Abstract
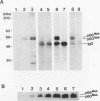
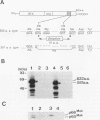
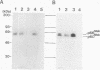
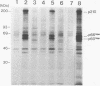
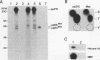
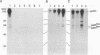
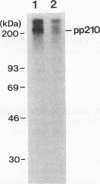
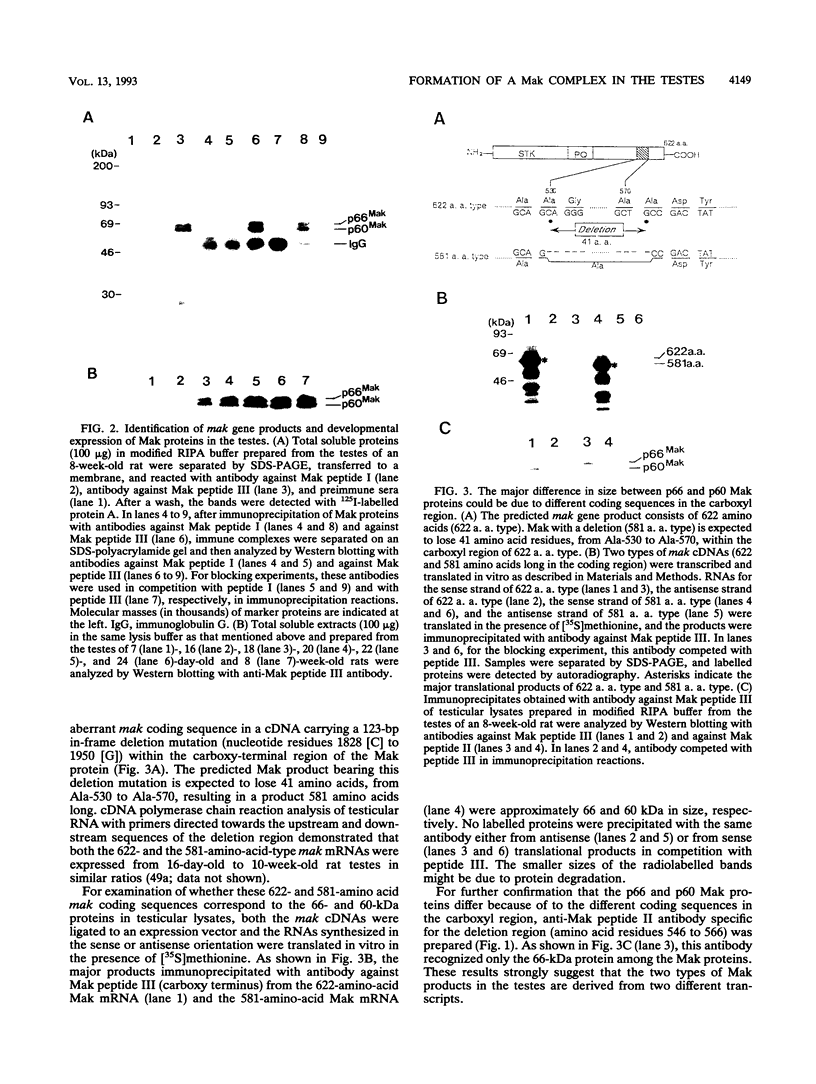
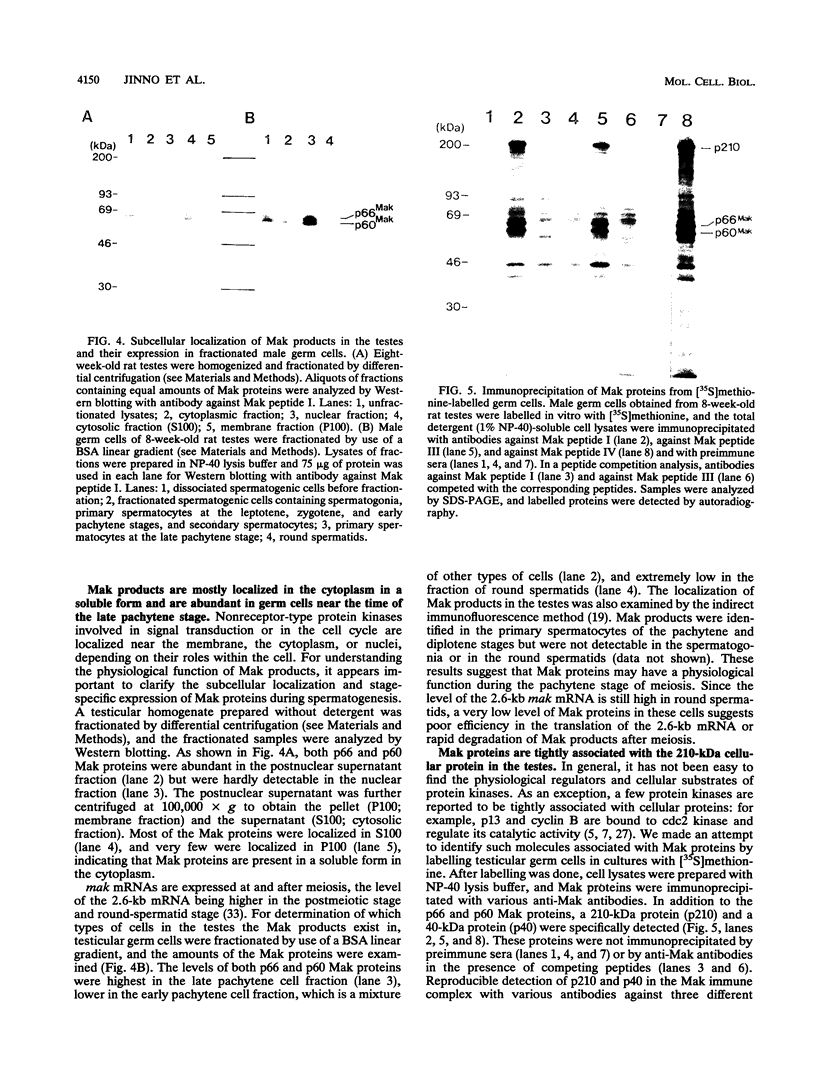
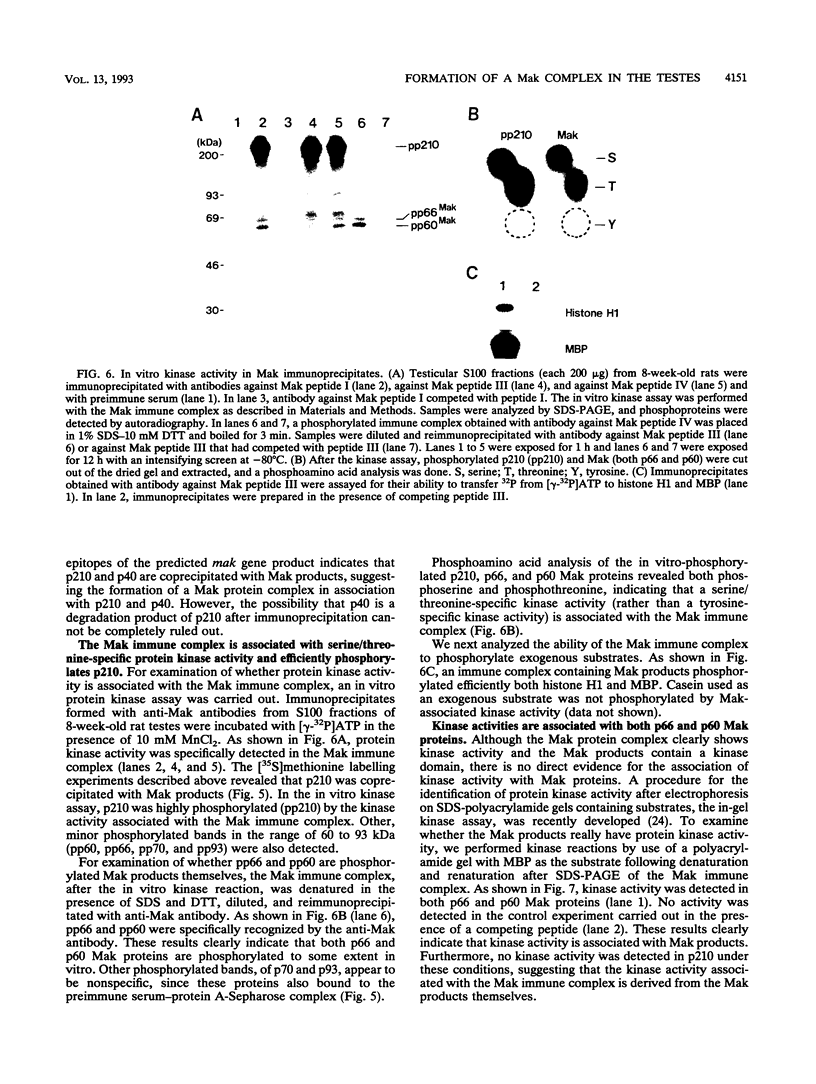
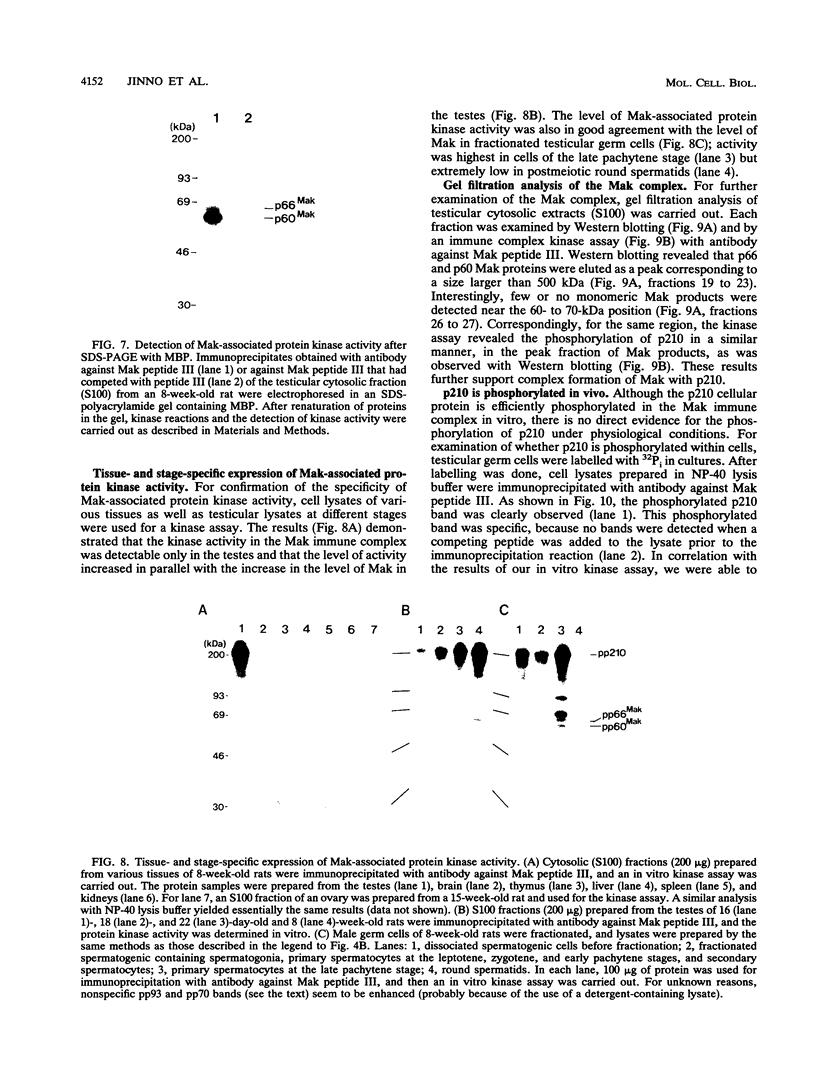
The mak gene encodes a new protein kinase distantly related to cdc2 kinase, and its transcripts are expressed exclusively in testicular germ cells at and after meiosis (H. Matsushime, A. Jinno, N. Takagi, and M. Shibuya, Mol. Cell. Biol. 10:2261-2268, 1990). In this study, we prepared a series of antibodies against synthetic peptides and fusion products of the mak gene and characterized the subcellular localization, protein kinase activity, and association with other cellular proteins of Mak. Mak products were identified as 66- and 60-kDa proteins that specifically appeared in rat testes after puberty. Testicular germ cell fractionation revealed that Mak products were most abundant in the fraction of the late pachytene stage and that their levels were dramatically decreased in postmeiotic haploid cells. Mak products were localized mostly in the cytoplasm as a soluble form. [35S]methionine labelling demonstrated that Mak products were associated with a 210-kDa cellular protein; in an in vitro kinase assay with immunoprecipitates of Mak products, the 210-kDa cellular protein was efficiently phosphorylated on serine and threonine residues. Furthermore, in a testicular cell culture system with 32Pi, the 210-kDa molecule associated with Mak was phosphorylated in vivo on serine and threonine residues. These results strongly suggest that the Mak complex may play a role in meiosis during spermatogenesis and that a phosphorylated 210-kDa protein is one of the physiological substrates for this protein kinase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Allen R. L., O'Brien D. A., Eddy E. M. A novel hsp70-like protein (P70) is present in mouse spermatogenic cells. Mol Cell Biol. 1988 Feb;8(2):828–832. doi: 10.1128/mcb.8.2.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. L., O'Brien D. A., Jones C. C., Rockett D. L., Eddy E. M. Expression of heat shock proteins by isolated mouse spermatogenic cells. Mol Cell Biol. 1988 Aug;8(8):3260–3266. doi: 10.1128/mcb.8.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989 Dec 20;8(13):3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R. N., Alfa C. E., Hyams J. S., Beach D. H. The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell. 1989 Aug 11;58(3):485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987 Nov;6(11):3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr, Karsenti E., Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991 Nov;10(11):3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Spottswood M. R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman K., Edman J. C., Shackleford G. M., Turner J. A., Rutter W. J., Nir U. A murine fer testis-specific transcript (ferT) encodes a truncated Fer protein. Mol Cell Biol. 1990 Jan;10(1):146–153. doi: 10.1128/mcb.10.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B., Fujimoto H., Kramer J. M., Erickson R. P., Hecht N. B. Haploid accumulation and translational control of phosphoglycerate kinase-2 messenger RNA during mouse spermatogenesis. Dev Biol. 1983 Aug;98(2):392–399. doi: 10.1016/0012-1606(83)90368-8. [DOI] [PubMed] [Google Scholar]
- Goto M., Koji T., Mizuno K., Tamaru M., Koikeda S., Nakane P. K., Mori N., Masamune Y., Nakanishi Y. Transcription switch of two phosphoglycerate kinase genes during spermatogenesis as determined with mouse testis sections in situ. Exp Cell Res. 1990 Feb;186(2):273–278. doi: 10.1016/0014-4827(90)90306-u. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Moriyama K., Matsuda S., Okumura E., Kishimoto T., Kawasaki H., Suzuki K., Yahara I., Sakai H., Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991 Sep;10(9):2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Yamashita T., Hoshi M., Kawakami M., Sakai H. Microtubule-associated-protein (MAP) kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneji T., Koide S. S. Identification of antigen in rat spermatogenic cells interacting with an anti-human sperm monoclonal antibody. Biol Reprod. 1987 Sep;37(2):467–477. doi: 10.1095/biolreprod37.2.467. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hao Q. L., Heisterkamp N., Groffen J. Isolation and sequence analysis of a novel human tyrosine kinase gene. Mol Cell Biol. 1989 Apr;9(4):1587–1593. doi: 10.1128/mcb.9.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Activation of a Ca2+-inhibitable protein kinase that phosphorylates microtubule-associated protein 2 in vitro by growth factors, phorbol esters, and serum in quiescent cultured human fibroblasts. J Biol Chem. 1988 Apr 15;263(11):5396–5401. [PubMed] [Google Scholar]
- Iino Y., Yamamoto M. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2447–2451. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Keshet E., Itin A., Fischman K., Nir U. The testis-specific transcript (ferT) of the tyrosine kinase FER is expressed during spermatogenesis in a stage-specific manner. Mol Cell Biol. 1990 Sep;10(9):5021–5025. doi: 10.1128/mcb.10.9.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letwin K., Mizzen L., Motro B., Ben-David Y., Bernstein A., Pawson T. A mammalian dual specificity protein kinase, Nek1, is related to the NIMA cell cycle regulator and highly expressed in meiotic germ cells. EMBO J. 1992 Oct;11(10):3521–3531. doi: 10.1002/j.1460-2075.1992.tb05435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letwin K., Yee S. P., Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene. 1988 Dec;3(6):621–627. [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Jinno A., Takagi N., Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol. 1990 May;10(5):2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M., Beach D. A specific inhibitor of the ran1+ protein kinase regulates entry into meiosis in Schizosaccharomyces pombe. Nature. 1988 Apr 7;332(6164):509–514. doi: 10.1038/332509a0. [DOI] [PubMed] [Google Scholar]
- McLeod M., Beach D. Homology between the ran1+ gene of fission yeast and protein kinases. EMBO J. 1986 Dec 20;5(13):3665–3671. doi: 10.1002/j.1460-2075.1986.tb04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Nomoto S., Yasuda H., Reed S. I., Matsumoto K. Cloning of a human cDNA encoding a CDC2-related kinase by complementation of a budding yeast cdc28 mutation. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9006–9010. doi: 10.1073/pnas.88.20.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- O'Brien D. A. Stage-specific protein synthesis by isolated spermatogenic cells throughout meiosis and early spermiogenesis in the mouse. Biol Reprod. 1987 Aug;37(1):147–157. doi: 10.1095/biolreprod37.1.147. [DOI] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romrell L. J., Bellvé A. R., Fawcett D. W. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976 Mar;49(1):119–131. doi: 10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- Rossi P., Albanesi C., Grimaldi P., Geremia R. Expression of the mRNA for the ligand of c-kit in mouse Sertoli cells. Biochem Biophys Res Commun. 1991 Apr 30;176(2):910–914. doi: 10.1016/s0006-291x(05)80272-4. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Shiina N., Moriguchi T., Ohta K., Gotoh Y., Nishida E. Regulation of a major microtubule-associated protein by MPF and MAP kinase. EMBO J. 1992 Nov;11(11):3977–3984. doi: 10.1002/j.1460-2075.1992.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. E., Mitchell A. P. A transcriptional cascade governs entry into meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1989 May;9(5):2142–2152. doi: 10.1128/mcb.9.5.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V., Giorgi M., Geremia R., Besmer P., Rossi P. Expression of the c-kit proto-oncogene in the murine male germ cells. Oncogene. 1991 Jan;6(1):149–151. [PubMed] [Google Scholar]
- Tamaru M., Nagao Y., Taira M., Tatibana M., Masamune Y., Nakanishi Y. Selective activation of testis-specific genes in cultured rat spermatogenic cells. Biochim Biophys Acta. 1990 Jul 30;1049(3):331–338. doi: 10.1016/0167-4781(90)90106-c. [DOI] [PubMed] [Google Scholar]
- Witte O. N. Steel locus defines new multipotent growth factor. Cell. 1990 Oct 5;63(1):5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Viviano C. M., Gizang-Ginsberg E., Frohman M. A., Joyner A. L., Martin G. R. Differential expression of the mouse homeobox-containing gene Hox-1.4 during male germ cell differentiation and embryonic development. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5813–5817. doi: 10.1073/pnas.84.16.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Kawaguchi H., Sakata Y., Kominami K., Hirano M., Shima H., Akada R., Yamashita I. Initiation of meiosis and sporulation in Saccharomyces cerevisiae requires a novel protein kinase homologue. Mol Gen Genet. 1990 Apr;221(2):176–186. doi: 10.1007/BF00261718. [DOI] [PubMed] [Google Scholar]
- Zakeri Z. F., Wolgemuth D. J., Hunt C. R. Identification and sequence analysis of a new member of the mouse HSP70 gene family and characterization of its unique cellular and developmental pattern of expression in the male germ line. Mol Cell Biol. 1988 Jul;8(7):2925–2932. doi: 10.1128/mcb.8.7.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn F. A., Spiegel J. E., Maylié-Pfenninger M. F., Nordeen S. K. A 43 kD c-mos protein is only expressed before meiosis during rat spermatogenesis. Oncogene. 1991 Jun;6(6):929–932. [PubMed] [Google Scholar]