Summary
Caloric restriction (CR) retards aging in laboratory rodents. No information is available on the effects of long-term CR on physiologic markers of aging and longevity in humans. Heart rate variability (HRV) is a marker for cardiac autonomic functioning. The progressive decline in HRV with aging and the association of higher HRV with better health outcomes are well established. Heart rate variability assessment is a reliable tool by which the effects of CR on autonomic function can be assessed. Time- and frequency-domain analyses compared 24-h HRV in 22 CR individuals aged 35–82 years and 20 age-matched controls eating Western diets (WD). The CR group was significantly leaner than the WD group. Heart rate was significantly lower, and virtually, all HRV values were significantly higher in the CR group than in the WD group (P < 0.002). Heart rate variability in the CR individuals was comparable with published norms for healthy individuals 20 years younger. In addition, when differences in HRAUTHOR: Please define HR. and HRV between CR and WD were compared with previously published changes in HRV induced in healthy adults given atenolol, percent differences in each measure were generally similar in direction and magnitude and suggested declines in sympathetic and increases in parasympathetic modulation of HR and increased circadian variability associated with CR. These findings provide evidence that CR has direct systemic effects that counter the expected age-associated changes in autonomic function so that HRV indexes in CR individuals are similar to those of individuals 20 years younger eating WDs.
Keywords: calorie restriction, heart rate variability, autonomic function, parasympathetic function, aging, cardiovascular health
Introduction
Caloric restriction without malnutrition (CR) slows aging, increases maximal life span, and protects against stress in many model organisms including yeast, worms, flies, mice, and rats (Weindruch & Walford, 1988; Fontana et al., 2010a,b)AUTHOR: Fontana et al., 2010 has been changed to Fontana et al., 2010a, 2010b so that this citation matches the Reference List. Please confirm that this is correct.. However, little is known regarding the long-term effects of CR on the age-associated deterioration of physiological parameters in humans. The autonomic nervous system (ANS) plays a key role in controlling and coordinating several important physiological functions (Llewellyn-Smith & Verberne, 2011).
Aging is associated with increasing homeostatic imbalance, and autonomic function is altered with aging (Pfeifer et al., 1983; Cowen, 1993) resulting in a progressive decline in heart rate variability (HRV), which is a well-accepted index of ANS function (O’Brien et al., 1986; Ferrari et al., 1991; Schwartz et al., 1991; Umetani et al., 1998; Zulfiqar et al., 2010). Heart rate variability is a composite reflection of the interactions among multiple physiologic systems (e.g., autonomic outflow and inflow and neuroendocrine function) at the cellular, tissue, and organ level. Heart rate variability decreases in various disease states, including heart disease, hypertension, obesity, and inflammatory disease, and higher HRV is generally associated with global health (Dekker et al., 1997; De Meersman & Stein, 2007). However, HRV also decreases progressively with age in normal, healthy individuals (O’Brien et al., 1986; Schwartz et al., 1991; Umetani et al., 1998; De Meersman & Stein, 2007; Zulfiqar et al., 2010). This has led to the suggestion that HRV could serve as a marker of biological age, as opposed to chronological age (Corino et al., 2007).
In rodents, long-term CR has been shown to improve autonomic function and, in particular, to increase the high-frequency component of the HRV spectrum, a marker for a respiration-mediated parasympathetic activity (Herlihy et al., 1992; Cowen et al., 2000; Mager et al., 2006). The purpose of the present study was to investigate whether CR counters the age-related decline in HRV in individuals who have been practicing long-term, strict CR. To this end, we compared indexes of HRV obtained from 24-h Holter recordings in the CR with those of age-matched, healthy individuals eating usual Western diets (WD). To further put these findings into perspective, we compared HRV measures in the CR group with published data on normative values for HRV at different ages (Umetani et al., 1998). We also compared differences in HRV between CR and WD with published changes in HRV induced by the administration of atenolol in healthy young adults (Cook et al., 1991).
Results
Study population characteristics
Clinical, demographic, and laboratory characteristics for the entire subject sample are summarized in Table 1. Age and gender were not different, but body mass index was significantly lower in the CR than the WD group (Table 1). Total body fat, measured by dual-energy X-ray absorptiometry, was also much lower in CR than in the WD group (Table 1).
Table 1.
Clinical and Demographic Characteristics of Study Population
| CR (n=22) μ±SD |
WD (n=20) μ±SD |
p-value | |
|---|---|---|---|
| Age (years) | 51.5±10.8 | 52±8.9 | NS |
| Gender (M/F) | 18/4 | 16/4 | NS |
| Height (m) | 1.74±0.1 | 1.79±0.1 | NS |
| Weight (kg) | 57±5.9 | 80.1±13.4 | <0.001 |
| Body mass index (kg/m2) | 18.8±1.1 | 25±3.1 | <0.001 |
| Lean mass (kg) | 49±6.7 | 56.8±11.3 | 0.009 |
| Total body fat (%) | 9.9±4.7 | 24.3±8.7 | <0.001 |
| Systolic blood pressure (mmHg) | 99±9 | 125±12 | <0.001 |
| Diastolic blood pressure (mmHg) | 61±6 | 79±10 | <0.001 |
| Total cholesterol (mg/dl) | 164±35 | 196±42 | 0.003 |
| High-density lipoprotein cholesterol (mg/dl) |
63±18 | 53±14 | NS |
| Total cholesterol/high-density lipoprotein cholesterol ratio |
2.7±0.5 | 4.0±1.2 | <0.001 |
Cardiometabolic risk factors
Serum total cholesterol and high-density lipoprotein cholesterol concentrations of the WD group (Table 1) fell close to the 50th percentile for people in their age group in the Unites States (National Heart, Lung, and Blood Institute, 2001). In contrast, the average serum total cholesterol concentrations of the CR group fell into the lowest 10% for people in their age group (National Heart, Lung, and Blood Institute, 2001) (Table 1). Unlike the decrease in high-density lipoprotein cholesterol that often occurs when individuals are placed on low-fat diets to lose weight, the CR group had high levels, in the 85th to 90th percentile range for middle-aged men in the United States. (National Heart, Lung, and Blood Institute, 2001) (Table 1). As a consequence, their total cholesterol/high-density lipoprotein cholesterol ratio was remarkably low. Blood pressure for the WD (Table 1) was also similar to the average values found in middle-aged healthy people in the United States (Burt et al., 1995; National Institute of Diabetes and Digestive and Kidney Diseases, 1995); however, both systolic and diastolic blood pressures were significantly lower in the CR group (P < 0.001) and fell into the range found in 10-year olds (Williams et al., 2002) (Table 1). Serum total cholesterol and high-density lipoprotein cholesterol concentrations and blood pressure levels of some of our CR subjects have been previously reported (Fontana et al., 2004).
Nutrient intake
Caloric restriction subjects consumed a variety of nutrient-dense unprocessed foods (i.e., vegetables, fruits, nuts, egg whites, fish, poultry, low-fat dairy products, whole grains, and beans) that supplied > 100% of the recommended daily intake for all essential nutrients. Refined foods rich in empty calories, trans-fatty acids, and salt were avoided. Energy intake was 30% lower in CR (1765 ± 328 kcal day−1) than in WD (2528 ± 463 kcal day−1) (P < 0.001). The percentage of total energy intake derived from protein, carbohydrate, fat, and alcohol was: 22%, 50%, 28%, and 0.1%, respectively, in the CR and 16%, 48%, 32%, and 4% in the WD.
Twenty-four-hour, daytime and nighttime HR, and 24-h heart rate variability analysis
All subjects were in normal sinus rhythm and therefore had recordings eligible for HRV analysis. N = 22 recordings for CR and 20 for WD were obtained. N = 21 recordings for CR and 20 recording for WD were eligible for 24-h time-domain HRV analysis. For the frequency-domain analysis, 20 CR and 18 WD had eligible 24-h data. Criteria for electrocardiogram data quality sufficient for 24-h time-domain HRV analysis and for frequency-domain analysis have been described in the Experimental procedures.
Table 2 compares 24-h, daytime and nighttime heart rate, and 24-h HRV measures between CR and WD. Twenty-four-hour, daytime, and nighttime heart rates were significantly lower in CR than in WD (P < 0.001). At the same time, virtually all 24-h HRV measures were significantly higher (P < 0.005) in CR vs. WD. The exception was low-frequency power where the difference in ln-transformed measures was of borderline significance (P = 0.06). Differences in the ratio of HRV measures were smaller between groups but remained significant (P < 0.02).
Table 2.
Comparison of heart rate and 24-hr HRV between long-term CR and WD
| CR μ±SD |
WD μ±SD |
p-value | |
|---|---|---|---|
| Average HR for 24 hr (bpm) | 57±6 | 76±9 | < 0.001 |
| Average daytime HR (bpm) | 61±7 | 81±10 | < 0.001 |
| Average nighttime HR (bpm) | 50±5 | 67±10 | < 0.001 |
| Maximum instantaneous HR (bpm) | 110±22 | 129±14 | 0.001 |
| Minimum instantaneous HR (bpm) | 37±4 | 45±10 | 0.002 |
| SDNN (ms) | 168±26 | 128±46 | 0.001 |
| SDANN (ms) | 149±20 | 119±38 | 0.005 |
| SDNNIDX (ms) | 67±15 | 49±15 | <0.001 |
| rMSSD (ms) | 36±12 | 23±11 | 0.001 |
| pNN50 (%) | 12±8 | 5±5 | 0.001 |
| Total power (ms2, Median[IQR]) | 26764[9456] | 14578[16665] | |
| Ln total power | 10.2±0.3 | 9.6±0.6 | 0.002 |
| Very-low-frequency power (ms2, Median[IQR]) | 2971[1709] | 1138[1111] | |
| Ln very-low-frequency power | 7.8±0.4 | 7.1±0.6 | <0.001 |
| Low-frequency power (ms2, Median[IQR]) | 986[1026] | 508[652] | |
| Ln low-frequency power | 6.8±0.7 | 6.3±0.8 | 0.06 |
| High-frequency power (ms2, Median[IQR]) | 284 [232] | 110 [165] | |
| Ln high-frequency power | 5.7±0.7 | 4.9±1.0 | 0.004 |
| Normalized low-frequency power (%) | 64±9 | 71±9 | 0.020 |
| Normalized high-frequency power (%) | 26±7 | 20±9 | 0.017 |
Ln= natural logarithm; N-N= normal-to-normal RR intervals; 24-hr HR= 60,000/average N-N for 24-hrs in bpm; daytime HR= average HR between 08:00 and 20:00; nighttime HR= average HR between 00:00 and 06:00; min HR= 60,000/minimum instantaneous N-N; max HR= 60,000/maximum instantaneous N-N; SDNN= 24-hr standard deviation of N-N intervals in ms; SDANN=24-hr SD of 5-min-averaged N-N intervals; SDNNIDX= 24-hr average of SD of 5-min N-N intervals; rMSSD= root mean square successive difference of N-N intervals in ms; pNN50= percent of successive N-N intervals differences > 50 ms in %; total power= total spectral power of HRV; very-low-frequency power = 24-hr average of power between 0.003-0.04 Hz; low-frequency power (24-hr average of 5-min power between 0.04-0.15 Hz); high-frequency power (24-hr average of 5-min power between 0.15-0.4 Hz); normalized low-frequency power (24-hr average of the proportion of total power in each 5-min interval accounted for by the low-frequency component); normalized high-frequency power (24-hr average of the proportion of total power in each 5-min interval accounted for by the high-frequency component). Values are expressed as mean ± SD or median [IRQ].
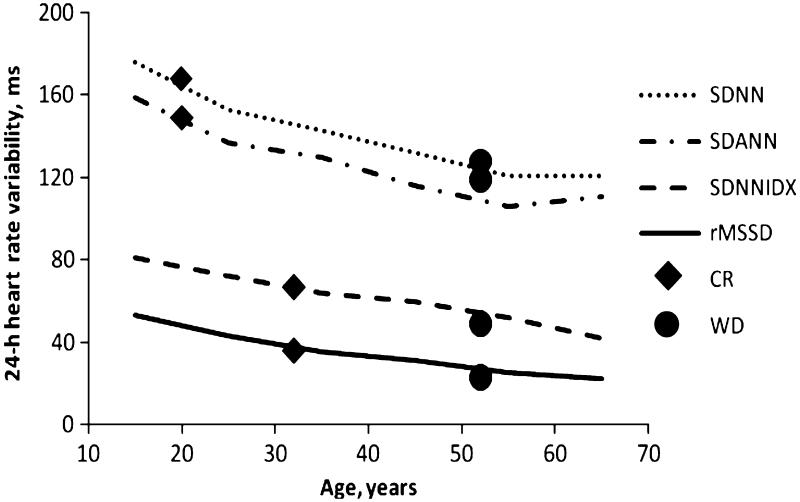
Comparison of caloric restriction-associated with age-related changes in heart rate variability Figure 1 shows plots of selected HRV measures by age taken from published norms (Umetani et al., 1998). Filled diamonds indicate mean values for CR, and filled circles indicate mean values for WD. As can be seen in the figure, mean 24-h standard deviation of normal-to-normal (N-N) intervals in ms (SDNN), 24-h SD of 5-min-averaged N-N intervals (SDANN), 24-h average of SD of 5-min N-N intervals (SDNNIDX), and root mean square successive difference of N-N intervals in ms (rMSSD) values of the CR group were similar to values found in healthy adults aged 20–30 year, whereas individuals in the WD group had HRV values consistent with published norms for their age (Umetani et al., 1998).
Fig. 1.
Mean heart rate variability (HRV) in caloric restriction (CR) (age, 51.5 ± 10.8 years) and Western diets (WD) (age, 52 ± 8.9 years) compared with previously published age-related norms. Curves show age-related norms for 24-h standard deviation of normal-to-normal (N-N) intervals in ms (SDNN), 24-h SD of 5-min-averaged N-N intervals (SDANN), 24-h average of SD of 5-min N-N intervals (SDNNIDX), and root mean square successive difference of N-N intervals in ms (rMSSD) (Umetani et al., 1998). Mean CR HRV values are indicated by filled diamonds and mean WD values by filled circles. See Table 2 legend for HRV definitions.
Comparison of heart rate variability differences between the caloric restriction and control groups with changes induced by beta-blockade
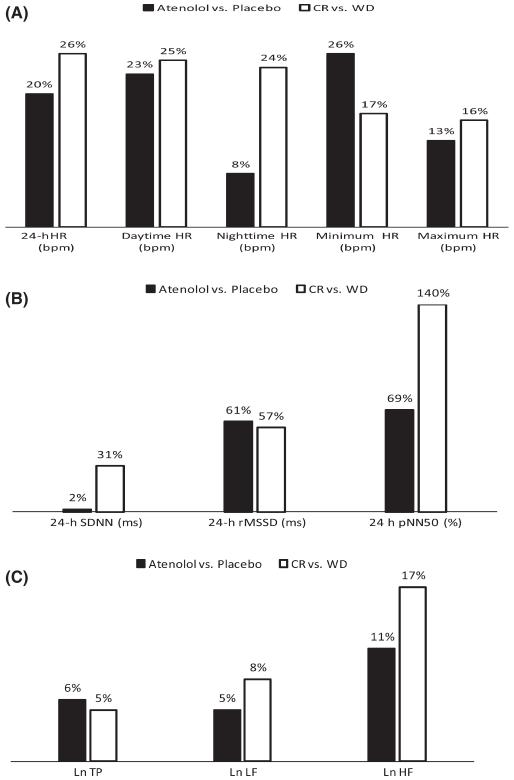
Cook et al. (1991) gave 50 mg of atenolol four times a day to 16 healthy adults (mean age 32 ± 7 years) in a randomized, double-blind, placebo-controlled trial to determine the effect of beta-blockade on 24-h Holter-based heart rate and HRV. We compared the percent change in these parameters induced by atenolol with percent differences in comparable HRV parameters between WD and CR. As can be seen in Fig. 2(A), the effect of atenolol on the heart rate was similar in magnitude and direction to differences between CR and WD, except for nighttime heart rate that was 8% lower than placebo with atenolol and 24% lower in the CR than in WD. Figure 2(B) compares the effect of atenolol administration on 24-h time-domain HRV with the difference in these parameters between WD and CR. Atenolol had no effect on SDNN, but SDNN in CR was 31% higher than in WD. Atenolol was associated with a 61% increase in rMSSD, similar to differences seen between CR and WD (57%). There was a 69% increase in percent of successive N-N intervals differences > 50 ms (pNN50) with atenolol administration and an almost doubled difference in CR (140%). Finally, in Fig. 2(C), increases in total power and low-frequency power appeared to be similar between atenolol and CR/WD, but, consistent with findings for rMSSD, difference in high-frequency power were greater for CR vs. WD than for atenolol vs. placebo.
Fig. 2.
(A) Comparison of the effect of atenolol vs. calorie restriction on heart rate. Comparison of % decreases in 24-h, daytime, and nighttime heart rate between atenolol and placebo and long-term caloric restriction (CR) vs. Western diets (WD). (B) Effect of atenolol vs. calorie restriction on time-domain heart rate variability (HRV). Comparison of % increases in time-domain HRV between atenolol vs. placebo and long-term CR vs. WD. SDNN, 24-hour standard deviation of normal-to-normal (N-N) intervals in ms; rMSSD, root mean square successive difference of N-N intervals in ms; pNN50, percent of successive N-N intervals differences > 50 ms in %. (C) Effect of atenolol vs. calorie restriction on frequency-domain HRV. Comparison of % increases in frequency-domain HRV between atenolol and placebo and long-term CR vs. WD. LnTP, normalized total power; LnLF, normalized low-frequency power; LnHF, normalized high-frequency power.
Discussion
Prospective data on the effects of long-term CR on ANS function, as assessed by 24-h HRV in healthy lean humans are not yet available. In this cross-sectional study, research-quality HRV assessment was performed in 22 men and women who had been on self-imposed CR for 3–15 years and compared with HRV in 20 age-matched healthy controls eating typical WD. Our study is the first, to our knowledge, to demonstrate that long-term CR individuals have markedly lower heart rate and a markedly better HRV profile than healthy people of the same age eating standard WD.
Caloric restriction has been shown to slow aging and prevent or delay several chronic diseases in rodents (Weindruch & Walford, 1988; Fontana et al., 2010a,b). Caloric restriction also protects against diabetes, cancer, and cardiovascular disease in Rhesus monkeys (Colman et al., 2009). In humans, CR is associated with metabolic changes that protect against these age-related pathologies and against left ventricular diastolic dysfunction (Fontana et al., 2004, 2006, 2010a,b; Meyer et al., 2006; Fontana & Klein, 2007; Cangemi et al., 2010; Soare et al., 2011). In particular, we have previously shown that long-term CR in humans protects against obesity, insulin resistance, hypertension, inflammation, and atherosclerosis and is associated with many of the same hormonal changes that are thought to mediate the anti-aging effects of CR in rodents (Fontana et al., 2004, 2006, 2010a,b; Meyer et al., 2006; Fontana & Klein, 2007; Cangemi et al., 2010; Soare et al., 2011).
Decreased HRV is associated with senescence in rodents, as well as in healthy humans (Pfeifer et al., 1983; O’Brien et al., 1986; Ferrari et al., 1991; Schwartz et al., 1991; Herlihy et al., 1992; Cowen, 1993; Umetani et al., 1998; Cowen et al., 2000; Mager et al., 2006; Corino et al., 2007; Zulfiqar et al., 2010). Mager et al. (2006) have shown that, in rats, several markers of ANS function were altered by CR. In particular, CR rats exhibited decreased low-frequency power in diastolic blood pressure variability and increased high-frequency power in HRV, suggesting that CR results in decreased sympathetic activity and augmented parasympathetic activity (Mager et al., 2006). Interestingly, the HRV differences we found in our CR subjects were similar to those found in long-lived CR rats, strongly suggesting that long-term CR also retards the age-associated deterioration of ANS function in humans.
Many bodily systems that deteriorate with aging (e.g., cardiovascular, gastrointestinal and neuroendocrine functions, regulation of body temperature, energy homeostasis, metabolism, and tissue defense) are dependent on the healthy functioning of the ANS (Llewellyn-Smith & Verberne, 2011). Novel insights into how long-term CR affects ANS function in healthy human individuals are provided by our study. First, CR individuals demonstrated profoundly increased parasympathetic activity as measured by rMSSD and high-frequency power. Moreover, lower levels of vagally modulated HRV have been demonstrated to be associated with increased inflammation and cardiovascular morbidity and mortality in the elderly (Tsuji et al., 1996; Dekker et al., 1997). In contrast, activation of the parasympathetic nervous system, through stimulation of the efferent vagus nerve, decreases systemic inflammation in several experimental models of acute systemic inflammation (Tracey, 2007). Furthermore, predicted values of rMSSD and pNN50 by age developed by Umetani et al. (1998) indicate that rMSSD and pNN50 levels found in the CR group would be expected to be seen in someone under the age of thirty, whereas rMSSD and pNN50 in the WD group was consistent with their being in the mid-fifties, which they were. Thus, as CR is associated with levels of ANS functioning seen in a younger cohort, and there is no evidence that individuals who choose CR already have better ANS function, our results suggest that CR reverses the age-associated decline in ANS function.
Atenolol is a beta-blocker and has been shown to both reduce sympathetic and increase relative parasympathetic control of heart rate. The HRV changes associated with atenolol administration, as shown by Cook et al. (1991), are consistent with these autonomic effects. Our data suggest that CR is associated with very similar autonomic effect on heart rate, because the direction of heart rate and HRV changes are generally consisted with those associated with atenolol administration. Interestingly, CR appears to be associated with even greater reduction in nighttime heart rate and greater increase in measures of parasympathetic activity, despite the study population being, on average, 20 years older.
In conclusion, results of this study on subjects following a strict CR diet for an average of 7 years provide the first evidence in humans that long-term CR is associated with similar effect to those already documented in animal studies, that is, better autonomic function than in matched controls. Our data suggest that long-term may, as has been show in animals, attenuate the deterioration of multiple HRV parameters associated with primary aging in humans. Prospective studies are needed to verify the causal relationship of CR with these markers. Findings would need to be verified in different age groups and in those with established clinical cardiovascular disease risk factors. In addition, the dose-response relationships for the anti-aging benefits of CR would be of great clinical interest.
Experimental procedures
Study participants
Twenty-two individuals practicing long-term CR for an average of 7 years (3–15 years) were recruited, mainly through the Caloric Restriction Society. Two were from the St. Louis area and the others came to the Washington University Medical Center from other cities in the United States, Canada, and UK. Their motivation for practicing CR is the strong desire to live as long as possible in good health and the belief, based on the findings on CR rodents and other species, that CR will markedly prolong their healthy life span. Their average age was 51.5 ± 10.8 years (range 35–82 years). Four CR subjects were women. Twenty individuals eating a conventional US diet, matched with the CR group in terms of age, gender, and socioeconomic status, were the comparison group. None of the subjects had evidence of chronic diseases, including cardiovascular, lung, gastrointestinal and autoimmune diseases, type 2 diabetes or cancer. None were smokers. In addition, none of the subjects were taking lipid-lowering or antihypertensive agents, or other medications that could have affected cardiometabolic or HRV measures. Subjects were enrolled after undergoing a physical examination, medical history, and laboratory evaluation that revealed no evidence of any health problems. All study participants were weight stable (i.e., < 2 kg weight change in the preceding 6 months) and did not perform more than 20 min of vigorous exercise twice per week. This study was approved by the Human Studies Committee of Washington University School of Medicine, and all subjects gave informed consent before their participation.
Anthropometrics and body composition
Height was measured without shoes to the nearest 0.1 cm. Body weight was obtained on a balance scale in the morning after a 12-h fast. Body mass index was calculated by dividing body weight (in kograms) by the square of height (in meters). Total body fat mass and fat-free mass were determined by dual-energy X-ray absorptiometry (QDR 1000/w; Hologic, Waltham, MA, USA).
Cardiometabolic risk factors
Blood pressure was measured with a mercury sphygmomanometer, with the participant in the sitting position after 5 min of rest in a quiet environment, according to the recommendations of the American Hypertension Society. Four measurements of systolic and diastolic blood pressure were made at ≈5-min intervals and averaged. A venous blood sample was taken to determine lipid concentrations after subjects had fasted for at least 12 h. Measurement of serum lipid concentrations was performed in the Core Laboratory for Clinical Studies at Washington University. Total cholesterol was measured by automated enzymatic commercial kits (Miles/Technicon, Tarrytown, NY, USA). High-density lipoprotein cholesterol was measured in plasma after precipitation of apolipoprotein B-containing lipoproteins by dextrane sulfate (50 000 molecular weight) and magnesium (Warnick et al., 1982).
Dietary assessment
Subjects were instructed by a research dietician to record all food and beverages consumed, preparation methods, and approximate portion sizes for seven consecutive days. To assist with portion size determinations, measuring spoon and cup sets were provided to all participants, and all food diaries had a ruler imprinted on the back cover. Food records were analyzed by using the Nutrition Data System for Research programAUTHOR: Please give manufacturer information for ‘Nutrition Data System for Research program’: company name, town, state (if USA), and country. (version 4.03_31) from the Nutrition Coordinating Center at the University of Minnesota.
Heart rate variability data collection
All subjects underwent 24-h ambulatory Holter electrocardiogram monitoring. Electrocardigrams were recorded on DMS 300-3M digital Holter recorders at a sampling rate of 250 Hz and downloaded to a personal computer for analysis using cardio scan software (V12.0; DMS Holter, Stateside, NV, USA). Recordings were processed at the Washington University School of Medicine Heart Rate Variability Laboratory. After the scanner automatically detected and labeled all QRSAUTHOR: Please define QRS. complexes, beat labels were edited using standard research Holter analysis procedures. The cardio scan software also displayed a beat-by-beat heart rate tachogram, and clicking on a specific segment brought up the associated electrocardiogram strip. This permitted detection of missed beats, missed ectopic beats, and other outliers that could then be corrected. All Holter analyses were reviewed in detail by PKS and AS with special attention paid to ensuring that only N-N beats with uniformly detected onsets were included in the HRV analysis. The longest and shortest true N-N intervals were identified for each recording and intervals outside of these limits, including blocked atrial premature contractions as well as ectopic or inserted beats, were excluded from all calculations. After editing, the labeled QRS data stream was transferred to a Sun workstation (Sun Microsystems, Palo Alto, CA, USA) for HRV analysis. For recording to be accepted for time-domain analysis (which is less sensitive to missing data), ≥ 18 h of data with at least 50% N-N interbeat intervals in each 5-min segment were required. For a recording to be used for frequency-domain analyses, a ≥ 80% N-N interval in each 5-min segment was required. Heart rate and HRV were calculated for the entire recording, for daytime (08:00–20:00) and nighttime (00:00–06:00).
Time-domain analysis of heart rate variability
Time-domain indices of HRV are derived from statistical calculations performed on the set of N-N intervals. A detailed definition for the HRV indices tested in the analysis can be found in legend for Table 2. Average heart rate was computed from N-N intervals only. Standard deviation of normal-to-normal (N-N) intervals in ms and SDANN are primarily influenced by circadian rhythms (Kleiger et al., 1992). SDNNIDX reflects intermediate-term HRV. Short-term HRV indices like pNN50 and rMSSD reflect beat-to-beat changes in heart rate, mediated by changes in parasympathetic activity (Kleiger et al., 1992).
Frequency-domain analysis of normal-to-normal intervals
Frequency-domain or power spectral analysis partitions the variance in heart rate (i.e., length of N-N interbeat intervals) signal into underlying frequencies. A full definition for the frequency-domain HRV measures tested in this study will be found in the Legend for Table 2. Total power is the sum of the variance of all of the components (Kleiger et al., 2005). Very-low-frequency power quantifies the variance in heart rate occurring at cycles of 25-s to 5 min and is unaffected by beta-blockade and abolished by atropine, suggesting that it reflects parasympathetic control of heart rate (Taylor et al., 1998). In addition, it is affected by angiotensin-converting enzyme inhibition, suggesting an influence of the renin-angiotensin system (Taylor et al., 1998). Thermoregulatory and peripheral vasomotor influences have also been suggested (Akselrod et al., 1985; Pomeranz et al., 1985). In addition, ratio measures of HRV, normalized low- and normalized high-frequency power, which have been suggested as rough surrogates of autonomic balance, were compared between groups (Pomeranz et al., 1985).
Statistical analysis
T-tests compared clinical and demographic factors, heart rate, and HRV between CR and WD. Frequency-domain HRV measures are highly skewed and are generally ln transformed to permit parametric comparisons and that was done in this case. A P < 0.05 was considered statistically significant. International Business Machines, statistical package for the social science 19 (SPSS, Inc, Chicago, IL, USA) software was used for these analyses.
Daytime HR, average HR between 08:00 and 20:00; Ln, natural logarithm; min HR, 60 000/minimum instantaneous N-N; max HR, 60 000/maximum instantaneous N-N; N-N, normal-to-normal RR intervals; nighttime HR, average HR between 00:00 and 06:00; pNN50, percent of successive N-N intervals differences > 50 ms in %; rMSSD, root mean square successive difference of N-N intervals in ms; SDNN, 24-h standard deviation of N-N intervals in ms; SDANN, 24-h SD of 5-min-averaged N-N intervals; SDNNIDX, 24-h average of SD of 5-min N-N intervals; total power, total spectral power of HRV; very-low-frequency power, 24-h average of power between 0.003 and 0.04 Hz; 24-h HR, 60 000/average N-N for 24-h in bpm.
Low-frequency power (24-h average of 5-min power between 0.04 and 0.15 Hz); high-frequency power (24-h average of 5-min power between 0.15 and 0.4 Hz); normalized low-frequency power (24-h average of the proportion of total power in each 5-min interval accounted for by the low-frequency component); normalized high-frequency power (24-h average of the proportion of total power in each 5-min interval accounted for by the high-frequency component).
Values are expressed as mean ± SD or median [IRQ].
Acknowledgments
Supported by grants from the National Center for Research Resources (UL1 RR024992; a component of the National Institutes of Health and NIH Roadmap for Medical Research), the National Institute of Diabetes And Digestive And Kidney Diseases (P30DK056341), the Longer Life Foundation (an RGA/Washington University Partnership), and a donation from the Bakewell Foundation and the Scott and Annie Appleby Charitable Trust. The funding agencies had no role in the analysis or interpretation of the data or in the decision to submit the report for publicationAUTHOR: Please check text under the heading ‘Acknowledgments’..
Footnotes
Financial disclosures
None.
Author contributions
P. K. Stein participated in the concept, design, implementation of the study, analysis and interpretation of data, and undertook plausibility testing, drafted the report, and has seen and approved the final version. A. Soare participated in the implementation of the study, analysis and interpretation of data, helped in undertaking plausibility testing, in the drafting of the report, and has seen and approved the final version. T. E. Meyer participated in the design, implementation of the study, in the revision of the manuscript, and has seen and approved the final version. R. Cangemi participated in the implementation of the study, analysis and interpretation of the data, in the revision of the manuscript, and has seen and approved the final version. J. O. Holloszy participated in the concept and design of the study, drafting of the report, and has seen and approved the final version. L. Fontana participated in the concept, design, implementation of the study, analysis and interpretation of the data, drafted the report, and has seen and approved the final version.
References
- Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am. J. Physiol. 1985;249:H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Cangemi R, Friedmann AJ, Holloszy JO, Fontana L. Effects of long-term calorie restriction on serum sex hormones concentration in men. Aging Cell. 2010;9:236–242. doi: 10.1111/j.1474-9726.2010.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kostmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JR, Bigger JT, Kleiger RE, Fleiss JL, Steinman RC, Rolnitzky LM. Effect of atenolol and diltiazem on heart period variability in normal persons. J Am Coll Cardiol. 1991;17:480–484. doi: 10.1016/s0735-1097(10)80119-6. [DOI] [PubMed] [Google Scholar]
- Corino VD, Matteucci M, Mainardi LT. Analysis of heart rate variability to predict patient age in a healthy population. Methods Inf. Med. 2007;46:191–195. [PubMed] [Google Scholar]
- Cowen T. Ageing in the autonomic nervous system: a result of nerve-target interactions? a review. Mech. Ageing Dev. 1993;68:163–173. doi: 10.1016/0047-6374(93)90148-k. [DOI] [PubMed] [Google Scholar]
- Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653–660. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meersman RE, Stein PK. Vagal modulation and aging. Biol. Psychol. 2007;74:165–173. doi: 10.1016/j.biopsycho.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am. J. Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Daffonchio A, Gerosa S, Mancia G. Alterations in cardiac parasympathetic function in aged rats. Am. J. Physiol. Heart Circ. Physiol. 1991;260:H647–H649. doi: 10.1152/ajpheart.1991.260.2.H647. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk of atherosclerosis in humans. Proc. Natl. Acad. Sci. USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action and adipokine production. Age. 2010 a;32:97–108. doi: 10.1007/s11357-009-9118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010 b;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy JT, Stacy C, Bertrand HA. Long-term calorie restriction enhances baroreflex responsiveness in Fischer 344 rats. Am. J. Physiol. 1992;263:H1021–1025. doi: 10.1152/ajpheart.1992.263.4.H1021. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bosner MS, Rottman JN. Time domain measurements of heart rate variability. Cardiol. Clin. 1992;10:478–487. [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bigger JT. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Verberne AJM. Central regulation of Autonomic Functions. 2nd edn. Oxford Press; New York: 2011. [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Collage Cardiology. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute . Third Report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Natl. Inst. Health; Bethesda: 2001. DHHS Publ. No. (NIH) 01-3670, Executive Summary, Appendix III-A. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases . Diabetes in America. 2nd edn. Natl. Inst. Health; Bethesda: 1995. DHHS Pub. No. (NIH) 95-1465. [Google Scholar]
- O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br. Heart J. 1986;55:348–354. doi: 10.1136/hrt.55.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB. Differential changes of autonomic nervous system function with age in man. Am. J. Med. 1983;75:249–258. doi: 10.1016/0002-9343(83)91201-9. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Macaulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Coehen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 1985;17:H151–153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Schwartz JB, Gibb WJ, Tran T. Aging effects on heart rate variation. J. Gerontol. 1991;46:M99–106. doi: 10.1093/geronj/46.3.m99. [DOI] [PubMed] [Google Scholar]
- Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging. 2011;3:374–379. doi: 10.18632/aging.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J. Clin. Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: the Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2 + precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Charles C Thomas Publisher; Springfield, IL: 1988. [Google Scholar]
- Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, Bazzarre T, American Heart Association Cardiovascular health in childhood: a statement for health professionals on atherosclerosis, hypertension, and obesity in the young (AHOY) of the Council on Cardiovascular Disease in the Young. Circulation. 2002;106:143–160. doi: 10.1161/01.cir.0000019555.61092.9e. [DOI] [PubMed] [Google Scholar]
- Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of high heart rate variability to healthy longevity. Am. J. Cardiol. 2010;105:1181–1185. doi: 10.1016/j.amjcard.2009.12.022. [DOI] [PubMed] [Google Scholar]