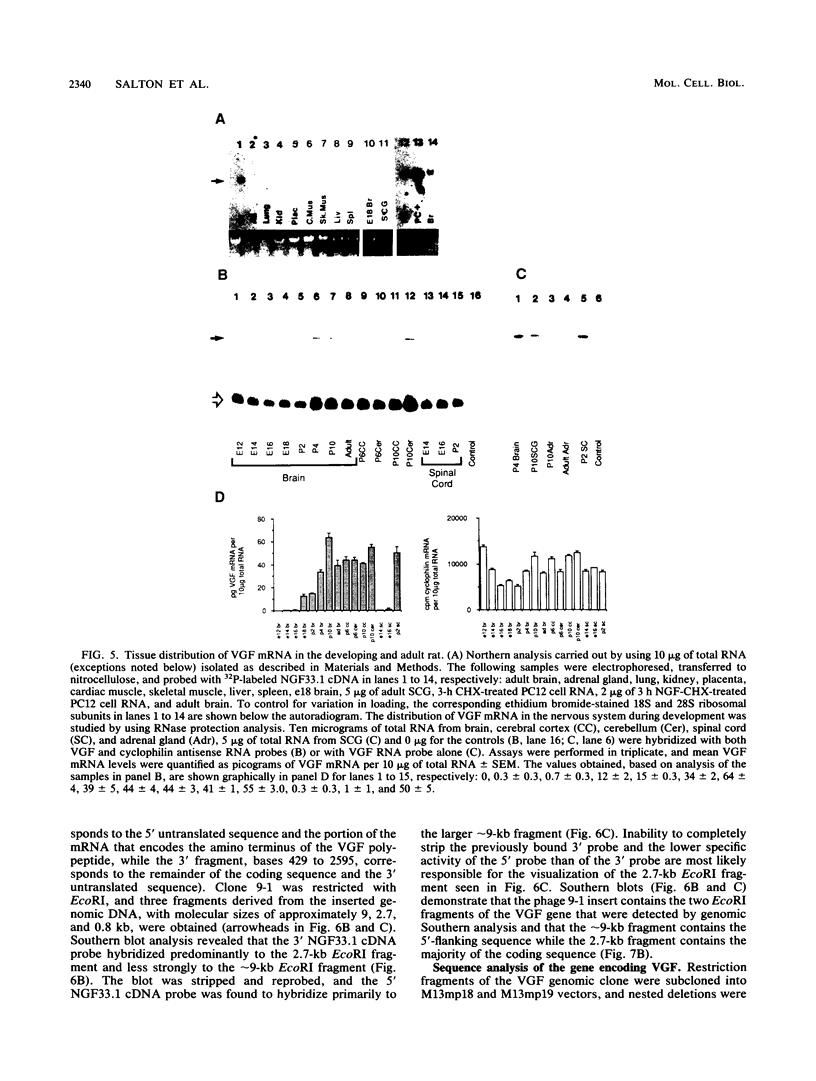
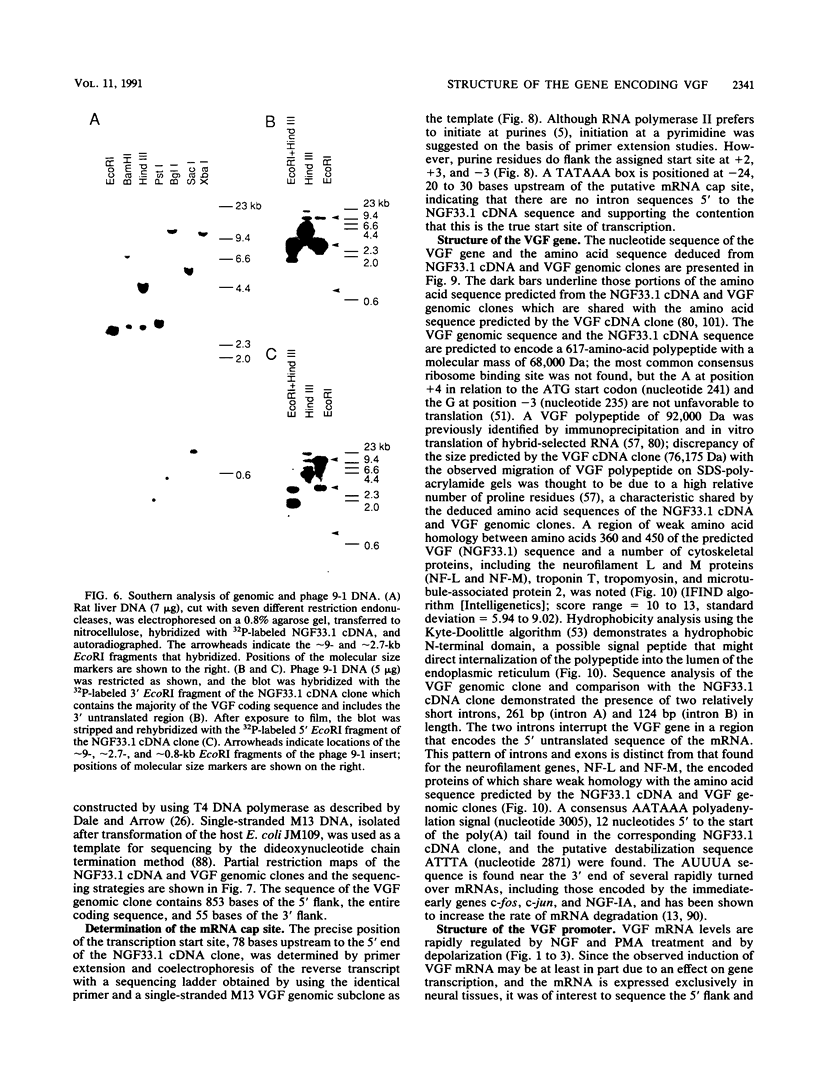
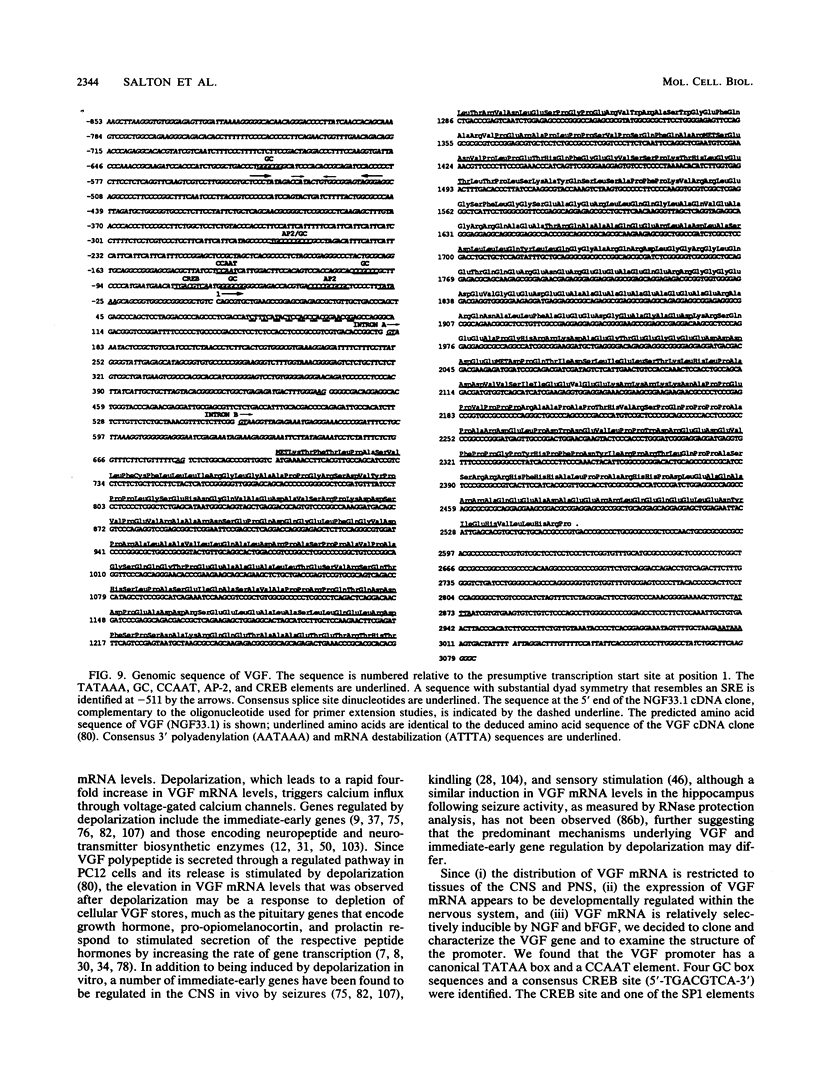
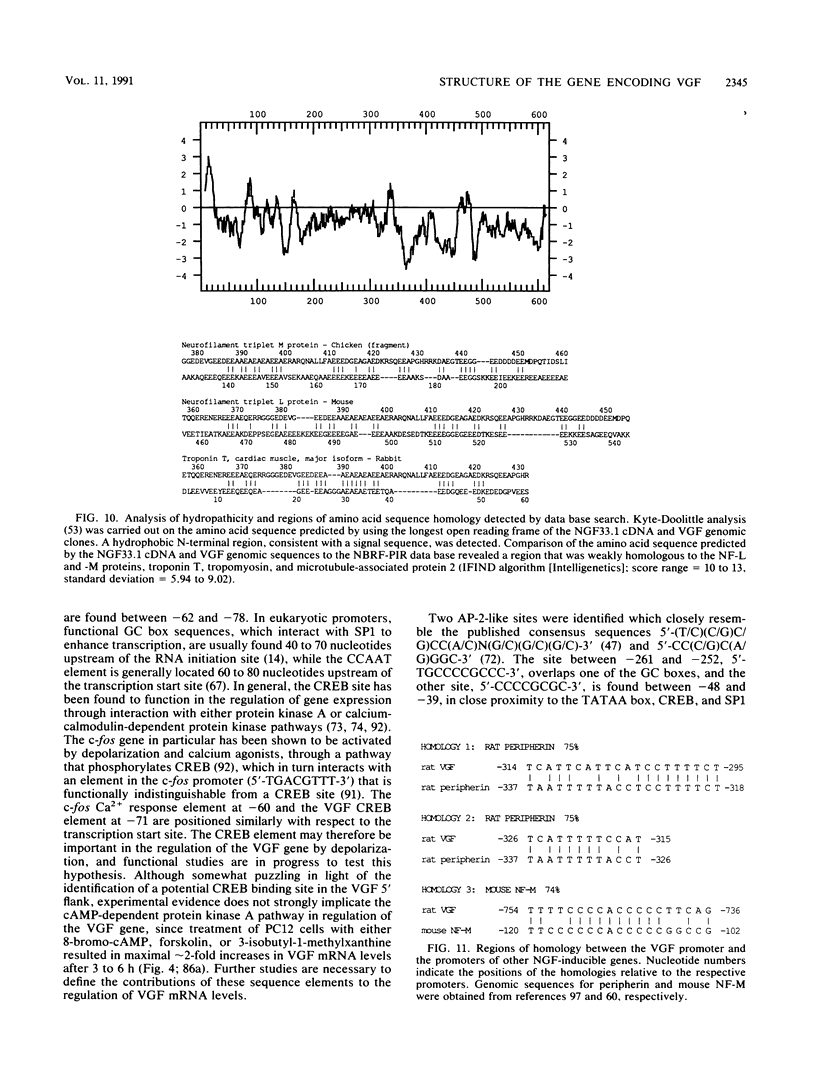
Abstract
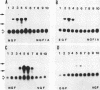
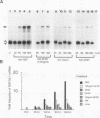
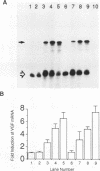
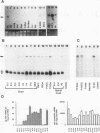
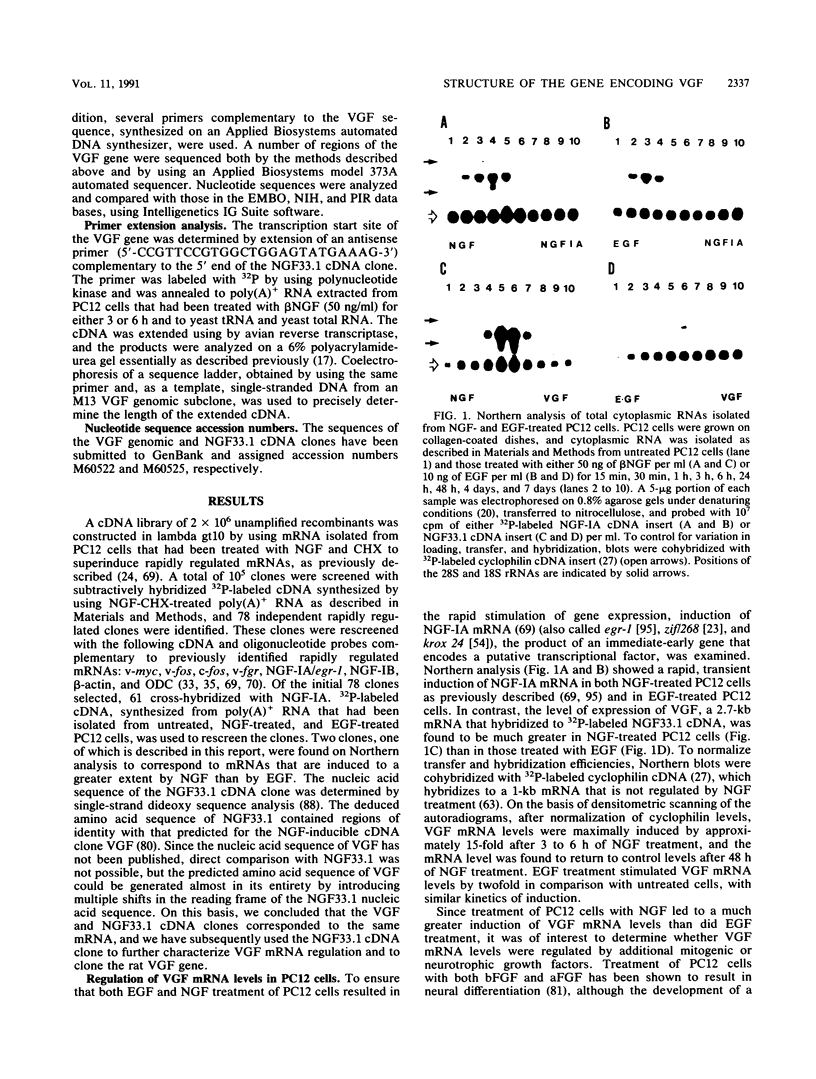
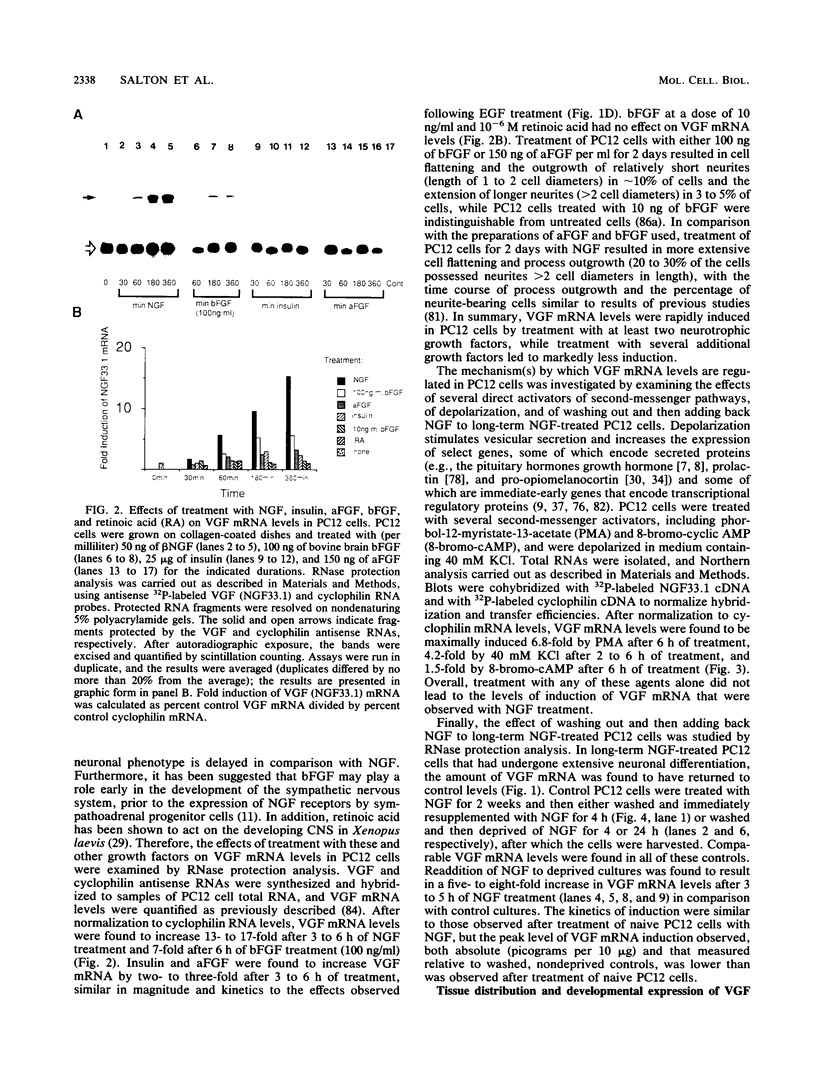
Nerve growth factor (NGF) plays a critical role in the development and survival of neurons in the peripheral nervous system. Following treatment with NGF but not epidermal growth factor, rat pheochromocytoma (PC12) cells undergo neural differentiation. We have cloned a nervous system-specific mRNA, NGF33.1, that is rapidly and relatively selectively induced by treatment of PC12 cells with NGF and basic fibroblast growth factor in comparison with epidermal growth factor. Analysis of the nucleic acid and predicted amino acid sequences of the NGF33.1 cDNA clone suggested that this clone corresponded to the NGF-inducible mRNA called VGF (A. Levi, J. D. Eldridge, and B. M. Paterson, Science 229:393-395, 1985; R. Possenti, J. D. Eldridge, B. M. Paterson, A. Grasso, and A. Levi, EMBO J. 8:2217-2223, 1989). We have used the NGF33.1 cDNA clone to isolate and characterize the VGF gene, and in this paper we report the complete sequence of the VGF gene, including 853 bases of 5' flank revealed TATAA and CCAAT elements, several GC boxes, and a consensus cyclic AMP response element-binding protein binding site. The VGF promoter contains sequences homologous to other NGF-inducible, neuronal promoters. We further show that VGF mRNA is induced in PC12 cells to a greater extent by depolarization and by phorbol-12-myristate-13-acetate treatment than by 8-bromo-cyclic AMP treatment. By Northern (RNA) and RNase protection analysis, VGF mRNA is detectable in embryonic and postnatal central and peripheral nervous tissues but not in a number of nonneural tissues. In the cascade of events which ultimately leads to the neural differentiation of NGF-treated PC12 cells, the VGF gene encodes the most rapidly and selectively regulated, nervous-system specific mRNA yet identified.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allendoerfer K. L., Shelton D. L., Shooter E. M., Shatz C. J. Nerve growth factor receptor immunoreactivity is transiently associated with the subplate neurons of the mammalian cerebral cortex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):187–190. doi: 10.1073/pnas.87.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Axel R. Molecular probes for the development and plasticity of neural crest derivatives. Cell. 1985 Sep;42(2):649–662. doi: 10.1016/0092-8674(85)90122-9. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Stein R., Axel R. Gene expression in differentiating and transdifferentiating neural crest cells. Cold Spring Harb Symp Quant Biol. 1985;50:855–863. doi: 10.1101/sqb.1985.050.01.103. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M., Bilezikjian L. M., Vale W. W., Rosenfeld M. G., Evans R. M. Independent effects of growth hormone releasing factor on growth hormone release and gene transcription. Nature. 1985 Mar 21;314(6008):279–281. doi: 10.1038/314279a0. [DOI] [PubMed] [Google Scholar]
- Barinaga M., Yamonoto G., Rivier C., Vale W., Evans R., Rosenfeld M. G. Transcriptional regulation of growth hormone gene expression by growth hormone-releasing factor. Nature. 1983 Nov 3;306(5938):84–85. doi: 10.1038/306084a0. [DOI] [PubMed] [Google Scholar]
- Bartel D. P., Sheng M., Lau L. F., Greenberg M. E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989 Mar;3(3):304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Basi G. S., Jacobson R. D., Virág I., Schilling J., Skene J. H. Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth. Cell. 1987 Jun 19;49(6):785–791. doi: 10.1016/0092-8674(87)90616-7. [DOI] [PubMed] [Google Scholar]
- Birren S. J., Anderson D. J. A v-myc-immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron. 1990 Feb;4(2):189–201. doi: 10.1016/0896-6273(90)90094-v. [DOI] [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Friedman W. F., LaGamma E. F., Roach A. H. Biochemistry of information storage in the nervous system. Science. 1987 Jun 5;236(4806):1263–1268. doi: 10.1126/science.2884727. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Buck C. R., Martinez H. J., Chao M. V., Black I. B. Differential expression of the nerve growth factor receptor gene in multiple brain areas. Brain Res Dev Brain Res. 1988 Dec 1;44(2):259–268. doi: 10.1016/0165-3806(88)90224-6. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Changelian P. S., Feng P., King T. C., Milbrandt J. Structure of the NGFI-A gene and detection of upstream sequences responsible for its transcriptional induction by nerve growth factor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):377–381. doi: 10.1073/pnas.86.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- Cho K. O., Minsk B., Wagner J. A. NICER elements: a family of nerve growth factor-inducible cAMP-extinguishable retrovirus-like elements. Proc Natl Acad Sci U S A. 1990 May;87(10):3778–3782. doi: 10.1073/pnas.87.10.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. O., Skarnes W. C., Minsk B., Palmieri S., Jackson-Grusby L., Wagner J. A. Nerve growth factor regulates gene expression by several distinct mechanisms. Mol Cell Biol. 1989 Jan;9(1):135–143. doi: 10.1128/mcb.9.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Arrow A. A rapid single-stranded cloning, sequencing, insertion, and deletion strategy. Methods Enzymol. 1987;155:204–214. doi: 10.1016/0076-6879(87)55017-0. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H. A. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987 Oct 1;329(6138):441–442. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Eberwine J. H., Jonassen J. A., Evinger M. J., Roberts J. L. Complex transcriptional regulation by glucocorticoids and corticotropin-releasing hormone of proopiomelanocortin gene expression in rat pituitary cultures. DNA. 1987 Oct;6(5):483–492. doi: 10.1089/dna.1987.6.483. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Dave J. R., Hotchkiss A. J., Affolter H. U. Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984 Dec 13;312(5995):661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feinstein S. C., Dana S. L., McConlogue L., Shooter E. M., Coffino P. Nerve growth factor rapidly induces ornithine decarboxylase mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5761–5765. doi: 10.1073/pnas.82.17.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagner J. P., Drouin J. Opposite regulation of pro-opiomelanocortin gene transcription by glucocorticoids and CRH. Mol Cell Endocrinol. 1985 Apr;40(1):25–32. doi: 10.1016/0303-7207(85)90154-6. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B., Greene L. A. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986 Oct 3;234(4772):80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- Greene L. A., McGuire J. C. Induction of ornithine decarboxylase by nerve growth factor dissociated from effects on survival and neurite outgrowth. Nature. 1978 Nov 9;276(5684):191–194. doi: 10.1038/276191a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U. A one tube reaction for the synthesis of blunt-ended double-stranded cDNA. Nucleic Acids Res. 1988 Mar 25;16(6):2726–2726. doi: 10.1093/nar/16.6.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroff G., Dickens G., End D. The induction of ornithine decarboxylase by nerve growth factor and epidermal growth factor in PC12 cells. J Neurochem. 1981 Aug;37(2):342–349. doi: 10.1111/j.1471-4159.1981.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Hatten M. E., Liem R. K. Astroglial cells provide a template for the positioning of developing cerebellar neurons in vitro. J Cell Biol. 1981 Sep;90(3):622–630. doi: 10.1083/jcb.90.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M. E., Liem R. K., Mason C. A. Two forms of cerebellar glial cells interact differently with neurons in vitro. J Cell Biol. 1984 Jan;98(1):193–204. doi: 10.1083/jcb.98.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987 Aug 13;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Karns L. R., Ng S. C., Freeman J. A., Fishman M. C. Cloning of complementary DNA for GAP-43, a neuronal growth-related protein. Science. 1987 May 1;236(4801):597–600. doi: 10.1126/science.2437653. [DOI] [PubMed] [Google Scholar]
- Kley N., Loeffler J. P., Pittius C. W., Höllt V. Involvement of ion channels in the induction of proenkephalin A gene expression by nicotine and cAMP in bovine chromaffin cells. J Biol Chem. 1987 Mar 25;262(9):4083–4089. [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., HAMBURGER V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951 Mar;116(2):321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard D. G., Gorham J. D., Cole P., Greene L. A., Ziff E. B. A nerve growth factor-regulated messenger RNA encodes a new intermediate filament protein. J Cell Biol. 1988 Jan;106(1):181–193. doi: 10.1083/jcb.106.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard D. G., Ziff E. B., Greene L. A. Identification and characterization of mRNAs regulated by nerve growth factor in PC12 cells. Mol Cell Biol. 1987 Sep;7(9):3156–3167. doi: 10.1128/mcb.7.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Booker B. DESTRUCTION OF THE SYMPATHETIC GANGLIA IN MAMMALS BY AN ANTISERUM TO A NERVE-GROWTH PROTEIN. Proc Natl Acad Sci U S A. 1960 Mar;46(3):384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A., Eldridge J. D., Paterson B. M. Molecular cloning of a gene sequence regulated by nerve growth factor. Science. 1985 Jul 26;229(4711):393–395. doi: 10.1126/science.3839317. [DOI] [PubMed] [Google Scholar]
- Levy E., Liem R. K., D'Eustachio P., Cowan N. J. Structure and evolutionary origin of the gene encoding mouse NF-M, the middle-molecular-mass neurofilament protein. Eur J Biochem. 1987 Jul 1;166(1):71–77. doi: 10.1111/j.1432-1033.1987.tb13485.x. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Anomalous placement of introns in a member of the intermediate filament multigene family: an evolutionary conundrum. Mol Cell Biol. 1986 May;6(5):1529–1534. doi: 10.1128/mcb.6.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum M. H., Carbonetto S., Grosveld F., Flavell D., Mushynski W. E. Transcriptional and post-transcriptional effects of nerve growth factor on expression of the three neurofilament subunits in PC-12 cells. J Biol Chem. 1988 Apr 25;263(12):5662–5667. [PubMed] [Google Scholar]
- MIALE I. L., SIDMAN R. L. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961 Oct;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- Machida C. M., Rodland K. D., Matrisian L., Magun B. E., Ciment G. NGF induction of the gene encoding the protease transin accompanies neuronal differentiation in PC12 cells. Neuron. 1989 Jun;2(6):1587–1596. doi: 10.1016/0896-6273(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Shooter E. M. Nerve growth factor induces the genes for two proteins related to a family of calcium-binding proteins in PC12 cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1277–1281. doi: 10.1073/pnas.85.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J. C., Greene L. A., Furano A. V. NGF stimulates incorporation of fucose or glucosamine into an external glycoprotein in cultured rat PC12 pheochromocytoma cells. Cell. 1978 Oct;15(2):357–365. doi: 10.1016/0092-8674(78)90004-1. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988 May;1(3):183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor rapidly induces c-fos mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4789–4793. doi: 10.1073/pnas.83.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986 Aug 7;322(6079):552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Mori N., Stein R., Sigmund O., Anderson D. J. A cell type-preferred silencer element that controls the neural-specific expression of the SCG10 gene. Neuron. 1990 Apr;4(4):583–594. doi: 10.1016/0896-6273(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Franco R., Evans R. M., Rosenfeld M. G. Polypeptide hormone regulation of gene expression. Thyrotropin-releasing hormone rapidly stimulates both transcription of the prolactin gene and the phosphorylation of a specific nuclear protein. J Biol Chem. 1983 Dec 25;258(24):15329–15335. [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Possenti R., Eldridge J. D., Paterson B. M., Grasso A., Levi A. A protein induced by NGF in PC12 cells is stored in secretory vesicles and released through the regulated pathway. EMBO J. 1989 Aug;8(8):2217–2223. doi: 10.1002/j.1460-2075.1989.tb08345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel R. E., Greene L. A. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci. 1987 Nov;7(11):3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffen D. W., Cole A. J., Worley P. F., Christy B. A., Ryder K., Baraban J. M. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K., Kushiya E., Takahashi Y., Suzuki Y. The structure and expression of neuron-specific enolase gene. Gene. 1987;60(1):103–113. doi: 10.1016/0378-1119(87)90218-6. [DOI] [PubMed] [Google Scholar]
- Salton S. R., Blum M., Jonassen J. A., Clayton R. N., Roberts J. L. Stimulation of pituitary luteinizing hormone secretion by gonadotropin-releasing hormone is not coupled to beta-luteinizing hormone gene transcription. Mol Endocrinol. 1988 Nov;2(11):1033–1042. doi: 10.1210/mend-2-11-1033. [DOI] [PubMed] [Google Scholar]
- Salton S. R., Richter-Landsberg C., Greene L. A., Shelanski M. L. Nerve growth factor-inducible large external (NILE) glycoprotein: studies of a central and peripheral neuronal marker. J Neurosci. 1983 Mar;3(3):441–454. doi: 10.1523/JNEUROSCI.03-03-00441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton S. R., Shelanski M. L., Greene L. A. Biochemical properties of the nerve growth factor-inducible large external (NILE) glycoprotein. J Neurosci. 1983 Dec;3(12):2420–2430. doi: 10.1523/JNEUROSCI.03-12-02420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D. Isolation of differentially expressed genes. Methods Enzymol. 1987;152:423–432. doi: 10.1016/0076-6879(87)52049-3. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Stein R., Mori N., Matthews K., Lo L. C., Anderson D. J. The NGF-inducible SCG10 mRNA encodes a novel membrane-bound protein present in growth cones and abundant in developing neurons. Neuron. 1988 Aug;1(6):463–476. doi: 10.1016/0896-6273(88)90177-8. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Bandtlow C., Heumann R. The physiological function of nerve growth factor in the central nervous system: comparison with the periphery. Rev Physiol Biochem Pharmacol. 1987;109:145–178. doi: 10.1007/BFb0031026. [DOI] [PubMed] [Google Scholar]
- Thompson M. A., Ziff E. B. Structure of the gene encoding peripherin, an NGF-regulated neuronal-specific type III intermediate filament protein. Neuron. 1989 Jan;2(1):1043–1053. doi: 10.1016/0896-6273(89)90228-6. [DOI] [PubMed] [Google Scholar]
- Tirone F., Shooter E. M. Early gene regulation by nerve growth factor in PC12 cells: induction of an interferon-related gene. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2088–2092. doi: 10.1073/pnas.86.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Vantini G., Schiavo N., Di Martino A., Polato P., Triban C., Callegaro L., Toffano G., Leon A. Evidence for a physiological role of nerve growth factor in the central nervous system of neonatal rats. Neuron. 1989 Sep;3(3):267–273. doi: 10.1016/0896-6273(89)90251-1. [DOI] [PubMed] [Google Scholar]
- White J. D., Gall C. M. Differential regulation of neuropeptide and proto-oncogene mRNA content in the hippocampus following recurrent seizures. Brain Res. 1987 Dec;427(1):21–29. doi: 10.1016/0169-328x(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Seiger A. The expression, localization and functional significance of beta-nerve growth factor in the central nervous system. Brain Res. 1987 Nov;434(4):439–464. doi: 10.1016/0165-0173(87)90008-7. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Errington M. L., Williams S., Dunnett S. B., Waters C., Hitchcock D., Evan G., Bliss T. V., Hunt S. P. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron. 1990 Apr;4(4):603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]
- Wu B. Y., Fodor E. J., Edwards R. H., Rutter W. J. Nerve growth factor induces the proto-oncogene c-jun in PC12 cells. J Biol Chem. 1989 May 25;264(15):9000–9003. [PubMed] [Google Scholar]
- van den Pol A. N., Decavel C., Levi A., Paterson B. Hypothalamic expression of a novel gene product, VGF: immunocytochemical analysis. J Neurosci. 1989 Dec;9(12):4122–4137. doi: 10.1523/JNEUROSCI.09-12-04122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dreden G., Loeffler J. P., Grimm C., Höllt V. Influence of calcium ions on proopiomelanocortin mRNA levels in clonal anterior pituitary cells. Neuroendocrinology. 1988 Jan;47(1):32–37. doi: 10.1159/000124887. [DOI] [PubMed] [Google Scholar]