Summary
An 87-year-old man was found in a state of cardiopulmonary arrest. Despite cardiopulmonary resuscitation (CPR) for over 1 hour by emergency technicians and physicians, the patient died. Immediate postmortem computed tomography showed cardiovascular gas in the right atrium, right ventricle, and left ventricle. Cardiovascular gas in the left ventricle was located in the myocardium and appeared as linear or branch-shaped suggesting the presence of myocardial intravascular gas. This is the first report describing the appearance and significance of myocardial intravascular gas of the left ventricle as a CPR-related change.
Keywords: Postmortem computed tomography (PMCT), Cardiopulmonary resuscitation (CPR), Cardiovascular gas (CVG), Supersaturation, Tribonucleation
Introduction
Due to the worldwide decline in conventional autopsy rates, the need for and frequency of postmortem imaging as a complementary, supplementary or alternative method for autopsy have increased worldwide (Brogdon 1998; Swift & Rutty 2006; Oesterhelweg & Thali 2009; Roberts et al. 2012; Wichmann et al. 2012; Takahashi et al. 2012; Okuda et al. 2012; Lundstrom et al. 2012; Daly et al. 2012). Interpreting whether findings are related to cause of death requires knowledge of normal postmortem computed tomography (PMCT) findings. PMCT findings are classified into three categories (Takahashi et al. 2011): (1) causes of death and associated changes, (2) postmortem changes, and (3) cardiopulmonary resuscitation (CPR)-related changes. Intravascular gas as CPR-related changes on early non-traumatic PMCT obtained within a few hours after confirmation of death have been reported including hepatic vascular gas, cerebral vascular gas, and cardiovascular gas (CVG) (Shiotani et al. 2004; Shiotani et al. 2005; Yokota et al. 2009; Takahashi et al. 2009; Shiotani et al. 2010; Zenda et al. 2011; Ishida et al. 2011). Laurent et al. verified that CPR induces intravascular gas using porcine experimental models (Laurent et al. 2013).
CVG on early non-traumatic PMCT often shows gas bubbles along the anterior walls of the right atrium (RA) and right ventricle (RV) (Shiotani et al. 2005). The causes of such CVG are venous catheterization that permits possible air inflow and pneumatization of dissolved gas in the blood as a result of chest compression (Shiotani et al. 2005). However, to our knowledge, there has been no paper which describes myocardial intravascular gas of the left ventricle (LV) as a CPR-related change. Herein, we report a sudden death case involving CPR and in which PMCT images showed gas of the LV immediately after death.
Case report
An 87-year-old man suddenly mis-swallowed food, impairing respiration, which caused him to collapse and lose consciousness. When emergency technicians arrived at the site, he was in a state of cardiopulmonary arrest (CPA). CPR was performed for 40 min by emergency technicians during transport to our emergency room (ER) and by emergency medical physicians for an additional 30 min in our ER. CPR was performed by continuous external chest compression, artificial respiration with bag-valve mask ventilation following endotracheal intubation, and peripheral intravenous catheterization following administration of epinephrine at 1 mg. However, the CPR was ineffective and death was confirmed.
After investigation of the location where the man was found in CPA, and subsequent cadaveric examination, the police rejected the cause of death due to traumatic accident or criminal reason. As the cause of death re-mained to be undetermined and the family did not want an autopsy done, PMCT was performed immediately after the confirmation of death using a clinical scanner in the Radiology Department of our institution with the prior approval of the institutional review board. The corpse exhibited no signs of putrefaction at the time of the scan.
PMCT was performed with a 64-channel multidetector-row CT scanner (Lightspeed VCT; GE Healthcare, Milwaukee, USA). The scan parameters for the thorax, abdomen, and pelvis were determined for helical scan mode with settings of auto mA (standard deviation value: 20), 120 kV, 1.0 second/rotation, 0.625 mm collimation, pitch 1.375, and contiguous 1.3-mm sections. The scan parameters for the heart were determined for conventional scan mode with settings of auto mA, 120 kV, 2.0 seconds/rotation, 0.625 mm collimation, and contiguous 1.3-mm sections. We obtained image reformations of the contiguous 1.3 mm sections in short-axis and horizontal long-axis plane from 0.625 mm axial reconstructions. All images were observed on a 21-inch monochrome monitor with a resolution of 1,600 × 1,200 pixels at appropriate window settings for each region. A board-certified diagnostic radiologist with 21 years of experience and a subspecialty in forensic radiology judged CVG to be present when air density, which is lower than that of the lung, was observed in the vessels at the lung window setting (window level/width = -600/1600).
PMCT of the thorax showed airless bilateral bronchi filled with fluid. Based on a comprehensive understanding of the patient’s clinical course and PMCT findings, cause of death was diagnosed mis-swallowing.
Multiple intravascular gases were shown by PMCT in the brain, bilateral jugular veins, bilateral brachiocephalic veins, RA, RV, LV, and the liver. CVG in the LV was located in the myocardium and appeared as a spotty, linear, or branch-shaped, suggesting the presence of myocardial intravascular gas (Figure 1). The exact location of these gases could not be differentiated as either arterial gas, venous gas, or both. Vessels in the epicardial fat connected with coronary sinus contained spotty gas, suggesting the gas was intravenous.
Figure 1.
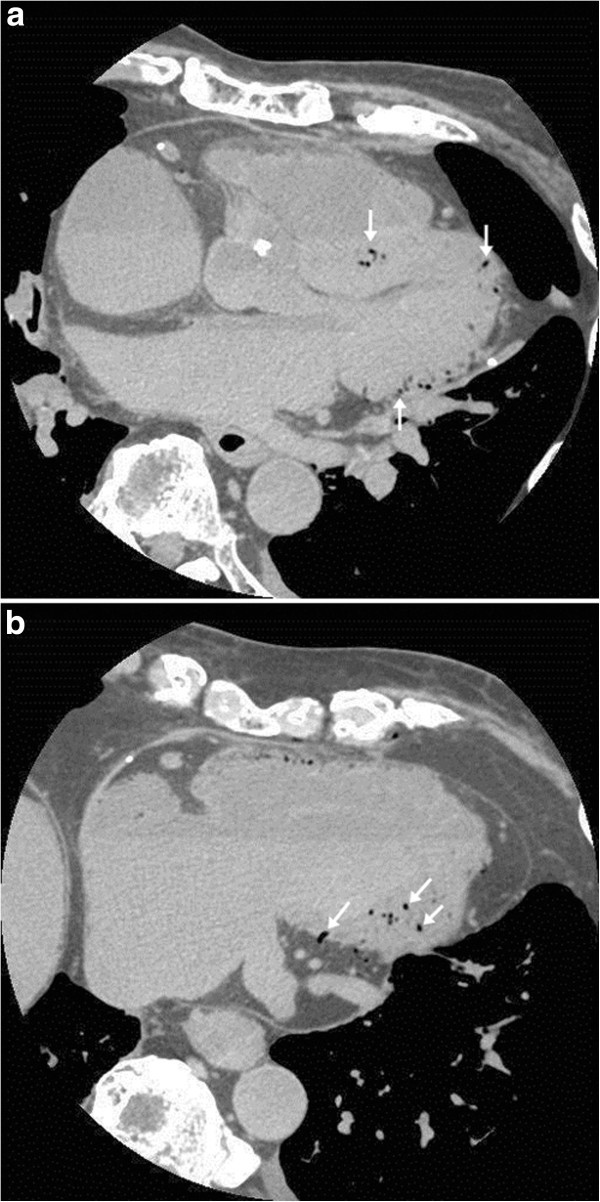
Myocardial intravascular gas of the left ventricle on postmortem CT of the heart. a. Axial image at the level of aortic root shows intravascular gas in the ventricular septum, apex, and posterior wall (arrows). b. Axial image at the level of inferior wall shows intravascular gas in the inferior wall (arrows).
Discussion
To evaluate intravascular gas as a CPR-related change, PMCT examination needs to be done as soon as possible after death, to avoid effects from putrefaction. The patient in this study underwent PMCT immediately after confirmation of death, and thus the effects from putrefaction are considered minimal.
One of the causes of CVG on early non-traumatic PMCT is pneumatization of dissolved gas in the blood as a result of chest compression (Shiotani et al. 2005). This physiological phenomenon is called tribonucleation (= exercise-induced cavitation) and can generate gas nuclei (Roston & Haines 1947; Ikels 1970). The veins are more likely to collapse and expand in response to CPR than the arteries, and gas nuclei tend to be produced (Ikels 1970). Therefore, myocardial intravascular gas of the LV is considered to have occurred intravenously. However, tribonucleation alone cannot explain why CVG is seen in the RA, RV, and myocardial vein of the LV, but not in the LA. The thoracic pump theory suggests that chest compression produces a rise in the intrathoracic pressure that is transmitted equally to all intrathoracic vascular structures (Criley et al. 1976; Radikoff et al. 1980; Babbs 1980).
The common factor among the RA, RV, and the myocardial vein of the LV is that these include venous blood and the partial pressure of carbon dioxide (PCO2) is high. Gas nuclei grow to become visible, and stable gas bubbles depend on the dissolved CO2 gas concentration (Wilbur et al. 2010; Lin et al. 2000). Partial venous pressure of CO2 (PvCO2) elevates during CPR and its causes are as follows (Suwa 1992; Halmagyi et al. 1970; Suwa et al. 1969; Chazan et al. 1968; Weil et al. 1986; Gudipati et al. 1990; Lindner et al. 1991): 1) increased arterial-venous difference of PCO2 due to low cardiac output, 2) increased CO2 production due to anaerobic metabolism progression, and 3) decreased CO2 transport by the blood. Normal partial arterial pressure (Pa) and partial venous pressure (Pv) are as follows (Hills 1985; Liew et al. 1993): PaH2O = PvH2O = 47 mmHg, PaN2 = PvN2 = 573 mmHg, PaCO2 = 40 mmHg, PvCO2 = 46 mmHg, PaO2 = 88 mmHg, PvO2 = 40 mmHg, Pa inherent unsaturation (IU) = 12 mmHg, and PvIU = 54 mmHg. During CPR, PH2O (47 mmHg) and PN2 (573 mmHg) are stable, but the PO2 and PCO2 change. In cases where the outcome of CPR is not successful, PvCO2 markedly increases, and this additional CO2 may be sufficient to exceed the IU, and the total pressure in the venous blood exceeds atmospheric pressure (760 mmHg); consequently the venous blood is supersaturated and releases its excess solute CO2 as gas bubbles (Hills 1977; Rasmussen et al. 1999). CVG in the RA, RV, and myocardial vein of the LV are changes related to CPR, and may be a sign of death.
Our study has some limitations. First, the rate of occurrence is still unknown regarding the appearance of myocardial intravascular gas in the LV on non-traumatic PMCT performed immediately after certification of death as a CPR-related change. Although this phenomenon is anticipated to occur as frequently as CVG in the RA and RV, the occurrence may depend on the strength and duration of chest compression. Second, PMCT findings could not morphologically differentiate whether the myocardial intravascular gas of the LV was in the artery or in the vein. However, tribonucleation and supersaturation of CO2, which are the two predominant theories of gas nuclei and bubbles origin (Wilbur et al. 2010; Lin et al. 2000), suggest that the gases were in the vein. Additionally, the presence of intravascular gas in the venula of pericardial fat may be indirect evidence. Third, it was not clear whether or not the major component of the myocardial intravascular gas of the LV was CO2. As collection and analysis of the gas during autopsy is difficult, it is not possible to determine the gas component. These limitations may be overcome by further experimental and clinical study. Meanwhile, the promising results presented here may serve to guide interpretation of PMCT following CPR.
Acknowledgments
This work was supported by a grant from the Daiwa Securities Health Foundation. The authors thank Ms. Yumiko Moriyama for assisting in the manuscript preparation.
Abbreviations
- RA
Right atrium
- RV
Right ventricle
- LA
Left atrium
- LV
Left ventricle
- P
Partial pressure
- Pa
Arterial partial pressure
- Pv
Venous partial pressure
- CO2
Carbon dioxide
- O2
Oxygen
- N2
Nitrogen
- H2O
Water
- IU
Inherent unsaturation.
Footnotes
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
TO, SS, and KS conceived and designed the study. SS, TK, and MK acquired the data. All authors analysed and interpreted the data. TO and SS drafted, edited and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Takahisa Okuda, Email: takahisa.okuda@gmail.com.
Seiji Shiotani, Email: shiotani@tmch.or.jp.
Tomoya Kobayashi, Email: t.kobayashi1001@gmail.com.
Mototsugu Kohno, Email: kono@tmch.or.jp.
Hideyuki Hayakawa, Email: hayakawa@tmch.or.jp.
Kazunori Kikuchi, Email: k-kikuchi@tmch.or.jp.
Kunio Suwa, Email: kunio.suwa@nifty.ne.jp.
References
- Babbs CF. New versus old theories of blood flow during cardiopulmonary resuscitation. Crit Care Med. 1980;8:191–195. doi: 10.1097/00003246-198003000-00026. [DOI] [PubMed] [Google Scholar]
- Brogdon BG. Research and applications of the new modalities. In: Brogdon BG, editor. Forensic Radiology. 1. Boca Raton: CBC Press; 1998. pp. 333–338. [Google Scholar]
- Chazan JA, Stenson R, Kurland GS. The acidosis of cardiac arrest. New Eng J Med. 1968;278:360–364. doi: 10.1056/NEJM196802152780703. [DOI] [PubMed] [Google Scholar]
- Criley JM, Blaufuss AH, Kissel GL. Cough-induced cardiac compression. Self-administration from of cardiopulmonary resuscitation. JAMA. 1976;236:1246–1250. [PubMed] [Google Scholar]
- Daly B, Abboud S, Ali Z, Sliker C, Fowler D. Comparison of whole-body post mortem 3D CT and autopsy evaluation in accidental blunt force traumatic death using the abbreviated injury scale classification. Forensic Sci Int. 2013;225:20–26. doi: 10.1016/j.forsciint.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Gudipati CV, Weil MH, Gazmuri RJ, Deshmukh HG, Bisera J, Rackow EC. Increase in coronary vein CO2 during cardiac resuscitation. J Appl Physiol. 1990;68:1405–1408. doi: 10.1152/jappl.1990.68.4.1405. [DOI] [PubMed] [Google Scholar]
- Halmagyi DFG, Kennedy M, Varga D. Hidden hypercapnea in hemorrhagic hypotension. Anesthesiology. 1970;33:594–601. doi: 10.1097/00000542-197012000-00003. [DOI] [PubMed] [Google Scholar]
- Hills BA. Supersaturation by counterperfusion and diffusion of gases. J App Physiol. 1977;42:758–760. doi: 10.1152/jappl.1977.42.5.758. [DOI] [PubMed] [Google Scholar]
- Hills BA. The pleural interface. Thorax. 1985;40:1–8. doi: 10.1136/thx.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikels KG. Production of gas bubbles in fluids by tribonucleation. J Appl Phys. 1970;28:524–527. [Google Scholar]
- Ishida M, Gonoi W, Hagiwara K, Takazawa Y, Akahane M, Fukayama M, Ohtomo K. Intravascular gas distribution in the upper abdomen of non-traumatic in-hospital death cases on postmortem computed tomography. Leg Med (Tokyo) 2011;13:174–179. doi: 10.1016/j.legalmed.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Laurent PE, Coulange M, Bartoli C, Boussuges A, Rostain JC, Luciano M, et al. Appearance of gas collections after scuba diving death: a computed tomography study in a porcine model. Int J Legal Med. 2013;127:177–184. doi: 10.1007/s00414-011-0662-6. [DOI] [PubMed] [Google Scholar]
- Liew V, Hugh D, Conkin J, Burkard ME. The oxygen window and decompression bubbles: estimates and significance. Aviation, Space, and Environmental Medicine. 1993;64:859–865. [PubMed] [Google Scholar]
- Lin HY, Bianccucci BA, Deutsch S, Fontaine AA, Tarbell JM. Observation and quantification of gas bubble formation on a mechanical heart valve. ASME J Biomech Eng. 2000;122:304–309. doi: 10.1115/1.1287171. [DOI] [PubMed] [Google Scholar]
- Lindner KH, Ahnefeld FW, Bowdler IM, Prengel AW. Influence of epinephrine on systemic, myocardial, and cerebral acid-base status during cardiopulmonary resuscitation. Anesthesiology. 1991;74:333–339. doi: 10.1097/00000542-199102000-00021. [DOI] [PubMed] [Google Scholar]
- Lundstrom C, Persson A, Ross S, Ljung P, Lindholm S, Gyllensvard F, Ynnerman A. State-of-the-art of visualization in post-mortem imaging. APMIS. 2012;120:316–326. doi: 10.1111/j.1600-0463.2011.02857.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelweg L, Thali MJ. Experiences with virtual autopsy approach worldwide. In: Thali MJ, Dirnhofer R, Vock P, editors. The virtopsy approach. 1. Boca Raton: CRC Press; 2009. pp. 475–477. [Google Scholar]
- Okuda T, Shiotani S, Sakamoto N, Kobayashi T. Background and current status of postmortem imaging in Japan: short history of ‘Autopsy imaging (Ai) Forensic Sci Int. 2013;225:3–8. doi: 10.1016/j.forsciint.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Radikoff MT, Maughan WL, Effron M, Freund P, Weisfeldt ML. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation. 1980;61:345–352. doi: 10.1161/01.CIR.61.2.345. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Kvarstein G, Johnsen H, Dirven H, Midtvedt T, Mirtaheri P, et al. Gas supersaturation in the cecal wall of mice due to bacterial CO2 production. J App Physiol. 1999;86:1311–1318. doi: 10.1063/1.370887. [DOI] [PubMed] [Google Scholar]
- Roberts IS, Benamore RE, Benbow EW, Lee SH, Harris JN, Jackson A, et al. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: a validation study. Lancet. 2012;379:136–142. doi: 10.1016/S0140-6736(11)61483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roston JB, Haines RW. Cracking in the metacarpo-phalangeal joint. J Anatomy. 1947;81:165–173. [PMC free article] [PubMed] [Google Scholar]
- Shiotani S, Kohno M, Ohashi N, Yamazaki K, Nakayama H, Watanabe K. Postmortem computed tomographic (PMCT) demonstration of the relation between gastrointestinal (GI) distension and hepatic portal venous gas (HPVG) Radiat Med. 2004;22:25–29. [PubMed] [Google Scholar]
- Shiotani S, Kohno M, Ohashi N, Atake S, Yamazaki K, Nakayama H. Cardiovascular gas on non-traumatic postmortem computed tomography (PMCT): the influence of cardiopulmonary resuscitation. Radiat Med. 2005;23:225–229. [PubMed] [Google Scholar]
- Shiotani S, Ueno Y, Atake S, Kohno M, Suzuki M, Kikuchi K, Hayakawa H. Nontraumatic postmortem computed tomographic demonstration of cerebral gas embolism following cardiopulmonary resuscitation. Jpn J Radiol. 2010;28:1–7. doi: 10.1007/s11604-009-0372-x. [DOI] [PubMed] [Google Scholar]
- Suwa K. Cardiopulmonary resuscitation and mixed venous partial pressure of carbon dioxcide. Kokyu to Junkan (Respiration and Circulation) 1992;40:759–763. [PubMed] [Google Scholar]
- Suwa K, Yamaguchi Y, Yamamura H. Arterial-alveolar CO2 gradient after cardiac resuscitation in the dog. Anesthesiology. 1969;30:37–42. doi: 10.1097/00000542-196901000-00016. [DOI] [PubMed] [Google Scholar]
- Swift B, Rutty GN. Recent advances in postmortem forensic radiology – computed tomography and magnetic resonance imaging applications. In: Tsokos M, editor. Forensic Pathology Reviews. 1. Totowa: Humana Press Inc; 2006. pp. 355–404. [Google Scholar]
- Takahashi N, Higuchi T, Shiotani M, Maeda H, Hirose Y. Intrahepatic gas at postmortem multislice computed tomography in cases of nontraumatic death. Jpn J Radiol. 2009;27:264–268. doi: 10.1007/s11604-009-0337-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Higuchi T, Shiotani M, Satou S, Hirose Y. Effectiveness of a worksheet for diagnosing postmortem computed tomography in emergency departments. Jpn J Radiol. 2011;29:701–706. doi: 10.1007/s11604-011-0618-2. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Higuchi T, Shiotani M, Hirose Y, Shibuya H, Yamanouchi H, et al. The effectiveness of postmortem multidetector computed tomography in the detection of fatal findings related to cause of non-traumatic death in the emergency department. Eur Radiol. 2012;22:152–160. doi: 10.1007/s00330-011-2248-6. [DOI] [PubMed] [Google Scholar]
- Weil MH, Rackow EC, Trevino R, Grundler W, Falk JL, Griffel MI. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med. 1986;315:153–156. doi: 10.1056/NEJM198607173150303. [DOI] [PubMed] [Google Scholar]
- Wichmann D, Obbelode F, Vogel H, Hoepker WW, Nierhaus A, Braune S, et al. Virtual autopsy as an alternative to traditional medical autopsy in the intensive care unit: a prospective study. Ann Intern Med. 2012;156:123–130. doi: 10.7326/0003-4819-156-2-201201170-00008. [DOI] [PubMed] [Google Scholar]
- Wilbur JC, Phillips SD, Donoghue TG, Alvarenga DL, Knaus DA, Magari PJ, et al. Signals consistent with microbubbles detected in legs of normal human subjects after exercise. J Appl Physiol. 2010;108:240–244. doi: 10.1152/japplphysiol.00615.2009. [DOI] [PubMed] [Google Scholar]
- Yokota H, Yamamoto S, Horikoshi T, Shimofusa R, Ito H. What is the origin of intravascular gas on postmortem computed tomography? Leg Med (Tokyo) 2009;11(Suppl 1):252–255. doi: 10.1016/j.legalmed.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Zenda T, Takayama T, Miyamoto M, Yamaguchi S, Endo T, Inaba H. Intravascular gas in multiple organs detected by postmortem computed tomography: effect of prolonged cardiopulmonary resuscitation on organ damage in patients with cardiopulmonary arrest. Jpn J Radiol. 2011;29:148–151. doi: 10.1007/s11604-010-0511-4. [DOI] [PubMed] [Google Scholar]


