Abstract
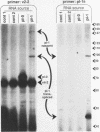
In nematodes, the RNA products of some genes are trans-spliced to a 22-nucleotide spliced leader (SL), while the RNA products of other genes are not. In Caenorhabditis elegans, there are two SLs, SL1 and SL2, donated by two distinct small nuclear ribonucleoprotein particles in a process functionally quite similar to nuclear intron removal. We demonstrate here that it is possible to convert a non-trans-spliced gene into a trans-spliced gene by placement of an intron missing only the 5' splice site into the 5' untranslated region. Stable transgenic strains were isolated expressing a gene in which 69 nucleotides of a vit-5 intron, including the 3' splice site, were inserted into the 5' untranslated region of a vit-2/vit-6 fusion gene. The RNA product of this gene was examined by primer extension and PCR amplification. Although the vit-2/vit-6 transgene product is not normally trans-spliced, the majority of transcripts from this altered gene were trans-spliced to SL1. We termed the region of a trans-spliced mRNA precursor between the 5' end and the first 3' splice site an "outron." Our results suggest that if a transcript begins with intronlike sequence followed by a 3' splice site, this alone may constitute an outron and be sufficient to demarcate a transcript as a trans-splice acceptor. These findings leave open the possibility that specific sequences are required to increase the efficiency of trans-splicing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bektesh S. L., Hirsh D. I. C. elegans mRNAs acquire a spliced leader through a trans-splicing mechanism. Nucleic Acids Res. 1988 Jun 24;16(12):5692–5692. doi: 10.1093/nar/16.12.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Squire M., Kirtland S., Cane J., Donegan M., Spieth J., Sharrock W. Cloning of a yolk protein gene family from Caenorhabditis elegans. J Mol Biol. 1984 Mar 25;174(1):1–18. doi: 10.1016/0022-2836(84)90361-9. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Thomas J. Cis and trans mRNA splicing in C. elegans. Trends Genet. 1988 Nov;4(11):305–308. doi: 10.1016/0168-9525(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzik J. P., Steitz J. A. Spliced leader RNA sequences can substitute for the essential 5' end of U1 RNA during splicing in a mammalian in vitro system. Cell. 1990 Sep 7;62(5):889–899. doi: 10.1016/0092-8674(90)90264-f. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Van Doren K., Hirsh D., Steitz J. A. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988 Oct 6;335(6190):559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cummins C., Anderson P. Regulatory myosin light-chain genes of Caenorhabditis elegans. Mol Cell Biol. 1988 Dec;8(12):5339–5349. doi: 10.1128/mcb.8.12.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A. Integrative transformation of Caenorhabditis elegans. EMBO J. 1986 Oct;5(10):2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Kondo K., Waterston R. Vectors for low copy transformation of C. elegans. Nucleic Acids Res. 1990 Jul 25;18(14):4269–4270. doi: 10.1093/nar/18.14.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi L. L., Albert P. S., Riddle D. L. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990 May 18;61(4):635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- Graham R. W., Jones D., Candido E. P. UbiA, the major polyubiquitin locus in Caenorhabditis elegans, has unusual structural features and is constitutively expressed. Mol Cell Biol. 1989 Jan;9(1):268–277. doi: 10.1128/mcb.9.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Huang X. Y., Barrios L. A., Vonkhorporn P., Honda S., Albertson D. G., Hecht R. M. Genomic organization of the glyceraldehyde-3-phosphate dehydrogenase gene family of Caenorhabditis elegans. J Mol Biol. 1989 Apr 5;206(3):411–424. doi: 10.1016/0022-2836(89)90490-7. [DOI] [PubMed] [Google Scholar]
- Huang X. Y., Hirsh D. A second trans-spliced RNA leader sequence in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8640–8644. doi: 10.1073/pnas.86.22.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. Protein structural domains in the Caenorhabditis elegans unc-54 myosin heavy chain gene are not separated by introns. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4253–4257. doi: 10.1073/pnas.80.14.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., Johnson J. J. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990 May;10(5):2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W. Trans splicing in trypanosomes--archaism or adaptation? Trends Genet. 1989 Jul;5(7):204–208. doi: 10.1016/0168-9525(89)90082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod A. R., Karn J., Brenner S. Molecular analysis of the unc-54 myosin heavy-chain gene of Caenorhabditis elegans. Nature. 1981 Jun 4;291(5814):386–390. doi: 10.1038/291386a0. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Shambaugh J., Denker J., Chubb G., Faser C., Putnam L., Bennett K. Characterization and expression of a spliced leader RNA in the parasitic nematode Ascaris lumbricoides var. suum. Mol Cell Biol. 1989 Aug;9(8):3543–3547. doi: 10.1128/mcb.9.8.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. S., Kramer J. M. Tandemly duplicated Caenorhabditis elegans collagen genes differ in their modes of splicing. J Mol Biol. 1990 Jan 20;211(2):395–406. doi: 10.1016/0022-2836(90)90360-X. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Spieth J., Blumenthal T. The Caenorhabditis elegans vitellogenin gene family includes a gene encoding a distantly related protein. Mol Cell Biol. 1985 Oct;5(10):2495–2501. doi: 10.1128/mcb.5.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth J., Denison K., Kirtland S., Cane J., Blumenthal T. The C. elegans vitellogenin genes: short sequence repeats in the promoter regions and homology to the vertebrate genes. Nucleic Acids Res. 1985 Jul 25;13(14):5283–5295. doi: 10.1093/nar/13.14.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth J., Denison K., Zucker E., Blumenthal T. The nucleotide sequence of a nematode vitellogenin gene. Nucleic Acids Res. 1985 Oct 11;13(19):7129–7138. doi: 10.1093/nar/13.19.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth J., MacMorris M., Broverman S., Greenspoon S., Blumenthal T. Regulated expression of a vitellogenin fusion gene in transgenic nematodes. Dev Biol. 1988 Nov;130(1):285–293. doi: 10.1016/0012-1606(88)90434-4. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs A. M., Denker J. A., Perrine K. G., Maroney P. A., Nilsen T. W. A 22-nucleotide spliced leader sequence in the human parasitic nematode Brugia malayi is identical to the trans-spliced leader exon in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7932–7936. doi: 10.1073/pnas.85.21.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. D., Conrad R. C., Blumenthal T. The C. elegans trans-spliced leader RNA is bound to Sm and has a trimethylguanosine cap. Cell. 1988 Aug 12;54(4):533–539. doi: 10.1016/0092-8674(88)90075-x. [DOI] [PubMed] [Google Scholar]
- Thomas J., Lea K., Zucker-Aprison E., Blumenthal T. The spliceosomal snRNAs of Caenorhabditis elegans. Nucleic Acids Res. 1990 May 11;18(9):2633–2642. doi: 10.1093/nar/18.9.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doren K., Hirsh D. Trans-spliced leader RNA exists as small nuclear ribonucleoprotein particles in Caenorhabditis elegans. Nature. 1988 Oct 6;335(6190):556–559. doi: 10.1038/335556a0. [DOI] [PubMed] [Google Scholar]
- Zeng W. L., Alarcon C. M., Donelson J. E. Many transcribed regions of the Onchocerca volvulus genome contain the spliced leader sequence of Caenorhabditis elegans. Mol Cell Biol. 1990 Jun;10(6):2765–2773. doi: 10.1128/mcb.10.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]