Abstract
A simple protocol for rapid quantitation of acylcarnitines in serum and whole blood has been developed using paper spray mass spectrometry. Dried serum and whole blood containing a mixture of ten acylcarnitines at various concentrations were analyzed as spots from paper directly without any sample pretreatment, separation, or derivatization. The composition of the spray solvent was found to be a critical factor: for serum samples, spray solvent of methanol/water/formic acid (80:20:0.1) gave the best signal intensity while for blood samples which contain more matrix components, acetonitrile/water (90:10) was a much more suitable spray solvent. For the paper type and size used, 0.5 μL of sample provided an optimal signal for both serum and whole blood samples. For quantitative profiling, the limits of quantitation obtained from both serum and blood were much lower than the clinically validated cutoff values for diagnosis of fatty acid oxidation disorders in newborn screening. Linearity (R2>0.95) and reproducibility (RSD ~10 %) were achieved in the concentration ranges from 100 nM to 5 μM for the C2 acylcarnitine, and for other acylcarnitines, these values were from 10 to 500 nM. Acylcarnitine profiles offer an effective demonstration of the fact that paper spray mass spectrometry is an appropriate, simple, rapid method with high sensitivity and high reproducibility applicable to newborn screening tests.
Keywords: Paper spray, Ambient ionization, Mass spectrometry, Acylcarnitines, Newborn screening
Introduction
Newborn screening for inborn metabolic errors is a well-established public health program. State-based newborn screening programs started 50 years ago in the USA, and more than 150 million infants have been screened [1, 2]. The screening tests and follow-up treatments allow early diagnosis and proper medical management to prevent significant and irreversible damage, such as mental retardation [3]. It has been suggested that mandatory screening be implemented using a panel of core biomarkers for 29 genetic conditions, including nine organic acidurias, six amino acidurias, five disorders of fat oxidation, three hemoglobin-opathies, and six others [4]. Fatty acid oxidation (FAO) disorders are one of the most common inherited metabolic disorders, and they are associated with the complex pathways of fatty acid transport and mitochondrial oxidation. The rate for combined incidence of these disorders is 1:9,300 among live births [5]. Early diagnosis of these conditions is critical because life-threatening symptoms could occur without warning during the newborn period and through adulthood. Once diagnosed, low-fat and high-carbohydrate dietary management and long-term monitoring of serum glucose can help patients live a normal life [6]. Acylcarnitines are a group of esters derived from reaction of carnitine with fatty acids, and their most important function is to transport fatty acids across the mitochondrial membrane for fatty acid β-oxidation. Increases in certain acylcarnitines in blood of infants are indicators of deficiencies in fatty acid oxidation enzymes [7], and they have been used as informative biomarkers to diagnose specific fatty acid oxidation disorders in patients.
Gas chromatography–mass spectrometry (GC–MS) was first used to identify acylcarnitines [8]; since then mass spectrometry has played an important role in the analysis of acylcarnitines, and it is rapidly becoming the primary analytical technology in clinical screening. At the early stage of the development, fast atom bombardment and liquid secondary ion mass spectrometry were utilized as the main ionization methods for polar analytes with high molecular weight [9–11]. The purified samples needed for these experiments were embedded in a non-volatile liquid matrix and ionized under vacuum, representing an inconvenience in sample preparation regimen. These methods were replaced by electrospray ionization (ESI) [12] which serves as a natural interface between MS and liquid chromatography [13]. Tandem mass spectrometry (MS/MS) and isotopic dilution allow quantitative analysis of metabolites to be achieved using ESI with less sophisticated separation methods [14–16].
Currently, flow injection ESI–MS/MS is the main technique for acylcarnitine analysis, due to low sample consumption with automated, rapid analyses and the high degree of chemical specificity achieved [17–19]. Although ESI–MS/MS analysis of acylcarnitines is claimed to take 2–3 min per sample, this does not take into account the much longer time for sample preparation [17]. For newborn screening, sample preparation includes punching out dried blood disks followed by analyte extraction and derivatization processes that are relatively labor intensive and time consuming. The recent development of ambient ionization techniques[20] has provided an alternative way to rapidly and efficiently ionize complex samples from a wide variety of surfaces with minimal sample preparation [21, 22]. Desorption electrospray ionization [23], direct analysis in real time [24], and many other methods [25–27] have been successfully applied to analyze pharmaceutical compounds in complex biological samples [28–32]. A new technique, paper spray ionization (PS) [33] has been demonstrated to be highly effective for the rapid, direct, and quantitative analysis of complex biological samples such as urine [34], dried blood spots (DBS) [35, 36], and tissue samples [37]. For therapeutic drug monitoring, a chemically diverse set of 15 drugs in blood was investigated, and the analytes were found to be detectable at 1 ng/mL using PS–MS [35, 38]. High precision in quantitative analysis has been achieved with isotopically labeled internal standards added directly to the samples or preprinted onto the paper spray substrate [36].
Cellulose-based paper has been used as the standard medium for DBS in newborn screening, and a major initiative is taking place to use DBS on paper for sample collection and storage for other clinical analyses [39]. The development of direct analysis using paper spray could pave the way for fast and quantitative analysis in clinical laboratories as well as at the point-of-care with highly simplified procedures. While acylcarnitines exist in many different biological fluids, plasma or serum has been the preferred sample matrix for acylcarnitine profiling in the diagnosis of fatty acid oxidation disorders [14]. Other samples, such as bile specimens and amniotic fluid, have been used in acylcarnitine analysis for postmortem diagnosis and prenatal diagnosis, respectively [40, 41]. Analysis of acylcarnitines in samples such as urine, bile, and other biological fluids is generally not comprehensively informative, and therefore, screening of these fluids is less frequently performed except for the diagnosis of some organic acidemias in certain situations [42]. Currently, DBS on paper have become the standard method of sample storage for neonatal screening of inborn errors of metabolism [17, 18]. Analysis using dried blood spots requires small sample amounts (~10–20 μL), so less invasive sampling techniques such as a finger or heel prick can be utilized, which is particularly advantageous for pediatric studies. Easier transport and long-term storage at ambient temperatures are additional advantages. Therefore, we have investigated the application of paper spray mass spectrometry for the rapid, direct, and quantitative analysis of underivatized acylcarnitines in dried serum and blood spot samples in this study. Quantitation of these biomarkers can be achieved in a single step leading to a potentially fast, simple, and inexpensive route for newborn screening and other clinical diagnostics.
Experimental
The instrumental setup for paper spray and the geometry of the paper substrate have been previously described [33, 34]. Briefly, Whatman grade 1 chromatography paper was cut into a triangle (5 mm base, 10 mm height, and 0.18 mm thick), in the center of which serum or blood spots containing acylcarnitine calibrators and internal standards were preloaded. For paper spray ionization, 15 μL of solvent and spray voltage optimized at 4.5 kV were applied to the paper to generate ions unless otherwise noted. All experiments were carried out using a TSQ Quantum Access Max (Thermo Scientific, San Jose, CA) with the positive precursor ion scan set to the major fragment product ion +CH2CH=CHCOOH at m/z 85 for each acylcarnitine. Calibration curves were constructed by plotting the ratios of signal intensities for the acylcarnitines to that of their internal standards against their theoretical concentrations.
Unlabeled acylcarnitine calibrators used included acetylcarnitine( C2), propionylcarnitine(C3), isovalerylcarnitine (C5), hexanoylcarnitine(C6), octanoylcarnitine(C8), decanoylcarnitine( C10), lauroylcarnitine(C12), myristoylcarnitine( C14), palmitoylcarnitine(C16), and stearoylcarnitine (C18), each of which was obtained from Sigma-Aldrich (St. Louis, MO). Stock solutions of standards were prepared by mixing the acylcarnitine standards in serum or blood with concentrations of 50 μM for C2 and 5 μM for all others. Samples at different concentrations were prepared with further dilution of the standard solutions for calibration. Deuterated acylcarnitine internal standards were purchased from Cambridge Isotopes Laboratories (Andover, MA, USA), including [2H3]acetylcarnitine(C2-d3), [2H3] propionylcarnitine(C3-d3), [2H9]isovalerylcarnitine(C5-d9), [2H3]octanoylcarnitine(C8-d3), and [2H3]palmitoylcarnitine (C16-d3) (Table 1). The stock solution of the internal standards was prepared at concentrations of 100 μM for C2-d3, 40 μM for C16-d3, and 20 μM for other isotope-labeled acylcarnitines in methanol/water (50:50). The IS stock solution was spiked into serum or blood samples at a ratio of 1:100. The stock solutions were stored at −20 °C. Methanol, acetonitrile, and formic acid were purchased from Mallinckrodt Baker (Phillipsburg, NJ, USA). Both human pooled serum and whole bovine blood stabilized with K2EDTAwere purchased from Innovative Research (Novi, MI, USA). The serum or blood sample spiked with unlabeled acylcarnitine calibration standards and deuterated acylcarnitine internal standards, was spotted on a paper triangle, dried overnight at room temperature, and stored at −20 °C before testing to avoid potential degradation of the acylcarnitines in the DBS [43].
Table 1.
Ten acylcarnitines and their corresponding internal standards (IS)
| Shorthand | Name | Mass | IS |
|---|---|---|---|
| C2 | Acetycarnitine | 204 | C2-d3 |
| C3 | Propionylcarnitine | 218 | C3-d3 |
| C5 | Isovalerylcarnitine | 246 | C5-d9 |
| C6 | Hexanoylcarnitine | 260 | |
| C8 | Octanoylcarnitine | 288 | C8-d3 |
| C10 | Decanoylcarnitine | 316 | |
| C12 | Lauroylcarnitine | 344 | |
| C14 | Myristoylcarnitine | 372 | C16-d3 |
| C16 | Palmitoylcarnitine | 400 | |
| C18 | Stearoylcarnitine | 428 |
Results and discussion
Current newborn screening of acylcarnitines involves a derivatization step before MS analysis. All the acylcarnitines are first converted into the corresponding methyl or n-butyl esters [19]. The derivatives have a net positive charge, which is expected to prevent the formation of zwitterions during ESI so that both stability and sensitivity of the analysis are improved [44]. However, there are some problems that are associated with the process, such as incomplete derivatization or subsequent hydrolysis, in addition to the time involved in sample preparation [45].
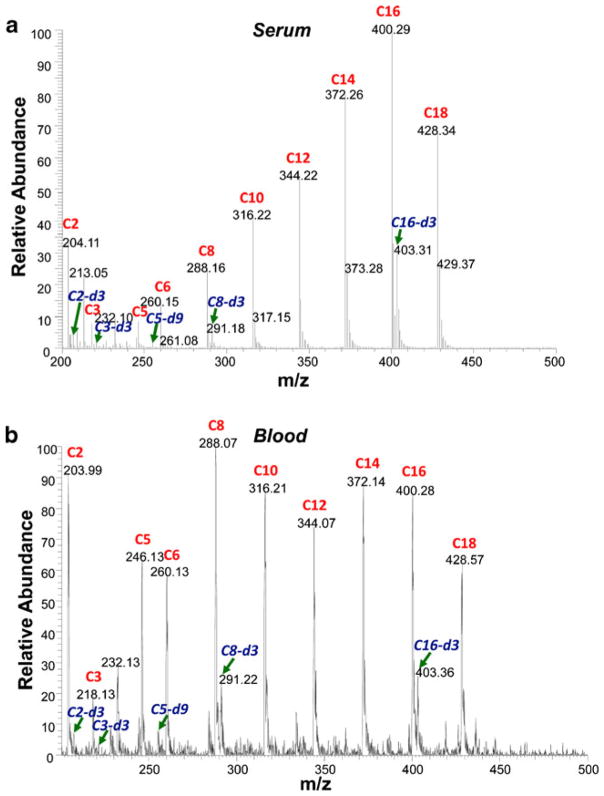
In this study, a simple method has been developed based on paper spray ionization to perform direct analysis of acylcarnitines without derivatization. Acylcarnitine calibration standards and their internal standards were spiked into serum or blood at concentrations within the range found in the normal population. Specifically, C2 acylcarnitine was spiked at 5 μM, and other acylcarnitines were spiked at 500 nM; the internal standards C2-d3, C16-d3, and other labeled internal standards were spiked at 1 μM, 400 nM, and 200 nM, respectively, for both serum and blood samples. Typically, 0.5 μL of sample was preloaded and dried on each triangle paper substrate to form the dried spots. After applying 15 μL spray solvent and 4.5 kV potential, fine droplets were generated at the sharp tip of the paper triangle, and the analyte ions underwent desolvation in the gas phase and were analyzed by MS/MS. Since a common product ion at m/z 85 was generated through CID for all the acylcarnitines by the loss of the acyl side chain, the precursor ion scan mode [46] was selected to monitor the intensities of all the acylcarnitines and internal standards simultaneously in a single scan. The spectrum recorded for a dried serum spot is shown in Fig. 1a. Interestingly, a trend of increasing intensities was observed for the C3 to C16 acylcarnitines. This presumably is due to the surfactant effect associated with increasing hydrophobicity previously observed for ESI of the pure analytes [47]. This serum trend was not observed for blood samples (Fig. 1b). The sample matrix in blood is much more complex, and the hydrophobicity effect might be suppressed by other matrix effects. Nevertheless, the acylcarnitines and the internal standards could all be observed readily in both dried serum and dried blood samples using paper spray.
Fig 1.
Precursor ion scans of acylcarnitine in a dried serum spot and in b dried blood spot using PS–MS. Acylcarnitine calibration standards are labeled in red (C2, 5 μM; other acylcarnitines, 500 nM), and internal standards are labeled in blue (C2-d3, 1 μM; C16-d3, 400 nM; other internal standards, 200 nM)
In the analysis of the acylcarnitines for inborn errors of fatty acid oxidation, the profile of all acylcarnitines, instead of an individual acylcarnitine, is the most reliable diagnostic measurement. Quantitative analysis of a single biomarker in blood samples, such as a drug compound, has been previously achieved using paper spray with selected reaction monitoring [33]. Good sensitivity and precision were obtained provided an internal standard was added to the samples. In this study, we explored a group of five selected internal standards for simultaneous quantitation of a panel of ten acylcarnitine biomarkers using parent ion scans. Even though acylcarnitines are chemical analogues, the acyl group side chain varies in both length and structure, which affects their ionization efficiency during MS analysis. In addition, different biological fluids have different components that can affect the extraction and ionization efficiency of the acylcarnitines during paper spray analysis. As a result, the paper spray conditions were optimized to improve the ionization efficiency for all of the acylcarnitines in order to maximize the range over which quantitation could be performed.
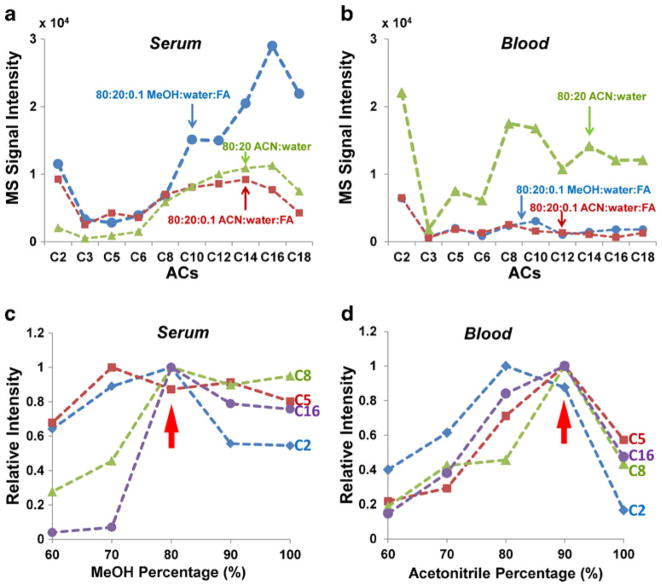
The solvent chosen for the paper spray has been shown to have a significant impact on the extraction of the analytes and the ionization efficiency [37, 48]. Methanol/water solvents have been shown to be generally applicable to the analysis of many types of samples [33] and were used in the initial trials of analyzing acylcarnitines in serum and blood. Stable signals were obtained for all acylcarnitines at the highest concentration; however, the signal intensity dropped quickly and standard deviations increased as the concentration of acylcarnitines decreased. The response was not linear in the concentration range we tested, especially for short-chain acylcarnitines. After adding 0.1 % formic acid to methanol/water (80:20), the signal became much more reproducible, the intensity increased by three to ten times, and the concentration dependence was much more linear (data not shown). Thus, excepting methanol/water (80:20), three spray solutions, methanol/water/formic acid, acetonitrile/water, and acetonitrile/water/formic acid, were systematically compared and the results are shown in Fig. 2. For dried spots prepared with 0.5 μL serum sample containing C2 at 5 μM and other acylcarnitines at 500 nM (Fig. 2a), the trend of increasing intensity from short chain to long chain (C3 to C16) was observed for all three solvents. Higher intensities were obtained for C2 to C6 for methanol/water/formic acid (80:20:0.1) and acetonitrile/water/formic acid (80:20:0.1) compared with those observed for acetonitrile/water (80:20). Significantly higher intensities were obtained for C10 to C18 with methanol/water/formic acid (80:20:0.1) with two- to four-fold increases over the other two solvents, so methanol/water/formic acid solvent was chosen as the best solvent for serum samples. However, for blood samples containing the acylcarnitines of the same corresponding concentrations (Fig. 2b), acetonitrile/water solvent gave much higher intensities for all the acylcarnitines except for C3 making acetonitrile/water the most suitable solvent for blood analysis.
Fig 2.
Effect of different solvent compositions for acylcarnitine analysis in serum and in blood; 80 % methanol with 0.1 % formic acid (blue, circles), 80 % acetonitrile with 0.1 % formic acid (red, squares), and 80 % acetonitrile (green, triangles) were tested for a serum and b blood samples. The effect of organic solvent percentage for acylcarnitine analysis was further investigated, by varying the percentage of methanol from 60 to 100 % with 0.1 % formic acid for serum sample (c), while the percentage of acetonitrile was varied from 60 to 100 % without adding acid for the blood sample (d)
To further optimize performance, the concentrations of the spray solvent components were varied for the serum and blood samples. For analysis of serum spots, the concentration of methanol in the methanol/water/formic acid solvent was varied, and the impact on the intensities of acylcarnitines with acyl group side chains of different length was monitored. The intensities for each acylcarnitine were normalized and plotted as a function of the percentage of methanol. Data for C2, C5, C8, and C16 are shown in Fig. 2c as representatives of short-, medium-, and long-chain acylcarnitines. It was found that the variation of the proportion of methanol in the solvent has a much more dramatic impact on the longer-chain acylcarnitines than those with short side chains. Solvent with 80 % methanol– 20 % water was found to be optimal for the entire set of acylcarnitines. Similar optimization was performed for analysis of blood spots by varying the concentration of acetonitrile in the acetonitrile/water solvent. The optimal concentration of acetonitrile was determined to be 90 % when considering all the acylcarnitines, and relatively small differences were observed with variation of the acyl side chains (Fig. 2d). In the experiments described below, the spray solvents of methanol/water/formic acid (80:20:0.1) and acetonitrile/water (90:10) were used for serum and blood dried spots, respectively.
The sample load is another factor found to influence the performance of paper spray [48, 49]. As the sample load increases, the intensities of the analyte peaks should increase; however, matrix effects can also become more serious and suppress the signal, so there is a tradeoff to be considered to set the optimal sample load. For the triangle paper substrate (5 mm base, 10 mm height, and 0.18 mm thickness) used in this study, different volumes of serum and blood samples, of 0.25, 0.5, 1, and 2 μL, were each spotted and dried in the center of the triangular substrate. The concentrations of acylcarnitines and internal standards were the same as in the above experiment. A fixed volume of 15 μL spray solvent was applied. The observed intensities were normalized with the maxima, and representative data are shown in Fig. 3 for C2, C5, C8, and C16. A relatively consistent trend in intensity as a function of sample load was observed for all acylcarnitines in blood samples, with an optimal sample load identified as 0.5 μL. However, there was no obvious trend observed for serum samples. As the sample load increased, C2 intensity stayed steady, C5 and C8 decreased, and C16 increased. The sample load of 0.5 μL was selected for both blood and serum samples in the following characterization of quantitation of acylcarnitines by paper spray.
Fig 3.
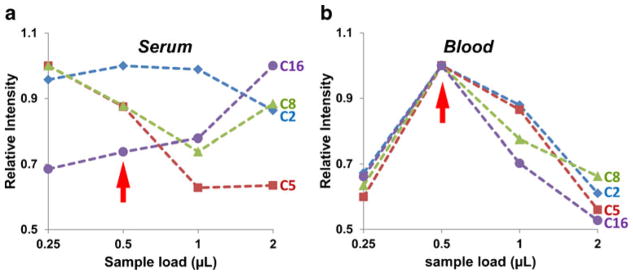
Effect of sample load on acylcarnitine analysis in a serum and b blood
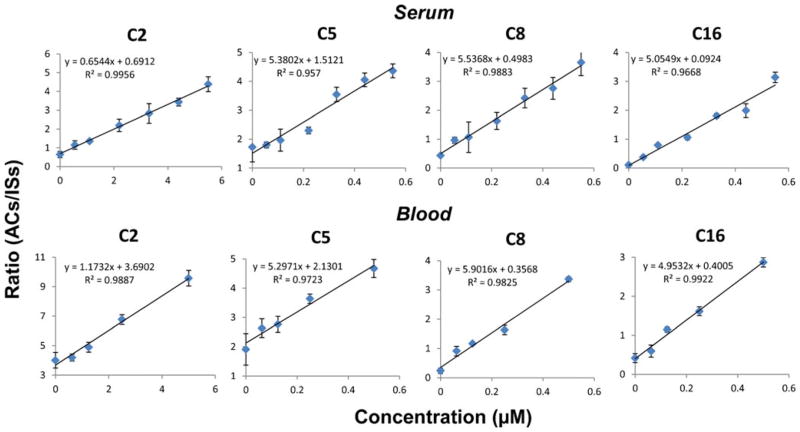
Quantitation of acylcarnitines was performed by analysis of dried serum and blood spots, each containing a mixture of acylcarnitine calibration samples, with C2 from 100 nM to 5 μM and the longer chain compounds from 10 to 500 nM. Isotopically labeled internal standards of fixed concentration (C2-d3, 1 μM; C16-d3, 400 nM; other internal standards, 200 nM) were spiked into the liquid serum or blood prior to spotting them on paper. The peak ratios of representative short-, medium-, and long-chain acylcarnitines (C2, C5, C8, and C16) relative to the corresponding internal standard (Table 1) were plotted as a function of their theoretical concentrations (Fig. 4). Good linearity was obtained for all the curves within the concentration range of 50 times, with a correlation coefficient better than 0.95 and a relative standard deviation of replicate measurements less than 10 % (n=4).
Fig 4.
Standard curves obtained for quantitative analysis of acylcarnitines in serum (top) and in blood (bottom)
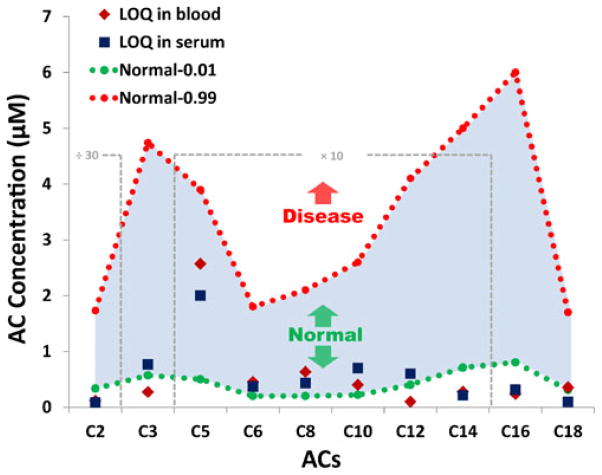
As a result of a worldwide collaborative project, new cutoff target ranges were published in 2011 for newborn screening of typical metabolic disorders using tandem mass spectrometry [50]. Cumulative percentiles of acylcarnitines for normal and fatty acid oxidation disorder ranges were clinically validated in dried blood spots of approximately 25–30 million normal newborns, with 10,742 identified as true-positive cases. These data were used as the reference to evaluate paper spray mass spectrometry for acylcarnitine screening, and the results are shown in graphical form in Fig. 5. The shaded area in blue indicates the range for a majority of the normal population and is edged with the dotted lines in green and red at the 1st and 99th percentile cumulative percentiles of normal acylcarnitine species, respectively. This means that only 1 % of the normal population falls below the green dotted line while 99 % of the normal population is covered by the red dotted line. In most cases of fatty acid oxidation disorder, the concentrations of one or more acylcarnitines are elevated [19]. Specific types of disorders can be identified based on the profile of elevated acylcarnitines, which is related to the deficiency or dysfunction of the corresponding enzymes. Therefore, the 99th percentile cumulative values are set as the cutoff for fatty acid oxidation disorder diagnosis in the clinic. For all the acylcarnitines analyzed in serum and blood samples using paper spray, the limits of quantitation (LOQs) obtained from calibration curves were much lower than the 99th percentile cumulative value of the normal ranges (Fig. 5). No significant differences in LOQs were found between serum and blood samples. This performance assures that paper spray mass spectrometry is sensitive enough for acylcarnitine profiling for clinical diagnosis of fatty acid oxidation disorders in both serum and blood spots.
Fig 5.
Limit of quantitation of acylcarnitines in serum and in blood using PS–MS. The shaded area in blue indicates the majority of acylcarnitine range for the normal population. The dotted green line at the bottom is the first percentile cumulative values of normal acylcarnitine species, and the dotted red line at the top is the 99th percentile cumulative values of normal acylcarnitines, which is also the cutoff for FAO disorder diagnosis in the clinic. Blue squares show the limit of quantitation of acylcarnitines in serum, and red diamonds are the limit of quantitation of acylcarnitines in blood. The data of C2 were zoomed out 30 times, and the data from C5 to C14 were zoomed in ten times
Conclusions
A simple protocol for quantitatively profiling acylcarnitines in serum and whole blood was developed using paper spray mass spectrometry. Dried serum and whole blood sample spots containing a mixture of ten acylcarnitines at various concentrations were analyzed directly without any sample pretreatment, separation, or derivatization. The quantitation of all the acylcarnitines was achieved using deuterated internal standards and serum or blood sample volumes as low as 0.5 μL. The composition of the spray solvent and the sample load was found to be a critical factor for high-performance paper spray of acylcarnitines. For quantitative profiling, the limits of detection obtained from both serum and blood are much lower than the clinically validated cutoff value, and acceptable linearity (R2>0.95) and good reproducibility (RSD, ~10 %) were achieved in the concentration ranges tested. PS–MS has the potential to be a new technique to rapidly assess acylcarnitine analysis in newborn screening.
While the promising capability of paper spray has been shown here for the clinical diagnosis with acylcarnitines as biomarkers, further development is necessary to address several critical issues for practical implementation. Simple sample handling without traditional laboratory techniques such as pipetting for taking samples of accurate amounts and mixing internal standards would be very valuable. The use of capillary tubes for transferring accurate amounts of blood samples onto the paper substrate containing preprinted internal standard has been shown to be effective [36], while other methods such as using capillaries with precoated internal standards remain attractive and are being explored [51, 52]. In this study, human pooled serum and whole bovine blood were used as the matrices, without testing relative matrix effects potentially caused by the differences among the blood samples from individuals. However, this is not expected to be problematic based on the results from a previous study on the quantitation of therapeutic drugs, where the relative matrix effects were found to be minimum [35].
Acknowledgments
This work was supported by the National Science Foundation (CHE 0847205 and CHE 0848650), National Science Foundation Instrumentation Development for Biological Research (DBI 0852740), National Center for Research Resources (5R21RR031246-02) and the National Institute of General Medical Sciences (8 R21 GM103454) from the National Institutes of Health, and the Alfred Mann Institute at Purdue University.
Contributor Information
Qian Yang, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
Nicholas E. Manicke, Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA
He Wang, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
Christopher Petucci, Metabolomics Core, Sanford-Burnham Medical Research Institute, Orlando, FL 32827, USA.
R. Graham Cooks, Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA, Center for Analytical Instrumentation Development, Purdue University, West Lafayette, IN 47907, USA.
Zheng Ouyang, Email: ouyang@purdue.edu, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA, Center for Analytical Instrumentation Development, Purdue University, West Lafayette, IN 47907, USA.
References
- 1.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32(3):338–343. [PubMed] [Google Scholar]
- 2.Kaye CI, Schaefer GB, Bull MJ, Enns GM, Gruen JR, Hersh JH, Mendelsohn NJ, Saal HM, Goldberg JD, Hanson JW, et al. Introduction to the newborn screening fact sheets. Pediatrics. 2006;118 (3):1304–1312. doi: 10.1542/peds.2006-1782. [DOI] [PubMed] [Google Scholar]
- 3.Sweetman L. Newborn screening by tandem mass spectrometry (MS-MS) Clin Chem. 1996;42(3):345–346. [PubMed] [Google Scholar]
- 4.Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR. Newborn screening: toward a uniform screening panel and system. Genet Med. 2006;8(Suppl 1):1S–252S. doi: 10.1097/01.gim.0000223891.82390.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindner M, Hoffmann GF, Matern D. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis. 2010;33(5):521–526. doi: 10.1007/s10545-010-9076-8. [DOI] [PubMed] [Google Scholar]
- 6.Vockley J, Singh RH, Whiteman DAH. Diagnosis and management of defects of mitochondrial beta-oxidation. Curr Opin Clin Nutr. 2002;5(6):601–609. doi: 10.1097/00075197-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Scriver CR. The metabolic and molecular bases of inherited disease. 7. McGraw-Hill; New York: 1995. [Google Scholar]
- 8.Bieber LL, Choi YR. Isolation and identification of aliphatic short-chain acylcarnitines from beef heart: possible role for carnitine in branched-chain amino acid metabolism. Proc Natl Acad Sci U S A. 1977;74(7):2795–2798. doi: 10.1073/pnas.74.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millington DS, Roe CR, Maltby DA. Application of high resolution fast atom bombardment and constant B/E ratio linked scanning to the identification and analysis of acylcarnitines in metabolic disease. Biomed Mass Spectrom. 1984;11(5):236–241. doi: 10.1002/bms.1200110508. [DOI] [PubMed] [Google Scholar]
- 10.Millington DS, Norwood DL, Kodo N, Roe CR, Inoue F. Application of fast atom bombardment with tandem mass-spectrometry and liquid-chromatography mass-spectrometry to the analysis of acylcarnitines in human-urine, blood, and tissue. Anal Biochem. 1989;180(2):331–339. doi: 10.1016/0003-2697(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 11.VianeySaban C, Guffon N, Delolne F, Guibaud P, Mathieu M, Divry P. Diagnosis of inborn errors of metabolism by acylcarnitine profiling in blood using tandem mass spectrometry. J Inherit Metab Dis. 1997;20(3):411–414. doi: 10.1023/a:1005306818025. [DOI] [PubMed] [Google Scholar]
- 12.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass-spectrometry of large biomolecules. Science. 1989;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 13.Ghoshal AK, Guo TD, Soukhova N, Soldin SJ. Rapid measurement of plasma acylcarnitines by liquid chromatography–tandem mass spectrometry without derivatization. Clin Chim Acta. 2005;358(1–2):104–112. doi: 10.1016/j.cccn.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Vreken P, van Lint AEM, Bootsma AH, Overmars H, Wanders RJA, van Gennip AH. Quantitative plasma acylcarnitine analysis using electrospray tandem mass spectrometry for the diagnosis of organic acidaemias and fatty acid oxidation defects. J Inherit Metab Dis. 1999;22(3):302–306. doi: 10.1023/a:1005587617745. [DOI] [PubMed] [Google Scholar]
- 15.Chace DH, DiPierna JC, Mitchell BL, Sgroi B, Hofman LF, Naylor EW. Electrospray tandem mass spectrometry for analysis of acylcarnitines in dried postmortem blood specimens collected at autopsy from infants with unexplained cause of death. Clin Chem. 2001;47(7):1166–1182. [PubMed] [Google Scholar]
- 16.Chace DH, Hillman SL, Shushan B, Corr JJ. Multiple metabolic profiles from dried filter paper blood spots using electrospray tandem mass spectrometry. Clin Chem. 1997;43(Suppl 6):436–436. [Google Scholar]
- 17.Rashed MS, Ozand PT, Bucknall MP, Little D. Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr Res. 1995;38(3):324–331. doi: 10.1203/00006450-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Rashed MS, Bucknall MP, Little D, Awad A, Jacob M, Alamoudi M, Alwattar M, Ozand PT. Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin Chem. 1997;43(7):1129–1141. [PubMed] [Google Scholar]
- 19.Smith EH, Matern D. Acylcarnitine analysis by tandem mass spectrometry. Curr Protoc Hum Genet. 2010;Chapter 17(Unit 17.8):1–20. doi: 10.1002/0471142905.hg1708s64. [DOI] [PubMed] [Google Scholar]
- 20.Cooks RG, Ouyang Z, Takáts Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311(5767):1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang Z, Zhang XR. Ambient mass spectrometry. Analyst. 2010;135(4):659–660. doi: 10.1039/c003812c. [DOI] [PubMed] [Google Scholar]
- 22.Harris GA, Galhena AS, Fernandez FM. Ambient sampling/ionization mass spectrometry: applications and current trends. Anal Chem. 2011;83(12):4508–4538. doi: 10.1021/ac200918u. [DOI] [PubMed] [Google Scholar]
- 23.Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 24.Cody RB, Laramee JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77(8):2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 25.Chen HW, Gamez G, Zenobi R. What can we learn from ambient ionization techniques? J Am Soc Mass Spectrom. 2009;20 (11):1947–1963. doi: 10.1016/j.jasms.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Van Berkel GJ, Pasilis SP, Ovchinnikova O. Established and emerging atmospheric pressure surface sampling/ionization techniques for mass spectrometry. J Mass Spectrom. 2008;43(9):1161–1180. doi: 10.1002/jms.1440. [DOI] [PubMed] [Google Scholar]
- 27.Venter A, Nefliu M, Cooks RG. Ambient desorption ionization mass spectrometry. Trends Anal Chem. 2008;27(4):284–290. [Google Scholar]
- 28.Nemes P, Vertes A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal Chem. 2007;79(21):8098–8106. doi: 10.1021/ac071181r. [DOI] [PubMed] [Google Scholar]
- 29.Sampson JS, Hawkridge AM, Muddiman DC. Construction of a versatile high precision ambient ionization source for direct analysis and imaging. J Am Soc Mass Spectrom. 2008;19(10):1527–1534. doi: 10.1016/j.jasms.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson JS, Hawkridge AM, Muddiman DC. Development and characterization of an ionization technique for analysis of biological macromolecules: liquid matrix-assisted laser desorption electrospray ionization. Anal Chem. 2008;80(17):6773–6778. doi: 10.1021/ac8001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiea J, Huang M-Z, HSu H-J, Lee C-Y, Yuan C-H, Beech I, Sunner J. Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids. Rapid Commun Mass Sp. 2005;19(24):3701–3704. doi: 10.1002/rcm.2243. [DOI] [PubMed] [Google Scholar]
- 32.Nemes P, Vertes A. Ambient mass spectrometry for in vivo local analysis and in situ molecular tissue imaging. Trends Anal Chem. 2012;34:22–34. [Google Scholar]
- 33.Wang H, Liu JJ, Cooks RG, Ouyang Z. Paper spray for direct analysis of complex mixtures using mass spectrometry. Angew Chem Int Ed. 2010;49(5):877–880. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- 34.Liu JJ, Wang H, Manicke NE, Lin JM, Cooks RG, Ouyang Z. Development, characterization, and application of paper spray ionization. Anal Chem. 2010;82(6):2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- 35.Manicke NE, Abu-Rabie P, Spooner N, Ouyang Z, Cooks RG. Quantitative analysis of therapeutic drugs in dried blood spot samples by paper spray mass spectrometry: an avenue to therapeutic drug monitoring. J Am Soc Mass Spectrom. 2011;22(9):1501–1507. doi: 10.1007/s13361-011-0177-x. [DOI] [PubMed] [Google Scholar]
- 36.Manicke NE, Yang Q, Wang H, Oradu S, Ouyang Z, Cooks RG. Assessment of paper spray ionization for quantitation of pharmaceuticals in blood spots. Int J Mass Spectrom. 2011;300(2–3):123–129. [Google Scholar]
- 37.Wang H, Manicke NE, Yang QA, Zheng LX, Shi RY, Cooks RG, Zheng OY. Direct analysis of biological tissue by paper spray mass spectrometry. Anal Chem. 2011;83(4):1197–1201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooks RG, Manicke NE, Dill AL, Ifa DR, Eberlin LS, Costa AB, Wang H, Huang G, Ouyang Z. New ionization methods and miniature mass spectrometers for biomedicine: DESI imaging for cancer diagnostics and paper spray ionization for therapeutic drug monitoring. Faraday Discuss. 2011;149:247–267. doi: 10.1039/c005327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spooner N, Lad R, Barfield M. Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of a quantitative bioanalytical method. Anal Chem. 2009;81(4):1557–1563. doi: 10.1021/ac8022839. [DOI] [PubMed] [Google Scholar]
- 40.Nada MA, Vianey-Saban C, Roe CR, Ding JH, Mathieu M, Wappner RS, Bialer MG, McGlynn JA, Mandon G. Prenatal diagnosis of mitochondrial fatty acid oxidation defects. Prenat Diag. 1996;16(2):117–124. doi: 10.1002/(SICI)1097-0223(199602)16:2<117::AID-PD820>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 41.Rashed MS, Ozand PT, Bennett MJ, Barnard JJ, Govindaraju DR, Rinaldo P. Inborn errors of metabolism diagnosed in sudden death cases by acylcarnitine analysis of postmortem bile. Clin Chem. 1995;41(8):1109–1114. [PubMed] [Google Scholar]
- 42.Lowes S, Rose ME. Simple urinary acylcarnitine profiling by gas-chromatography mass-spectrometry. Philos T Roy Soc A. 1990;333(1628):169–170. [Google Scholar]
- 43.Fingerhut R, Ensenauer R, Rochinger W, Arnecke R, Olgemoller B, Roscher AA. Stability of acylcarnitines and free carnitine in dried blood samples: implications for retrospective diagnosis of inborn errors of metabolism and neonatal screening for carnitine transporter deficiency. Anal Chem. 2009;81(9):3571–3575. doi: 10.1021/ac8022235. [DOI] [PubMed] [Google Scholar]
- 44.Chace DH. Mass spectrometry in the clinical laboratory. Chem Rev. 2001;101(2):445–477. doi: 10.1021/cr990077+. [DOI] [PubMed] [Google Scholar]
- 45.Osorio JH, Pourfarzam M. Hydrolysis of acylcarnitines during measurement in blood and plasma by tandem mass spectrometry. Acta Bioquim Clin L. 2010;44(2):189–193. [Google Scholar]
- 46.Millington DS, Kodo N, Terada N, Roe D, Chace DH. The analysis of diagnostic markers of genetic-disorders in human blood and urine using tandem mass-spectrometry with liquid secondary ion mass-spectrometry. Int J Mass Spectrom. 1991;111:211–228. [Google Scholar]
- 47.Briand G, Fontaine M, Schubert R, Ricart G, Degand P, Vamecq J. Direct analysis by electrospray ionization and matrix-assisted laser desorption ionization mass spectrometry of standard and urinary acylcarnitines—comparison with fast atom bombardment and gas chromatography chemical ionization mass spectrometry. J Mass Spectrom. 1995;30(12):1731–1741. [Google Scholar]
- 48.Zhang Z, Xu W, Mancke NE, Cooks RG, Ouyang Z. Silica coated paper substrate for paper-spray analysis of therapeutic drugs in dried blood spots. Anal Chem. 2012;84(2):931–938. doi: 10.1021/ac202058w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Q, Wang H, Maas JD, Chappell WJ, Manicke NE, Cooks RG, Ouyang Z. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int J Mass Spectrom. 2012;312:201–207. doi: 10.1016/j.ijms.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McHugh DMS, Cameron CA, Abdenur JE, Abdulrahman M, Adair O, Al Nuaimi SA, Ahlman H, Allen JJ, Antonozzi I, Archer S, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet Med. 2011;13 (3):230–254. doi: 10.1097/GIM.0b013e31820d5e67. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang Z, Cooks GR, Hertig J, Chen T, Manicke NE, Li L, Chen C, Liu JJ. Proof-of-concept development of a personal mass spectrometer. 60th ASMS Conference on Mass Spectrometry and Allied Topics; Vacouver, CA. May 20–24, 2012.2012. [Google Scholar]
- 52.Manicke NE, Espy R, Liu JJ, Hertig J, Ouyang Z, Cooks GR. Analysis of biological samples by paper spray-MS: toward point of care mass spectrometry. 60th ASMS Conference on Mass Spectrometry and Allied Topics; Vancouver, Canada. May 20–24, 2012.2012. [Google Scholar]