Abstract
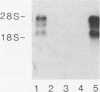
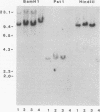
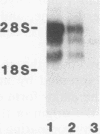
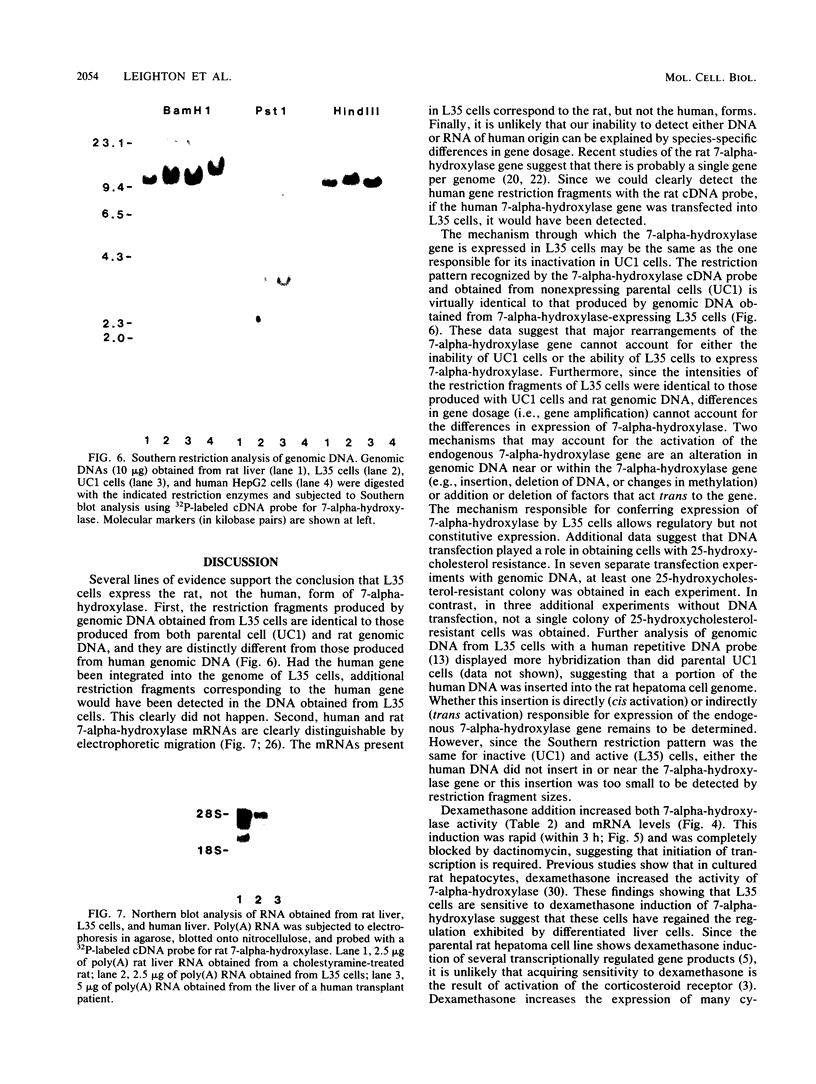
The oxysterol 25-hydroxycholesterol acts both as a regulatory sterol determining the expression of genes governed by sterol regulatory elements and as a substrate for 7-alpha-hydroxylase, the first and rate-limiting enzyme in the bile acid synthetic pathway. Most wild-type nonhepatic cells are killed by the cytotoxic action of 25-hydroxycholesterol. In contrast, liver cells, which express 7-alpha-hydroxylase activity, are resistant to killing by 25-hydroxycholesterol. We examined the possibility that selection for resistance to 25-hydroxycholesterol might lead to the derivation of a cell line expressing 7-alpha-hydroxylase. A rat hepatoma cell line (7-alpha-hydroxylase minus) was transfected with human DNA and screened for resistance to 25-hydroxycholesterol. Although parental hepatoma cells were all killed within a week, a 25-hydroxycholesterol-resistant cell line (L35 cells) which showed stable expression of 7-alpha-hydroxylase activity and mRNA was obtained. These cells exhibited normal inhibition of cholesterol biosynthesis by 25-hydroxycholesterol. Blocking 7-alpha-hydroxylase activity with ketoconazole also blocked the resistance of L35 cells to 25-hydroxycholesterol. Isolation of microsomes from these cells showed levels of 7-alpha-hydroxylase activity (22.9 pmol/min/mg of protein) that were comparable to the activity (33.2 pmol/min/mg) of microsomes isolated from the livers of rats killed during the high point of the diurnal cycle. Parental cells had no detectable activity. These data show a new complementation group for 25-hydroxycholesterol resistance: expression of 7-alpha-hydroxylase. Dexamethasone increased both the activity and the cellular content of mRNA coding for 7-alpha-hydroxylase. Since dactinomycin blocked the ability of dexamethasone to induce mRNA, active transcription is required. Southern analysis of genomic DNA showed that L35 cells contain the rat (endogenous) gene but not the human gene. Furthermore, the RNA expressed by L35 cells is similar in size to rat RNA and is distinct from the human form of 7-alpha-hydroxylase. The combined data indicate that L35 cells are resistant to 25-hydroxycholesterol because they express 7-alpha-hydroxylase. The mechanism responsible involves activation of the endogenous (silent) gene of the parental rat hepatoma cell.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaki Y., Kok E., Javitt N. B. Cholic acid synthesis from 26-hydroxycholesterol and 3-hydroxy-5-cholestenoic acid in the rabbit. J Biol Chem. 1989 Mar 5;264(7):3818–3821. [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Borchardt R. A., Davis R. A. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987 Dec 5;262(34):16394–16402. [PubMed] [Google Scholar]
- Chang E., Perlman A. J. Multiple hormones regulate angiotensinogen messenger ribonucleic acid levels in a rat hepatoma cell line. Endocrinology. 1987 Aug;121(2):513–519. doi: 10.1210/endo-121-2-513. [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Chang C. C. Revertants of a Chinese hamster ovary cell mutant resistant to suppression by an analogue of cholesterol: isolation and partial biochemical characterization. Biochemistry. 1982 Oct 12;21(21):5316–5323. doi: 10.1021/bi00264a030. [DOI] [PubMed] [Google Scholar]
- Chen H. W., Cavenee W. K., Kandutsch A. A. Sterol synthesis in variant Chinese hamster lung cells selected for resistance to 25-hydroxycholesterol. Cross-resistance to 7-ketocholesterol, 20alpha-hydroxycholesterol, and serum. J Biol Chem. 1979 Feb 10;254(3):715–720. [PubMed] [Google Scholar]
- Chiang J. Y., Miller W. F., Lin G. M. Regulation of cholesterol 7 alpha-hydroxylase in the liver. Purification of cholesterol 7 alpha-hydroxylase and the immunochemical evidence for the induction of cholesterol 7 alpha-hydroxylase by cholestyramine and circadian rhythm. J Biol Chem. 1990 Mar 5;265(7):3889–3897. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke C. F., Fogelman A. M., Edwards P. A. Diurnal rhythm of rat liver mRNAs encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase. Correlation of functional and total mRNA levels with enzyme activity and protein. J Biol Chem. 1984 Aug 25;259(16):10439–10447. [PubMed] [Google Scholar]
- Danielsson H. Relationship between diurnal variations in biosynthesis of cholesterol and bile acids. Steroids. 1972 Jul;20(1):63–72. doi: 10.1016/0039-128x(72)90118-3. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Hyde P. M., Kuan J. C., Malone-McNeal M., Archambault-Schexnayder J. Bile acid secretion by cultured rat hepatocytes. Regulation by cholesterol availability. J Biol Chem. 1983 Mar 25;258(6):3661–3667. [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Drevon C. A., Weinstein D. B., Steinberg D. Regulation of cholesterol esterification and biosynthesis in monolayer cultures of normal adult rat hepatocytes. J Biol Chem. 1980 Oct 10;255(19):9128–9137. [PubMed] [Google Scholar]
- Everson G. T., Polokoff M. A. HepG2. A human hepatoblastoma cell line exhibiting defects in bile acid synthesis and conjugation. J Biol Chem. 1986 Feb 15;261(5):2197–2201. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes L. M., Verboom H., de Wit E., Yap S. H., Princen H. M. Regulation of low density lipoprotein receptor activity in primary cultures of human hepatocytes by serum lipoproteins. Hepatology. 1986 Nov-Dec;6(6):1356–1360. doi: 10.1002/hep.1840060623. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Gurley E. C., Kubaska W. M., Whitehead T. R., Guzelian P. S., Vlahcevic Z. R. Suitability of primary monolayer cultures of adult rat hepatocytes for studies of cholesterol and bile acid metabolism. J Biol Chem. 1985 Jan 25;260(2):1015–1019. [PubMed] [Google Scholar]
- Jelinek D. F., Andersson S., Slaughter C. A., Russell D. W. Cloning and regulation of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J Biol Chem. 1990 May 15;265(14):8190–8197. [PMC free article] [PubMed] [Google Scholar]
- Jelinek D. F., Russell D. W. Structure of the rat gene encoding cholesterol 7 alpha-hydroxylase. Biochemistry. 1990 Aug 28;29(34):7781–7785. doi: 10.1021/bi00486a001. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C., Wang D. P., Chiang J. Y. Regulation of cholesterol 7 alpha-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7 alpha-hydroxylase mRNA. J Biol Chem. 1990 Jul 15;265(20):12012–12019. [PubMed] [Google Scholar]
- Metherall J. E., Goldstein J. L., Luskey K. L., Brown M. S. Loss of transcriptional repression of three sterol-regulated genes in mutant hamster cells. J Biol Chem. 1989 Sep 15;264(26):15634–15641. [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S. The role of glucocorticoids in the regulation of the diurnal rhythm of hepatic beta-hydroxy-beta-methylglutaryl-coenzyme A reductase and cholesterol 7 alpha-hydroxylase. Biochem J. 1976 Oct 15;160(1):49–55. doi: 10.1042/bj1600049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myant N. B., Mitropoulos K. A. Cholesterol 7 alpha-hydroxylase. J Lipid Res. 1977 Mar;18(2):135–153. [PubMed] [Google Scholar]
- Noshiro M., Okuda K. Molecular cloning and sequence analysis of cDNA encoding human cholesterol 7 alpha-hydroxylase. FEBS Lett. 1990 Jul 30;268(1):137–140. doi: 10.1016/0014-5793(90)80992-r. [DOI] [PubMed] [Google Scholar]
- Osborne T. F., Gil G., Goldstein J. L., Brown M. S. Operator constitutive mutation of 3-hydroxy-3-methylglutaryl coenzyme A reductase promoter abolishes protein binding to sterol regulatory element. J Biol Chem. 1988 Mar 5;263(7):3380–3387. [PubMed] [Google Scholar]
- Polokoff M. A., Everson G. T. Hepatocyte-hepatoma cell hybrids. Characterization and demonstration of bile acid synthesis. J Biol Chem. 1986 Mar 25;261(9):4085–4089. [PubMed] [Google Scholar]
- Princen H. M., Huijsmans C. M., Kuipers F., Vonk R. J., Kempen H. J. Ketoconazole blocks bile acid synthesis in hepatocyte monolayer cultures and in vivo in rat by inhibiting cholesterol 7 alpha-hydroxylase. J Clin Invest. 1986 Oct;78(4):1064–1071. doi: 10.1172/JCI112662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Hofstee B. Dexamethasone regulates bile acid synthesis in monolayer cultures of rat hepatocytes by induction of cholesterol 7 alpha-hydroxylase. Biochem J. 1989 Aug 15;262(1):341–348. doi: 10.1042/bj2620341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., JAYKO M. E., CHAIKOFF I. L., DAUBEN W. G. Nature of the metabolic products of C14-cholesterol excreted in bile and feces. Proc Soc Exp Biol Med. 1952 Dec;81(3):720–724. doi: 10.3181/00379727-81-19999. [DOI] [PubMed] [Google Scholar]
- Silver G., Reid L. M., Krauter K. S. Dexamethasone-mediated regulation of 3-methylcholanthrene-induced cytochrome P-450d mRNA accumulation in primary rat hepatocyte cultures. J Biol Chem. 1990 Feb 25;265(6):3134–3138. [PubMed] [Google Scholar]
- Simmons D. L., McQuiddy P., Kasper C. B. Induction of the hepatic mixed-function oxidase system by synthetic glucocorticoids. Transcriptional and post-transcriptional regulation. J Biol Chem. 1987 Jan 5;262(1):326–332. [PubMed] [Google Scholar]
- Sinensky M. Defective regulation of cholesterol biosynthesis and plasma membrane fluidity in a Chinese hamster ovary cell mutant. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1247–1249. doi: 10.1073/pnas.75.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Straka M. S., Junker L. H., Zacarro L., Zogg D. L., Dueland S., Everson G. T., Davis R. A. Substrate stimulation of 7 alpha-hydroxylase, an enzyme located in the cholesterol-poor endoplasmic reticulum. J Biol Chem. 1990 May 5;265(13):7145–7149. [PubMed] [Google Scholar]
- Swell L., Schwartz C. C., Gustafsson J., Danielsson H., Vlahcevic Z. R. A quantitative evaluation of the conversion of 25-hydroxycholesterol to bile acids in man. Biochim Biophys Acta. 1981 Jan 26;663(1):163–168. doi: 10.1016/0005-2760(81)90202-2. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Russell D. W., Brown M. S., Goldstein J. L. 42 bp element from LDL receptor gene confers end-product repression by sterols when inserted into viral TK promoter. Cell. 1987 Mar 27;48(6):1061–1069. doi: 10.1016/0092-8674(87)90713-6. [DOI] [PubMed] [Google Scholar]