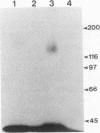
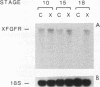
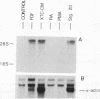
Abstract
The heparin-binding growth factors constitute a family of homologous polypeptides including basic and acidic fibroblast growth factors (FGFs). These factors participate in a variety of processes, including wound healing, angiogenesis, neuronal survival, and inductive events in the early amphibian embryo. We have isolated three closely related species of cDNA clones for Xenopus FGF receptors. One of these, designated XFGFR-A1, encodes an open reading frame of 814 amino acids. A second class encodes an identical amino acid sequence with the exception of an 88-amino-acid deletion near the 5' end. This species probably arises through alternative splicing. A third class of cDNA corresponding to the shorter form of XFGFR-A1 was isolated and shown to be 95% homologous and is designated XFGFR-A2. Xenopus FGF receptors are similar to FGF receptors from other species in that they contain a transmembrane domain, a tyrosine kinase domain split by a 14-amino-acid insertion, and a unique conserved stretch of eight acidic residues in the extracellular domain. Overexpression of Xenopus FGF receptor protein by transfection of COS1 cells with the corresponding cDNA in a transient expression vector leads to the appearance of new FGF binding sites on transfected cells, consistent with these cDNAs encoding for FGF receptors. RNA gel blot analysis demonstrates that Xenopus FGF receptor mRNA is a maternal message and is expressed throughout early development. When blastula-stage ectoderm is cultured in control amphibian salt solutions, Xenopus FGF receptor mRNA declines to undetectable levels by late neurula stages. However, when cultured in the presence of FGF of XTC mesoderm-inducing factor, Xenopus FGF receptor RNA expression is maintained.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Barr P. J., Cousens L. S., Fretto L. J., Williams L. T. Acidic and basic fibroblast growth factors stimulate tyrosine kinase activity in vivo. J Biol Chem. 1988 Jan 15;263(2):988–993. [PubMed] [Google Scholar]
- Dawid I. B., Sargent T. D., Rosa F. The role of growth factors in embryonic induction in amphibians. Curr Top Dev Biol. 1990;24:261–288. doi: 10.1016/s0070-2153(08)60090-3. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Dionne C. A., Crumley G., Bellot F., Kaplow J. M., Searfoss G., Ruta M., Burgess W. H., Jaye M., Schlessinger J. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J. 1990 Sep;9(9):2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Friesel R., Burgess W. H., Maciag T. Heparin-binding growth factor 1 stimulates tyrosine phosphorylation in NIH 3T3 cells. Mol Cell Biol. 1989 May;9(5):1857–1865. doi: 10.1128/mcb.9.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesel R., Burgess W. H., Mehlman T., Maciag T. The characterization of the receptor for endothelial cell growth factor by covalent ligand attachment. J Biol Chem. 1986 Jun 15;261(17):7581–7584. [PubMed] [Google Scholar]
- Gillespie L. L., Paterno G. D., Slack J. M. Analysis of competence: receptors for fibroblast growth factor in early Xenopus embryos. Development. 1989 May;106(1):203–208. doi: 10.1242/dev.106.1.203. [DOI] [PubMed] [Google Scholar]
- Green J. B., Howes G., Symes K., Cooke J., Smith J. C. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990 Jan;108(1):173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S. Association of bovine brain-derived growth factor receptor with protein tyrosine kinase activity. J Biol Chem. 1986 Jul 25;261(21):9568–9571. [PubMed] [Google Scholar]
- Hébert J. M., Basilico C., Goldfarb M., Haub O., Martin G. R. Isolation of cDNAs encoding four mouse FGF family members and characterization of their expression patterns during embryogenesis. Dev Biol. 1990 Apr;138(2):454–463. doi: 10.1016/0012-1606(90)90211-z. [DOI] [PubMed] [Google Scholar]
- Imamura T., Tokita Y., Mitsui Y. Purification of basic FGF receptors from rat brain. Biochem Biophys Res Commun. 1988 Sep 15;155(2):583–590. doi: 10.1016/s0006-291x(88)80534-5. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Lee P. L., Lu J., Williams L. T. Diverse forms of a receptor for acidic and basic fibroblast growth factors. Mol Cell Biol. 1990 Sep;10(9):4728–4736. doi: 10.1128/mcb.10.9.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan M., DiSorbo D., Hou J. Z., Hoshi H., Mansson P. E., McKeehan W. L. High and low affinity binding of heparin-binding growth factor to a 130-kDa receptor correlates with stimulation and inhibition of growth of a differentiated human hepatoma cell. J Biol Chem. 1988 Aug 15;263(23):11306–11313. [PubMed] [Google Scholar]
- Kimelman D., Abraham J. A., Haaparanta T., Palisi T. M., Kirschner M. W. The presence of fibroblast growth factor in the frog egg: its role as a natural mesoderm inducer. Science. 1988 Nov 18;242(4881):1053–1056. doi: 10.1126/science.3194757. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Paulson K. E., Hanafusa H. Novel tyrosine kinase identified by phosphotyrosine antibody screening of cDNA libraries. Mol Cell Biol. 1988 Dec;8(12):5541–5544. doi: 10.1128/mcb.8.12.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Gravel R. A. Rapid and reliable dideoxy sequencing of double-stranded DNA. Gene. 1985;40(2-3):317–323. doi: 10.1016/0378-1119(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Friesel R., Jaye M., Lyall R. M., Westermark B., Drohan W., Schmidt A., Maciag T., Schlessinger J. An angiogenic growth factor is expressed in human glioma cells. EMBO J. 1987 Jun;6(6):1627–1632. doi: 10.1002/j.1460-2075.1987.tb02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Marics I., Adelaide J., Raybaud F., Mattei M. G., Coulier F., Planche J., de Lapeyriere O., Birnbaum D. Characterization of the HST-related FGF.6 gene, a new member of the fibroblast growth factor gene family. Oncogene. 1989 Mar;4(3):335–340. [PubMed] [Google Scholar]
- Moenner M., Chevallier B., Badet J., Barritault D. Evidence and characterization of the receptor to eye-derived growth factor I, the retinal form of basic fibroblast growth factor, on bovine epithelial lens cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5024–5028. doi: 10.1073/pnas.83.14.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci T. J., Amaya E., Kirschner M. W. Regulation of the fibroblast growth factor receptor in early Xenopus embryos. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8365–8369. doi: 10.1073/pnas.87.21.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. Basic and acidic fibroblast growth factors interact with the same cell surface receptors. J Biol Chem. 1986 Apr 25;261(12):5631–5637. [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Olwin B. B., Hauschka S. D. Identification of the fibroblast growth factor receptor of Swiss 3T3 cells and mouse skeletal muscle myoblasts. Biochemistry. 1986 Jun 17;25(12):3487–3492. doi: 10.1021/bi00360a001. [DOI] [PubMed] [Google Scholar]
- Otte A. P., Koster C. H., Snoek G. T., Durston A. J. Protein kinase C mediates neural induction in Xenopus laevis. Nature. 1988 Aug 18;334(6183):618–620. doi: 10.1038/334618a0. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Singer S. J. Identification of a developmentally regulated protein-tyrosine kinase by using anti-phosphotyrosine antibodies to screen a cDNA expression library. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5449–5453. doi: 10.1073/pnas.86.14.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterno G. D., Gillespie L. L., Dixon M. S., Slack J. M., Heath J. K. Mesoderm-inducing properties of INT-2 and kFGF: two oncogene-encoded growth factors related to FGF. Development. 1989 May;106(1):79–83. doi: 10.1242/dev.106.1.79. [DOI] [PubMed] [Google Scholar]
- Reid H. H., Wilks A. F., Bernard O. Two forms of the basic fibroblast growth factor receptor-like mRNA are expressed in the developing mouse brain. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1596–1600. doi: 10.1073/pnas.87.4.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Kondaiah P., Rosa F., Watanabe S., Good P., Danielpour D., Roche N. S., Rebbert M. L., Dawid I. B., Sporn M. B. Mesoderm induction in Xenopus laevis distinguishes between the various TGF-beta isoforms. Growth Factors. 1990;3(4):277–286. doi: 10.3109/08977199009003670. [DOI] [PubMed] [Google Scholar]
- Rosa F. M. Mix.1, a homeobox mRNA inducible by mesoderm inducers, is expressed mostly in the presumptive endodermal cells of Xenopus embryos. Cell. 1989 Jun 16;57(6):965–974. doi: 10.1016/0092-8674(89)90335-8. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Interaction between peptide growth factors and homoeobox genes in the establishment of antero-posterior polarity in frog embryos. Nature. 1989 Sep 7;341(6237):33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- Ruta M., Burgess W., Givol D., Epstein J., Neiger N., Kaplow J., Crumley G., Dionne C., Jaye M., Schlessinger J. Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci U S A. 1989 Nov;86(22):8722–8726. doi: 10.1073/pnas.86.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safran A., Avivi A., Orr-Urtereger A., Neufeld G., Lonai P., Givol D., Yarden Y. The murine flg gene encodes a receptor for fibroblast growth factor. Oncogene. 1990 May;5(5):635–643. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Jamrich M., Dawid I. B. Cell interactions and the control of gene activity during early development of Xenopus laevis. Dev Biol. 1986 Mar;114(1):238–246. doi: 10.1016/0012-1606(86)90399-4. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C. Mesoderm induction and mesoderm-inducing factors in early amphibian development. Development. 1989 Apr;105(4):665–677. doi: 10.1242/dev.105.4.665. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Melton D. A. Induction of mesoderm by a viral oncogene in early Xenopus embryos. Science. 1989 May 19;244(4906):803–806. doi: 10.1126/science.2658054. [DOI] [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]