Abstract
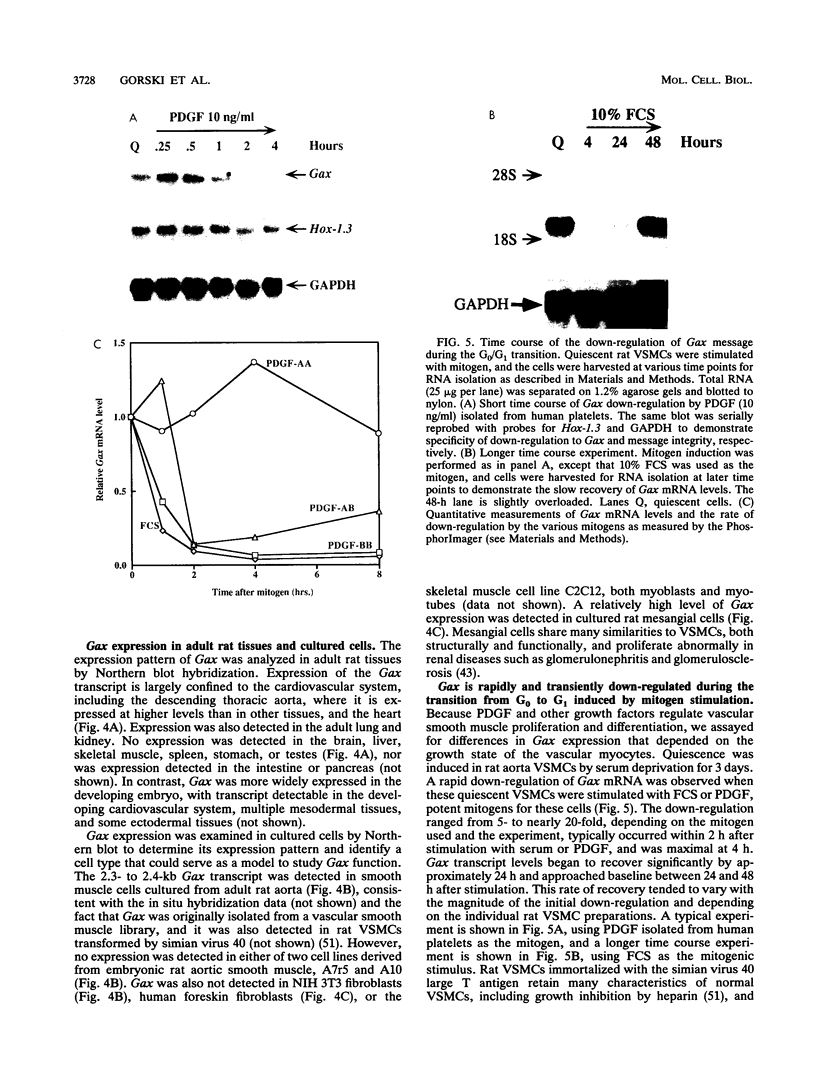
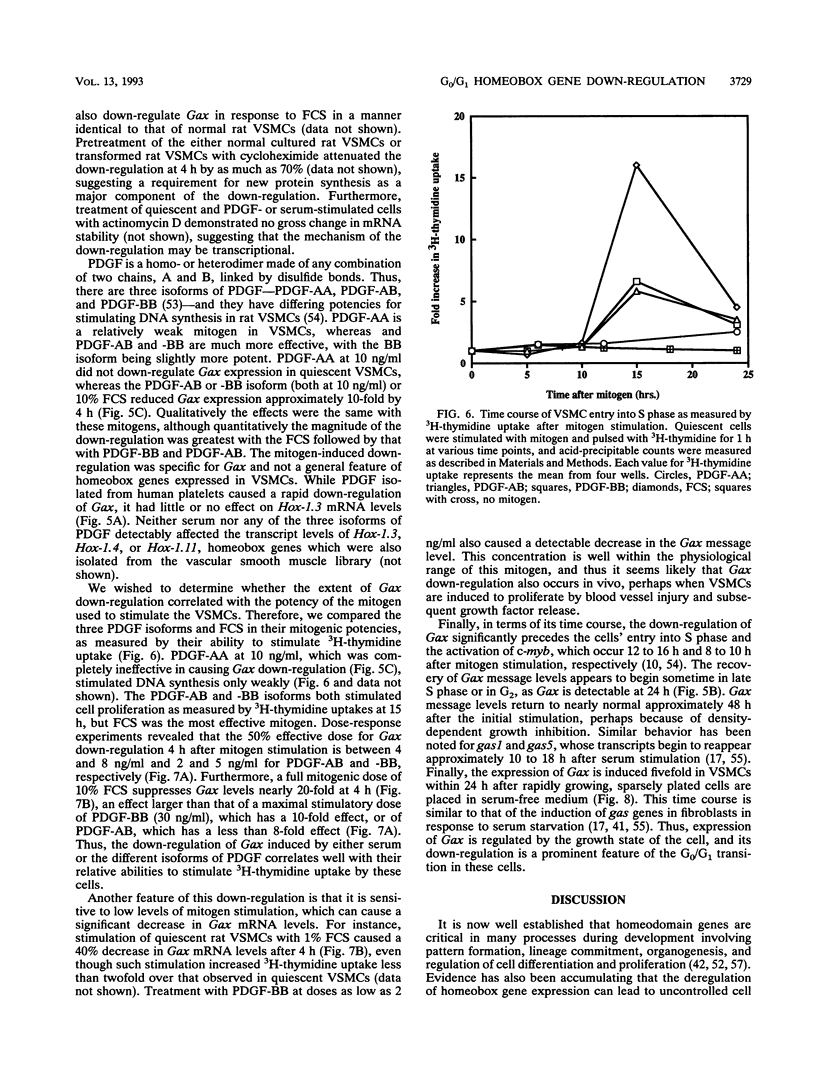
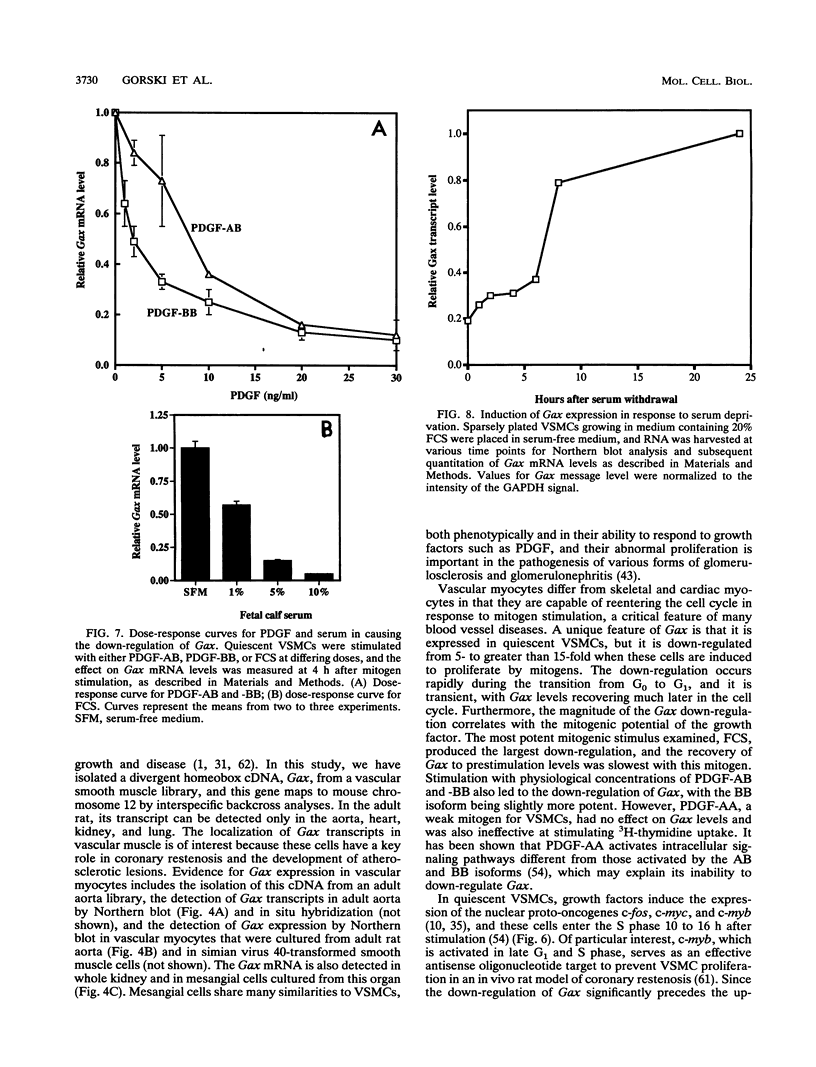
Adult vascular smooth muscle cells dedifferentiate and reenter the cell cycle in response to growth factor stimulation. Here we describe the molecular cloning from vascular smooth muscle, the structure, and the chromosomal location of a diverged homeobox gene, Gax, whose expression is largely confined to the cardiovascular tissues of the adult. In quiescent adult rat vascular smooth muscle cells, Gax mRNA levels are down-regulated as much as 15-fold within 2 h when these cells are induced to proliferate with platelet-derived growth factor (PDGF) or serum growth factors. This reduction in Gax mRNA is transient, with levels beginning to rise between 8 and 24 h after mitogen stimulation and returning to near normal by 24 to 48 h. The Gax down-regulation is dose dependent and can be correlated with the mitogen's ability to stimulate DNA synthesis. PDGF-AA, a weak mitogen for rat vascular smooth muscle cells, did not affect Gax transcript levels, while PDGF-AB and -BB, potent mitogens for these cells, were nearly as effective as fetal bovine serum. The removal of serum from growing cells induced Gax expression fivefold within 24 h. These data suggest that Gax is likely to have a regulatory function in the G0-to-G1 transition of the cell cycle in vascular smooth muscle cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberdam D., Negreanu V., Sachs L., Blatt C. The oncogenic potential of an activated Hox-2.4 homeobox gene in mouse fibroblasts. Mol Cell Biol. 1991 Jan;11(1):554–557. doi: 10.1128/mcb.11.1.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acampora D., D'Esposito M., Faiella A., Pannese M., Migliaccio E., Morelli F., Stornaiuolo A., Nigro V., Simeone A., Boncinelli E. The human HOX gene family. Nucleic Acids Res. 1989 Dec 25;17(24):10385–10402. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Avraham K. B., Prezioso V. R., Chen W. S., Lai E., Sladek F. M., Zhong W., Darnell J. E., Jr, Jenkins N. A., Copeland N. G. Murine chromosomal location of four hepatocyte-enriched transcription factors: HNF-3 alpha, HNF-3 beta, HNF-3 gamma, and HNF-4. Genomics. 1992 Jun;13(2):264–268. doi: 10.1016/0888-7543(92)90241-j. [DOI] [PubMed] [Google Scholar]
- Barad M., Jack T., Chadwick R., McGinnis W. A novel, tissue-specific, Drosophila homeobox gene. EMBO J. 1988 Jul;7(7):2151–2161. doi: 10.1002/j.1460-2075.1988.tb03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank R. S., Owens G. K. Platelet-derived growth factor regulates actin isoform expression and growth state in cultured rat aortic smooth muscle cells. J Cell Physiol. 1990 Mar;142(3):635–642. doi: 10.1002/jcp.1041420325. [DOI] [PubMed] [Google Scholar]
- Blank R. S., Thompson M. M., Owens G. K. Cell cycle versus density dependence of smooth muscle alpha actin expression in cultured rat aortic smooth muscle cells. J Cell Biol. 1988 Jul;107(1):299–306. doi: 10.1083/jcb.107.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Bottega S., Schneider C. Gas2, a growth arrest-specific protein, is a component of the microfilament network system. J Cell Biol. 1992 Jun;117(6):1251–1261. doi: 10.1083/jcb.117.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. E., Kindy M. S., Sonenshein G. E. Expression of the c-myb proto-oncogene in bovine vascular smooth muscle cells. J Biol Chem. 1992 Mar 5;267(7):4625–4630. [PubMed] [Google Scholar]
- Campan M., Desgranges C., Gadeau A. P., Millet D., Belloc F. Cell cycle dependent gene expression in quiescent stimulated and asynchronously cycling arterial smooth muscle cells in culture. J Cell Physiol. 1992 Mar;150(3):493–500. doi: 10.1002/jcp.1041500309. [DOI] [PubMed] [Google Scholar]
- Candia A. F., Hu J., Crosby J., Lalley P. A., Noden D., Nadeau J. H., Wright C. V. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992 Dec;116(4):1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Theill L. E., Karin M. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science. 1991 Jul 12;253(5016):197–199. doi: 10.1126/science.1677216. [DOI] [PubMed] [Google Scholar]
- Cho K. W., De Robertis E. M. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990 Nov;4(11):1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli C., Philipson L., Sorrentino V. Regulation of expression of growth arrest-specific genes in mouse fibroblasts. Mol Cell Biol. 1990 Apr;10(4):1525–1529. doi: 10.1128/mcb.10.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia E. M., Cicala C., Charlesworth A., Ciccarelli C., Rossi G. B., Philipson L., Sorrentino V. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol. 1992 Aug;12(8):3514–3521. doi: 10.1128/mcb.12.8.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991 Apr;7(4):113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Cribbs D. L., Pultz M. A., Johnson D., Mazzulla M., Kaufman T. C. Structural complexity and evolutionary conservation of the Drosophila homeotic gene proboscipedia. EMBO J. 1992 Apr;11(4):1437–1449. doi: 10.1002/j.1460-2075.1992.tb05188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992 Aug;115(4):1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Moroney J. F., Wilson G. L., Fox C. H., Winter H. S., Kehrl J. H. Cloning of a human homeobox gene that resembles a diverged Drosophila homeobox gene and is expressed in activated lymphocytes. New Biol. 1991 Apr;3(4):353–363. [PubMed] [Google Scholar]
- Del Sal G., Ruaro M. E., Philipson L., Schneider C. The growth arrest-specific gene, gas1, is involved in growth suppression. Cell. 1992 Aug 21;70(4):595–607. doi: 10.1016/0092-8674(92)90429-g. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Rubbia-Brandt L., Gabbiani G. Modulation of actin isoform expression in cultured arterial smooth muscle cells by heparin and culture conditions. Arterioscler Thromb. 1991 Mar-Apr;11(2):244–253. doi: 10.1161/01.atv.11.2.244. [DOI] [PubMed] [Google Scholar]
- Falzon M., Chung S. Y. The expression of rat homeobox-containing genes is developmentally regulated and tissue specific. Development. 1988 Jul;103(3):601–610. doi: 10.1242/dev.103.3.601. [DOI] [PubMed] [Google Scholar]
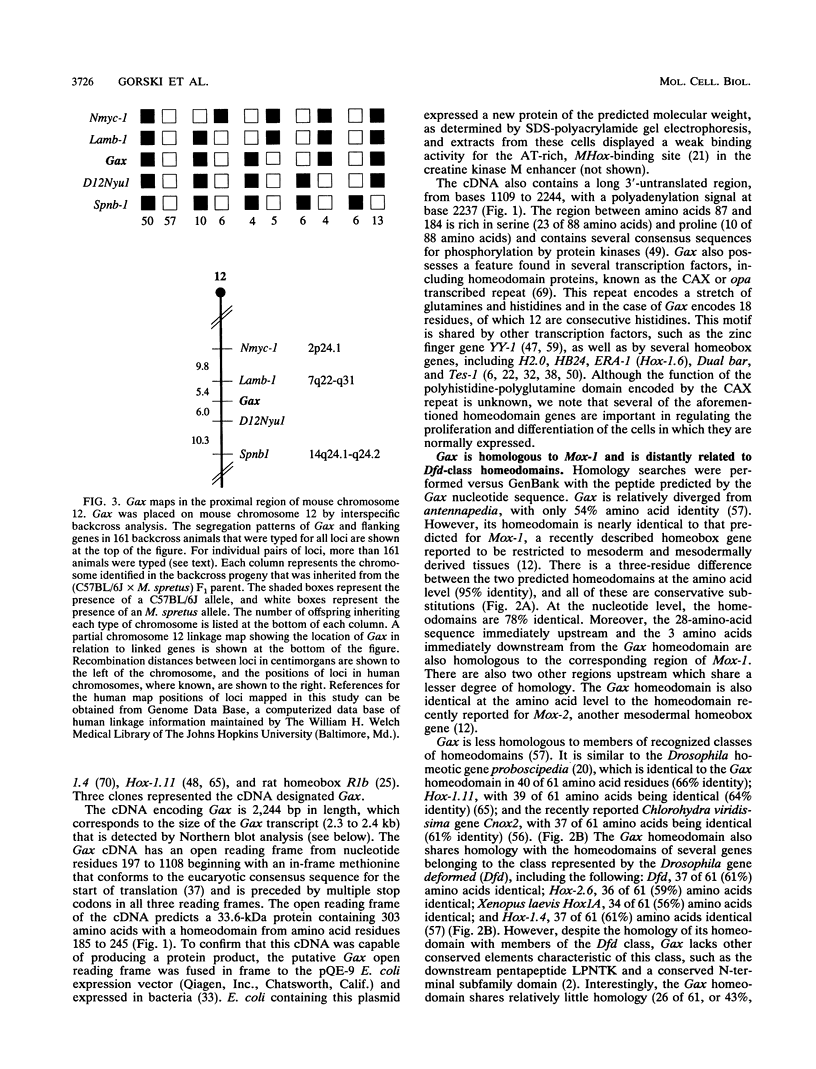
- Fibi M., Zink B., Kessel M., Colberg-Poley A. M., Labeit S., Lehrach H., Gruss P. Coding sequence and expression of the homeobox gene Hox 1.3. Development. 1988 Feb;102(2):349–359. doi: 10.1242/dev.102.2.349. [DOI] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueneberg D. A., Natesan S., Alexandre C., Gilman M. Z. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992 Aug 21;257(5073):1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Hatano M., Roberts C. W., Minden M., Crist W. M., Korsmeyer S. J. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science. 1991 Jul 5;253(5015):79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- Higashijima S., Kojima T., Michiue T., Ishimaru S., Emori Y., Saigo K. Dual Bar homeo box genes of Drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes Dev. 1992 Jan;6(1):50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindy M. S., Sonenshein G. E. Regulation of oncogene expression in cultured aortic smooth muscle cells. Post-transcriptional control of c-myc mRNA. J Biol Chem. 1986 Sep 25;261(27):12865–12868. [PubMed] [Google Scholar]
- Koch W. J., Ellinor P. T., Schwartz A. cDNA cloning of a dihydropyridine-sensitive calcium channel from rat aorta. Evidence for the existence of alternatively spliced forms. J Biol Chem. 1990 Oct 15;265(29):17786–17791. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader S. L. Influence of animal age on the beta-adrenergic system in cultured rat aortic and mesenteric artery smooth muscle cells. J Gerontol. 1992 Mar;47(2):B32–B36. doi: 10.1093/geronj/47.2.b32. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Manfioletti G., Ruaro M. E., Del Sal G., Philipson L., Schneider C. A growth arrest-specific (gas) gene codes for a membrane protein. Mol Cell Biol. 1990 Jun;10(6):2924–2930. doi: 10.1128/mcb.10.6.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mené P., Simonson M. S., Dunn M. J. Physiology of the mesangial cell. Physiol Rev. 1989 Oct;69(4):1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Müller M. M., Ruppert S., Schaffner W., Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. doi: 10.1038/336544a0. [DOI] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C. V., Gorski D. H., LePage D. F., Lincecum J., Walsh K. Molecular cloning of a homeobox transcription factor from adult aortic smooth muscle. J Biol Chem. 1992 Dec 25;267(36):26085–26090. [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Porteus M. H., Bulfone A., Ciaranello R. D., Rubenstein J. L. Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron. 1991 Aug;7(2):221–229. doi: 10.1016/0896-6273(91)90260-7. [DOI] [PubMed] [Google Scholar]
- Reilly C. F. Rat vascular smooth muscle cells immortalized with SV40 large T antigen possess defined smooth muscle cell characteristics including growth inhibition by heparin. J Cell Physiol. 1990 Feb;142(2):342–351. doi: 10.1002/jcp.1041420217. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G. POU-domain transcription factors: pou-er-ful developmental regulators. Genes Dev. 1991 Jun;5(6):897–907. doi: 10.1101/gad.5.6.897. [DOI] [PubMed] [Google Scholar]
- Ross R., Bowen-Pope D. F., Raines E. W. Platelet-derived growth factor and its role in health and disease. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):155–169. doi: 10.1098/rstb.1990.0051. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Interaction between peptide growth factors and homoeobox genes in the establishment of antero-posterior polarity in frog embryos. Nature. 1989 Sep 7;341(6237):33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- Sachinidis A., Locher R., Vetter W., Tatje D., Hoppe J. Different effects of platelet-derived growth factor isoforms on rat vascular smooth muscle cells. J Biol Chem. 1990 Jun 25;265(18):10238–10243. [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Schummer M., Scheurlen I., Schaller C., Galliot B. HOM/HOX homeobox genes are present in hydra (Chlorohydra viridissima) and are differentially expressed during regeneration. EMBO J. 1992 May;11(5):1815–1823. doi: 10.1002/j.1460-2075.1992.tb05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Nigro V., Faiella A., D'Esposito M., Stornaiuolo A., Mavilio F., Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991 Mar;33(3):215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Song K., Wang Y., Sassoon D. Expression of Hox-7.1 in myoblasts inhibits terminal differentiation and induces cell transformation. Nature. 1992 Dec 3;360(6403):477–481. doi: 10.1038/360477a0. [DOI] [PubMed] [Google Scholar]
- Spreyer P., Kuhn G., Hanemann C. O., Gillen C., Schaal H., Kuhn R., Lemke G., Müller H. W. Axon-regulated expression of a Schwann cell transcript that is homologous to a 'growth arrest-specific' gene. EMBO J. 1991 Dec;10(12):3661–3668. doi: 10.1002/j.1460-2075.1991.tb04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. H., Copeland N. G., Jenkins N. A., Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991 Nov;11(11):5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D. P., Ferrante J., Nazarali A., Shao X., Kozak C. A., Guo V., Nirenberg M. Murine Hox-1.11 homeobox gene structure and expression. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6280–6284. doi: 10.1073/pnas.89.14.6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Welcher A. A., Suter U., De Leon M., Snipes G. J., Shooter E. M. A myelin protein is encoded by the homologue of a growth arrest-specific gene. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7195–7199. doi: 10.1073/pnas.88.16.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Viviano C. M., Gizang-Ginsberg E., Frohman M. A., Joyner A. L., Martin G. R. Differential expression of the mouse homeobox-containing gene Hox-1.4 during male germ cell differentiation and embryonic development. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5813–5817. doi: 10.1073/pnas.84.16.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]