGCTGCCT). Promoter segments containing this sequence down-regulated a heterologous simian virus 40 promoter. Site-specific mutagenesis of the TNFRE eliminated TNF down-regulation. Mobility shift assays demonstrated that a constitutively expressed nuclear factor bound to the TNFRE; this factor was tentatively identified as the p50 homodimer of NF-kappa B. TNF stimulation induced a second TNFRE-binding protein which displaced the constitutive factor. The TNF-induced protein was not inhibitable by the NF-kappa B consensus sequence and was unreactive with anti-NF-kappa B antiserum. DNase footprinting demonstrated that both factors protected the -522/-501 portion of the promoter, consistent with the results of mapping studies and competitive mobility shift assays. It is hypothesized that the generalized catabolic activities of TNF in infectious and malignant diseases may be regulated via this novel element.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Morishima J. K., Devarayalu S., Gelinas R. E. Regulatory elements in the first intron contribute to transcriptional control of the human alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8869–8873. doi: 10.1073/pnas.84.24.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvagnet P. F., Strehler E. E., White G. E., Strehler-Page M. A., Nadal-Ginard B., Mahdavi V. Multiple positive and negative 5' regulatory elements control the cell-type-specific expression of the embryonic skeletal myosin heavy-chain gene. Mol Cell Biol. 1987 Dec;7(12):4377–4389. doi: 10.1128/mcb.7.12.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Baeuerle P., Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990 Apr;10(4):1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P. D., Malaval L., Arlot M. E., Meunier P. J. Serum bone Gla-protein compared to bone histomorphometry in endocrine diseases. Bone. 1985;6(5):339–341. doi: 10.1016/8756-3282(85)90326-6. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer M. H., Aho S., Gerstenfeld L. C., Boedtker H., Doty P. Unusual DNA sequences located within the promoter region and the first intron of the chicken pro-alpha 1(I) collagen gene. J Biol Chem. 1987 Sep 25;262(27):13323–13332. [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Barber A., Manogue K., Tracey K. J., Kuo G., Fischman D. A., Cerami A. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol. 1989 Mar;256(3 Pt 2):R659–R665. doi: 10.1152/ajpregu.1989.256.3.R659. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Wu R. New rapid methods for DNA sequencing based in exonuclease III digestion followed by repair synthesis. Nucleic Acids Res. 1982 Mar 25;10(6):2065–2084. doi: 10.1093/nar/10.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël A., Le Bail O., Hatat D., Piette J., Kieran M., Logeat F., Wallach D., Fellous M., Kourilsky P. TNF stimulates expression of mouse MHC class I genes by inducing an NF kappa B-like enhancer binding activity which displaces constitutive factors. EMBO J. 1989 Dec 1;8(12):3793–3800. doi: 10.1002/j.1460-2075.1989.tb08556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Shakhov A. N., Nedospasov S. A., Cerottini J. C. Molecular control of tissue-specific expression at the mouse TNF locus. Eur J Immunol. 1989 Mar;19(3):549–552. doi: 10.1002/eji.1830190321. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
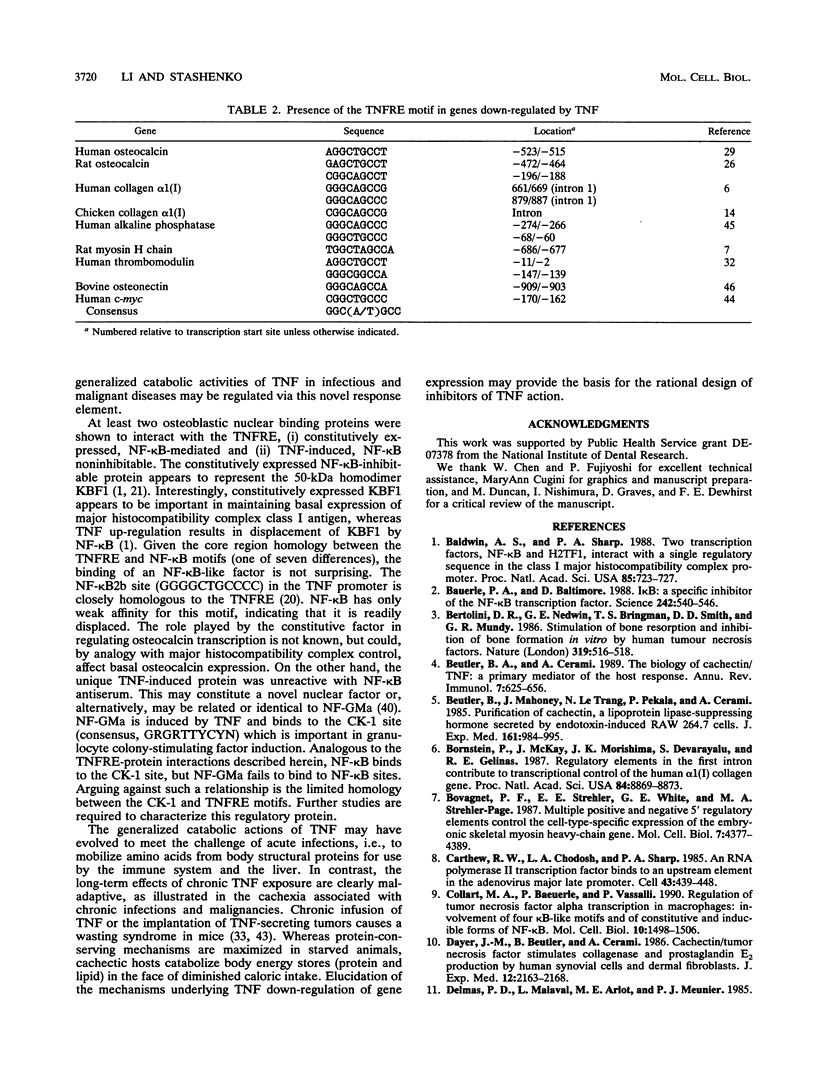
- Li Y. P., Stashenko P. Proinflammatory cytokines tumor necrosis factor-alpha and IL-6, but not IL-1, down-regulate the osteocalcin gene promoter. J Immunol. 1992 Feb 1;148(3):788–794. [PubMed] [Google Scholar]
- Lian J., Stewart C., Puchacz E., Mackowiak S., Shalhoub V., Collart D., Zambetti G., Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Böhnlein E., Greene W. C. Tumor necrosis factor alpha induces proteins that bind specifically to kappa B-like enhancer elements and regulate interleukin 2 receptor alpha-chain gene expression in primary human T lymphocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2331–2335. doi: 10.1073/pnas.86.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldawer L. L., Georgieff M., Lundholm K. Interleukin 1, tumour necrosis factor-alpha (cachectin) and the pathogenesis of cancer cachexia. Clin Physiol. 1987 Aug;7(4):263–274. doi: 10.1111/j.1475-097x.1987.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Morrison N. A., Shine J., Fragonas J. C., Verkest V., McMenemy M. L., Eisman J. A. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989 Dec 1;246(4934):1158–1161. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- Nanes M. S., Rubin J., Titus L., Hendy G. N., Catherwood B. Tumor necrosis factor-alpha inhibits 1,25-dihydroxyvitamin D3-stimulated bone Gla protein synthesis in rat osteosarcoma cells (ROS 17/2.8) by a pretranslational mechanism. Endocrinology. 1991 May;128(5):2577–2582. doi: 10.1210/endo-128-5-2577. [DOI] [PubMed] [Google Scholar]
- Ohdama S., Takano S., Ohashi K., Miyake S., Aoki N. Pentoxifylline prevents tumor necrosis factor-induced suppression of endothelial cell surface thrombomodulin. Thromb Res. 1991 Jun 15;62(6):745–755. doi: 10.1016/0049-3848(91)90378-a. [DOI] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozono K., Liao J., Kerner S. A., Scott R. A., Pike J. W. The vitamin D-responsive element in the human osteocalcin gene. Association with a nuclear proto-oncogene enhancer. J Biol Chem. 1990 Dec 15;265(35):21881–21888. [PubMed] [Google Scholar]
- Pfizenmaier K., Scheurich P., Schlüter C., Krönke M. Tumor necrosis factor enhances HLA-A,B,C and HLA-DR gene expression in human tumor cells. J Immunol. 1987 Feb 1;138(3):975–980. [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Shannon M. F., Pell L. M., Lenardo M. J., Kuczek E. S., Occhiodoro F. S., Dunn S. M., Vadas M. A. A novel tumor necrosis factor-responsive transcription factor which recognizes a regulatory element in hemopoietic growth factor genes. Mol Cell Biol. 1990 Jun;10(6):2950–2959. doi: 10.1128/mcb.10.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]
- Stashenko P., Obernesser M. S., Dewhirst F. E. Effect of immune cytokines on bone. Immunol Invest. 1989 Jan-May;18(1-4):239–249. doi: 10.3109/08820138909112240. [DOI] [PubMed] [Google Scholar]
- Watt R., Nishikura K., Sorrentino J., ar-Rushdi A., Croce C. M., Rovera G. The structure and nucleotide sequence of the 5' end of the human c-myc oncogene. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6307–6311. doi: 10.1073/pnas.80.20.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Henthorn P. S., Lamb B., Kadesch T., Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12002–12010. [PubMed] [Google Scholar]
- Young M. F., Findlay D. M., Dominguez P., Burbelo P. D., McQuillan C., Kopp J. B., Robey P. G., Termine J. D. Osteonectin promoter. DNA sequence analysis and S1 endonuclease site potentially associated with transcriptional control in bone cells. J Biol Chem. 1989 Jan 5;264(1):450–456. [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]