Abstract
In the last several decades polybrominated diphenyl ethers (PBDEs) have replaced the previously banned polychlorinated biphenyls (PCBs) in multiple flame retardant utilities. As epidemiological and laboratory studies have suggested PCBs as a risk factor for Parkinson’s disease (PD), the similarities between PBDEs and PCBs suggest that PBDEs have the potential to be neurotoxic to the dopamine system. The purpose of this study was to evaluate the neurotoxic effects of the PBDE mixture, DE-71, on the nigrostriatal dopamine system and address the role of altered dopamine handling in mediating this neurotoxicity. Using an in vitro model system we found DE-71 effectively caused cell death in a dopaminergic cell line as well as reducing the number of TH+ neurons isolated from VMAT2 WT and LO animals. Assessment of DE-71 neurotoxicity in vivo demonstrated significant deposition of PBDE congeners in the brains of mice, leading to reductions in striatal dopamine and dopamine handling, as well as reductions in the striatal dopamine transporter (DAT) and VMAT2. Additionally, DE-71 elicited a significant locomotor deficit in the VMAT2 WT and LO mice. However, no change was seen in TH expression in dopamine terminal or in the number of dopamine neurons in the substantia nigra pars compacta (SNpc). To date, these are the first data to demonstrate that exposure to PBDEs disrupts the nigrostriatal dopamine system. Given their similarities to PCBs, additional laboratory and epidemiological research should be considered to assess PBDEs as a potential risk factor for PD and other neurological disorders.
Keywords: Polybrominated diphenyl ethers, dopamine, striatum, vesicular monoamine transporter 2, DE-71, Parkinson’s disease
INTRODUCTION
Several epidemiological studies have implicated the environment as a significant risk factor for Parkinson’s disease (PD). Although specific environmental contaminants responsible have yet to be clearly identified, multiple reports have demonstrated an association between exposure to several organochlorine compounds and PD (Corrigan, et al., 1996, Corrigan, et al., 1998, Corrigan, et al., 2000, Fleming, et al., 1994, Hatcher, et al., 2008). Of these, the industrial toxicants, polychlorinated biphenyls (PCBs), have been repeatedly shown to alter the nigrostriatal dopamine system and influence the risk for PD (Caudle, et al., 2006, Seegal, et al., 1994, Seegal, et al., 2010, Steenland, et al., 2006). As these compounds were phased out, new compounds have been introduced to take their place. Specifically, polybrominated diphenyl ethers (PBDEs) have been extensively produced and used over the last several decades in many of the same applications as PCBs (de Wit, 2002). Unfortunately, the effects of PBDEs on the nervous system, particularly the dopamine system have not been fully appraised. Given the similarities in physiochemical properties, as well as distribution and deposition of PDBEs and PCBs, it is imperative to understand the potential role PBDE exposure may play in the risk of PD in the human population that is exposed to these compounds.
Polybrominated diphenyl ethers (PBDEs) are a class of brominated compounds chemically similar to PCBs, composed of varying degrees and positions of bromine substitutions to give 209 different structures or congeners. These compounds are utilized as additive flame retardants in plastic products used in the manufacturing of electronic equipment, polyurethane foam used in carpeting, furniture cushions and home insulation (Darnerud, et al., 2001, de Wit, 2002). The major commercial PBDE products produced were marketed as one of three mixtures, with the pentabrominated BDE mixture, DE-71, being the most widely used. The additive binding of these compounds, rather than the chemical incorporation into the plastic products, allows PDBEs to migrate out of the plastic and enter the environment. Furthermore, like PCBs, PBDE’s lipophilicity, resistance to degradation, and ability to biomagnify make them extremely persistent in the environment (Norstrom, et al., 2002). However, in contrast to PCBs and other organochlorines whose environmental levels have been decreasing, levels of PBDEs, particularly tetra- and pentabrominated PBDEs, have significantly increased, raising concern over the safety of PBDEs and their potential adverse health effects (de Wit, 2002).
While data concerning the neurological effects of PBDEs on the human population is lacking, several studies have characterized the neurotoxicological outcomes of exposure to PBDEs in in vitro and in vivo systems. Most notably, several in vitro studies have demonstrated the ability of PBDEs and other brominated compounds to elicit an increase in oxidative stress (Madia, et al., 2004, Reistad, et al., 2006, Reistad, et al., 2005, Reistad, et al., 2007). In addition to ROS production, several brominated compounds appear to cause disruption of the calcium signaling pathway, causing an influx in intracellular calcium (Kodavanti and Derr-Yellin, 2002, Kodavanti and Ward, 2005, Reistad, et al., 2005). PBDEs may also target the dopamine system, causing inhibition of vesicular uptake of dopamine through the vesicular monoamine transporter 2 (VMAT2), as well as altering dopamine handling in isolated synaptosomes, suggesting that VMAT2 may be a critical target for PBDEs (Dreiem, et al., 2010, Mariussen and Fonnum, 2003). In the nigrostriatal system, cytosolic dopamine is sequestered into synaptic vesicles by VMAT2 prior to release from the presynaptic terminal. Thus, VMAT2 serves as a key mediator of cytosolic dopamine levels, and the aberrant dysfunction has been demonstrated to be critically involved in PD pathogenesis (Caudle, et al., 2008, Caudle, et al., 2007). Indeed, mice generated to express approximately 5% of normal VMAT2 (VMAT2 LO) levels have been used to elucidate the importance of VMAT2 in dopamine handling and dopaminergic neurotoxicity (Caudle, et al., 2007, Taylor, et al., 2009). Given the ability of PBDEs to inhibit VMAT2 we sought to evaluate whether deficits in VMAT2 expression and function would influence the potential dopaminergic neurotoxicity of DE-71. Here, we demonstrate that DE-71 alone elicits significant reductions to the expression of DAT and VMAT2 in the striatum, as well as decreases in striatal dopamine and locomotor activity. These alterations in the nigrostriatal dopamine system are exacerbated when DE-71 is given to animals that are deficient for VMAT2. These findings suggest that exposure to DE-71 can have detrimental effects on the nigrostriatal dopamine system, and that dopamine homeostasis plays an imperative role in mediating some of these neurotoxic effects.
METHODS
Chemicals and Reagents
The commercial PBDE mixture DE-71 was purchased from Wellington Labs (Guelph, Ontario, Canada). HEK and SK-N-SH cells were obtained from American Type Culture Collection (ATCC; Manassas, VA). Hibernate A and Hibernate A- Calcium were purchased from BrainBits (Springfield, IL). B27, DNase1, and Neurobasal A were purchased from Life Technologies (Life Technologies). Papain was obtained from Sigma (St. Louis, MO). Dispase II was purchased from Roche (Nutley, NJ). Aphidicolin was purchased from A.G scientific (San Diego, CA). Whatman GF/F filter papers were obtained from Brandel, Inc. (Plantation, FL). The BCA protein assay kit was obtained from Pierce (Rockford, IL). Monoclonal anti-rat dopamine transporter and polyclonal anti-rabbit tyrosine hydroxylase antibodies were purchased from EMD Millipore (Billerica, MA) or Pel Freez Biologicals (Rogers, AR). Polyclonal anti-rabbit VMAT2 antibodies were generated by Covance to the C-terminal sequence in mouse (CTQNNVQPYPVGDDEESESD). Monoclonal anti-mouse α-tubulin antibodies were purchased from Sigma (St. Louis, MO). Mouse anti-MAP2 antibodies were purchased from Abcam (San Francisco, CA). Secondary antibodies conjugated to horseradish peroxidase were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Secondary antibodies conjugated to fluorescent tags were obtained from Life Technologies. SuperSignal West Dura Extended duration substrate and stripping buffer were obtained from Pierce. 3,3′ Diaminobenzidine (DAB) was purchased from Sigma. The LDH Assay Kit was obtained from Cayman Chemicals (Ann Arbor, MI). Monoamine standards for DA, dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were purchased from Sigma. Mobile phase was obtained from ESA Inc (Chelmsford, MA). 3H-Dopamine (3H-DA) purchased from Perkin-Elmer (Boston, MA). Cold tetrabenazine was obtained from Sigma. Polybrominated diphenyl ether (PBDE) standards were purchased as individual congener standard solutions in iso-octane from Accustandard (New Haven, CT). Hexane (99.9%), acetone (99.5%) and sodium sulfate anhydrous (99.3%) were purchased from Fisher Scientific (Fairlawn, NJ) as certified ACS grade. Pesticide residue grade iso-octane (99.5%) was obtained from Acros Organics (Fairlawn, NJ).
Culturing of SK-N-SH cells
Cells were cultured in DMEM F12 media supplemented with 100 units/mL penicillin and 100 units/ml streptomycin and 10% fetal bovine serum. Cells were cultured at 37°C in a humidified atmosphere with 5% CO2 and propagated according to the protocol provided by the supplier. When cells were confluent they were passaged to working concentrations in the appropriate culture plate for treatment with DE-71.
Culturing of HEK-VMAT2 cells
HEK293 cells (ATCC) stably expressing human-VMAT2 constructs were cultured at 37°C and 5% CO2 in DMEM with 10% FBS. Constructs were made in pcDNA3.1 and contained a zeocin resistance gene. Plasmids were transfected into HEK293 cells with Lipofectamine 2000. Stable cell lines were generated by repetitive rounds of limiting dilutions in selective media.
Cell Viability
Cell death was assessed using the lactate dehydrogenase cell viability assay per the standard procedures provided by the supplier.
Vesicular 3H-DA uptake in HEK-VMAT2 cells
Cells grown to confluency were harvested, centrifuged at 1000 × g and resuspended in 0.32 M sucrose, 5 mM HEPES, at pH 7.4 with 1X Protease Inhibitor Cocktail. The cell suspensions were centrifuged at 8000 × g and the supernatant was removed and added to VMAT2 uptake buffer (2 mM ATP-Mg2+, 1.7 mM ascorbate, 25 mM HEPES, 100 mM potassium tartrate, 0.1 mM EDTA, 0.05 mM EGTA, pH 7.4) or uptake buffer containing 200 μM tetrabenazine (TBZ). Cell fractions were incubated with increasing amounts of DE-71 dissolved in DMSO or control solutions lacking DE-71, followed by the addition of a 10 mM dopamine stock with a 6% 3H-DA tracer and incubated for 5 mins. Reactions were terminated and samples harvested with a Brandel Cell Harvester with Whatman GF/F Filter Paper. Bound activity on membranes was measured using a scintillation counter and specific activity was determined by subtracting total DPMs from TBZ-sensitive (Total-Nonspecific) DPMs and normalized to the total protein per well as determined by BCA protein assay.
Primary culture of mesencephalic neurons
The primary culture protocol was modified from the procedure used in Dr. Malu Tansey’s laboratory (Lee, et al., 2011). Briefly, ventral mesencephalic neuron cultures were prepared from postnatal mice (postnatal day 1–3). Mouse brains were dissected in ice cold Hibernate A supplemented with B27. Following isolation of the relevant region and the removal of meninges, tissue pieces were chemically treated with a dissociation solution containing Papain (1 mg/ml), Dispase II (1.2U/ml), and DNase 1 (1ul/ml) dissolved in Hibernate A- Calcium for 20 min at 37°C and gently agitated every 5 min. Tissue was then rinsed in plating media containing Neurobasal-A, 10% heat inactivated fetal bovine serum, pen-strep, and mechanically dissociated using gentle trituration. Cells were plated on poly-d-lysine pre-coated 96 well plates at 40,000 cells per well. Plating media was removed and immediately switched to Neurobasal-A based culture media containing B27, 1% L-glutamine and 1% penicillin-streptomycin after 2 hrs, in vitro. The following day, culture media containing aphidicolin (1ug/ml) was added to reduce the proliferation of glial cells in culture. Approximately one half of the culture media from each well was replaced every 4 days. Primary cultures were treated on day 8 in vitro with five concentrations of DE-71 dissolved in cell culture media. After 24 hrs, cells were fixed in 4% PFA for 20 mins and incubated overnight in rabbit anti-TH and mouse anti-MAP2 at 4°C. The following day, cultures were incubated with fluorescent secondary antibodies, goat anti-rabbit 488 and goat anti-mouse 572 for 1hr at room temperature. After staining with DAPI, cells were rinsed and stored in PBS. Images of treated cultures were obtained using an Array Scan VTI HCS (Cellomics; Pittsburgh, PA). Forty-nine contiguous fields were taken per well and TH+ neurons were counted and analyzed using GraphPad analysis software.
Animals and Treatment
VMAT2 WT and LO mice were generated as previously described (Caudle, et al., 2007, Taylor, et al., 2009). All mice were generated through redundant breeding of mice that were heterozygous for the VMAT2 allele and genotype of all mice was confirmed by PCR of DNA extracted from tail samples. Four month old mice were orally gavaged with 30 μl of 30 mg/kg of DE-71 dissolved in corn oil vehicle daily for 30 days, as previously described (Caudle, et al., 2006). This dosing paradigm was intended to represent the primary route of human exposure to DE-71 and is based upon previously published no observable adverse effect levels (NOAEL) and lowest observable adverse effect levels (LOAEL) for tetra-BDEs (Darnerud, et al., 2001, Hallgren and Darnerud, 2002, Hallgren, et al., 2001). Furthermore, DE-71 was chosen based upon its composition of tetra- and penta PBDE congeners, which represents a mixture of the most prevalent congeners found in the environment, as well as in human tissue (Darnerud, 2003, de Wit, 2002). One day following the last dose, mice were subjected to behavioral analysis and then sacrificed with brain tissue collected for subsequent experimental assays. Standard rodent chow and tap water were available ad libitum. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and have been approved by the Institutional Animal Care and Use Committee at Emory University.
High Performance Liquid Chromatography (HPLC) determination of striatal neurochemistry
HPLC analysis of neurochemistry was performed as previously described (Caudle, et al., 2007). Briefly, dissected striata was sonicated in 0.1 M perchloric acid. Homogenates were centrifuged at 15,000 × g and the supernatant filtered through a 0.22 μm filter by centrifugation at 15,000 × g. The supernatants were analyzed for levels of DA, DOPAC, HVA. Quantification was made by reference to calibration curves made with individual standards.
Gas Chromatography
Levels of selected polybrominated diphenyl ether (PBDE) were measured in post-mortem mouse brain tissue to assess accumulation of the major congeners in the DE-71 mixture. The congeners selected for quantification, based on the numbering system of Ballschmiter and Zell (1980), were PBDE 28 (2,4,4′-TriBDE), 47 (2,2′,4,4′-TetraBDE), 66 (2,3′,4,4′-TetraBDE), 85 (2,2′,3,4,4′-PentaBDE), 99 (2,2′,4,4′,5-PentaBDE), 100 (2,2′,4,4′,6-PentaBDE), 153 (2,2′,4,4′,5,5′-HexaBDE), and 154 (2,2′,4,4′,5,6′-HexaBDE). These congeners were chosen due to their higher percentages in the DE-71 technical mixtures (Konstantinov, et al., 2008) and their tendency to bioaccumulate.
Extraction of PBDEs from brain tissue samples followed the procedure described by (Corrigan, et al., 1998), as adapted by Caudle et al. (2006). It has previously been determined that PBDEs distribute ubiquitously throughout the brain (Kodavanti, et al., 2010); thus, we analyzed whole-brain samples lacking only the striatum, which were taken for neurochemical analysis. Approximately 150 mg of brain tissue were added to an amber vial containing 5 mL of 1:1 hexane and acetone to which 20 μL of 4000 μg/L PBDE 77 prepared in iso-octane as the internal standard. The vials were then vortex mixed for 2 min and homogenized for 2 min using a Tissue Tearor (Biospec Products, Bartlesville, OK) and the resulting homogenate was placed in a sonication bath at 22 ± 2°C for 15 min. Samples were vortex mixed for 2 min, and then centrifuged at 1300 × g (Eppendorf 5810R, Hauppauge, NY). The resulting supernatant was transferred to a pre-weighed 35 mL borosilicate glass centrifuge tube, and an additional 5 mL of 1:1 hexane and acetone were added to the amber glass vials. This process was repeated four additional times, resulting in a total extract volume of approximately 25 mL. The resulting extract was then dried completely at 50 °C under a gentle stream of high purity (99.999%) N2 gas in a Mulitvap 118 Nitrogen Evaporator (Organomotion Associates Inc., Berlin, MA), weighed to determine lipid weight, and reconstituted in 1 ml of hexane. To remove lipids and other background interferences, the 1 mL extract plus two 0.5 mL rinses were transferred to a 5000 mg florisil cartridge (PR grade, UCT, Bristol, PA) containing 1 g 10–60 mesh anhydrous sodium sulfate that was preconditioned with 20 ml of hexane. The cartridge was eluted with 25 ml hexane under vacuum in a Visiprep 12 port vacuum manifold (Supelco, St. Louis, MO). The eluent was collected in a 40 ml glass vial, dried completely and re-suspended in ca. 2 mL of isooctane. The extract was then transferred to a 2 mL amber glass chromatography vial, capped and analyzed immediately.
Tissue extracts were analyzed for PBDEs using an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA) equipped with a micro-electron capture detector (GC-μECD). He (99.999% purity) and N2 (99.999% purity) were used for the carrier and make-up gas, respectively. A 2 μL aliquot of each extract was injected using an autosampler into a 300°C inlet operated in pulsed split-less mode. The selected PBDE congeners were separated on an Agilent DB-5MS Ultra Inert capillary column (30m length × 0.25 mm inner diameter × 0.25 μm film thickness) at a constant carrier gas flow rate of 1 mL/min and oven temperature program consisting of an initial temperature of 100 °C for 1 min, increased to 180 °C at 25 °C/min; after which the temperature was ramped to 250 °C at 5 °C/min and then subsequently increased to 275 °C at 25°C/min and held for 15 min, resulting in a total run time of 36.2 min. Retention times of the individual congeners were determined by comparison to retention times for individual standards and confirmed by a 7000A Triple Quadrupole Mass Spectrometer (Agilent Technologies, Santa Clara, CA) operating in full scan mode with an electron ionization (EI) source. The resulting chromatographic separation enabled complete, baseline separation of the selected PBDE congeners, and no co-eluting interferences were present based on comparisons to blank extracts.
Final PBDE concentrations in the extracted samples were determined by comparison of the unknown sample peak area to a 5-point calibration curve prepared from external standards containing all nine congeners. The resulting extraction and analytical method resulted in an average recovery of approximately 80% or greater for the nine PBDE congeners and a method detection limit based on a signal to noise ratio of 3 of approximately 0.4 ng/g.
Western Blot Analysis
Western blots were used to quantify the amount of DAT, TH, VMAT2, and α-tubulin present in samples of striatal tissue from treated and control mice. Analysis was performed as previously described (Caudle, et al., 2006, Caudle, et al., 2007). Briefly, striata samples were homogenized and samples subjected to polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes. Nonspecific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline and then membranes incubated overnight in a monoclonal antibody to the N-terminus of DAT. DAT antibody binding was detected using a goat anti-rat horseradish peroxidase secondary antibody (1:10,000) and enhanced chemiluminescence. The luminescence signal was captured on an Alpha Innotech Fluorochem imaging system and stored as a digital image. Densitometric analysis was performed and calibrated to coblotted dilutional standards of pooled striata from all control samples. Membranes were stripped for 15 min at room temperature with Pierce Stripping Buffer and sequentially reprobed with α-tubulin (1:1,000), TH (1:1,000), and VMAT2 (1:10,000) antibodies. α-Tubulin blots were used to ensure equal protein loading across samples.
Immunohistochemistry and Stereological Neuron Counts
Tissue staining and cell counts were performed as described previously (Caudle, et al., 2007). Briefly, midbrain blocks from VMAT2 WT and LO mice were immersion fixed in 4% paraformaldehyde and serially sectioned at 40 μm. Sections were incubated with a polyclonal anti-TH antibody overnight and then incubated in a biotinylated goat anti-rabbit secondary antibody for 1 hr at room temperature. Visualization was performed using DAB for 3 mins at room temperature. After DAB, all sections were counterstained in 0.5% cresyl violet, dehydrated, and coverslipped. Neurons were counted using the optical fractionator method, and the substantia nigra pars compacta (SNpc) was delineated using previously described criteria (West, et al., 1991). After delineation at low magnification, every sixth section was sampled at higher magnification using the Cast grid system (Olympus, Albertslund, Denmark).
Locomotor Activity
Mice were placed in polycarbonate locomotor boxes (25.4 × 50.8 × 25.4 cm), and horizontal distance was quantified over time using Noldus Ethovision 3.0 (Noldus Information Technology, Wageningen, The Netherlands) (Caudle, et al., 2007). General locomotion for treated and untreated VMAT2 WT and LO mice were observed for a total of 4 hr. The first 30 min were considered the habituation period to ensure stabilization of the horizontal activity signal.
Statistical Analysis
All analysis was performed on raw data for each treatment group by one-way or two-way ANOVA. Post hoc analysis was performed using Tukey’s post hoc test. Significance is reported at the p < 0.05 level.
RESULTS
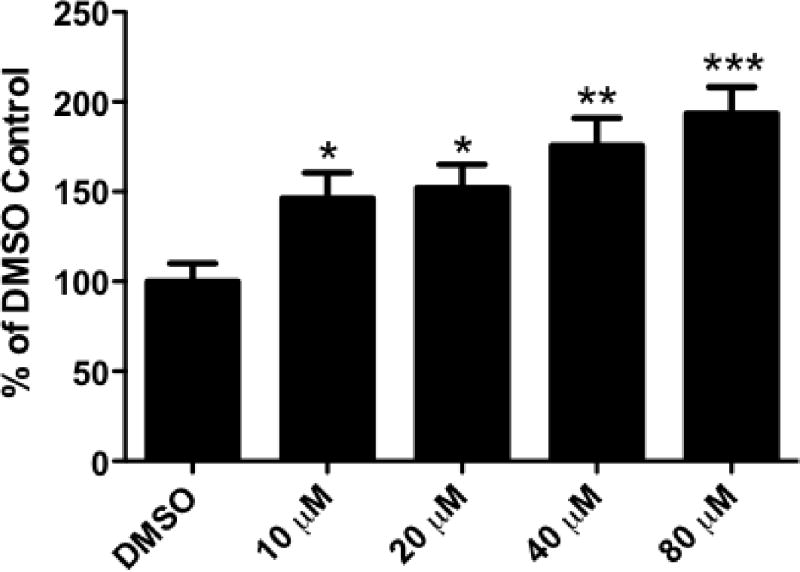
Since we were interested in evaluating the effects of DE-71 on the dopamine system we first assessed the neurotoxic properties of this compound using the SK-N-SH neuroblastoma cell line, which is known to exhibit dopaminergic properties (Kidd and Schneider, 2010, Richards and Sadee, 1986). Cells were treated acutely with various concentrations of DE-71 dissolved in cell culture media and then cytoxicity was measured by lactate dehydrogenase (LDH) leakage from the cell body. As seen in Figure 1, exposure to DE-71 for 24 hrs resulted in a dose dependent increase in LDH release. These data demonstrate the ability of DE-71 to be an efficient neurotoxicant in a dopaminergic cell line. These results were used to further inform our dosing paradigm in additional in vitro models.
Figure 1.
Exposure of SK-N-SH neuroblastoma cells to DE-71 caused a dose-dependent increase in cellular toxicity, as measured by release of LDH. Beginning at 10 μM, DE-71 causes a statistically significant increase in toxicity, compared with DMSO treated cells. The amount of toxicity continued to increase with increasing concentrations of DE-71. Columns represent percent change from DMSO control. Data represent the mean ± SEM of 12 experimental replicates per treatment group performed over 3 separate experiments. *Values that are significantly different from control (p < 0.05). **Values that are significantly different from control (p < 0.01). ***Values that are significantly different from control (p < 0.001).
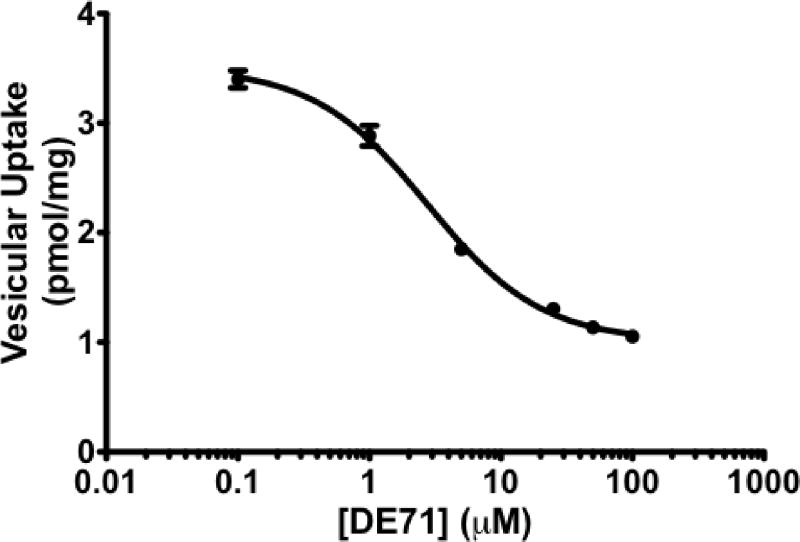
Previous work has demonstrated that DE-71 and other brominated flame retardants can affect the ability of the VMAT2 to effectively sequester monoamines, such as dopamine (Mariussen and Fonnum, 2003). Using human kidney cells stably expressing the human VMAT2, we evaluated the inhibitory properties of DE-71 on VMAT2. As seen in Figure 2, DE-71 effectively inhibits VMAT2 function with an IC50 of 2.8 μM, suggesting DE-71 has the ability to potently alter the ability of VMAT2 to package dopamine in the neuron.
Figure 2.
3H-dopamine uptake in HEK-hVMAT2 cells following exposure to DE-71 caused a dose-dependent inhibition of dopamine through VMAT2. Fractions of HEK-hVMAT2 cells were incubated with DE-71 or DMSO followed by incubation with 3H-dopamine. Resultant radioactive uptake into vesicles is shown as percent of vehicle treated control. Results demonstrate DE-71 inhibits dopamine uptake by VMAT2 with an IC50=2.8 μM.
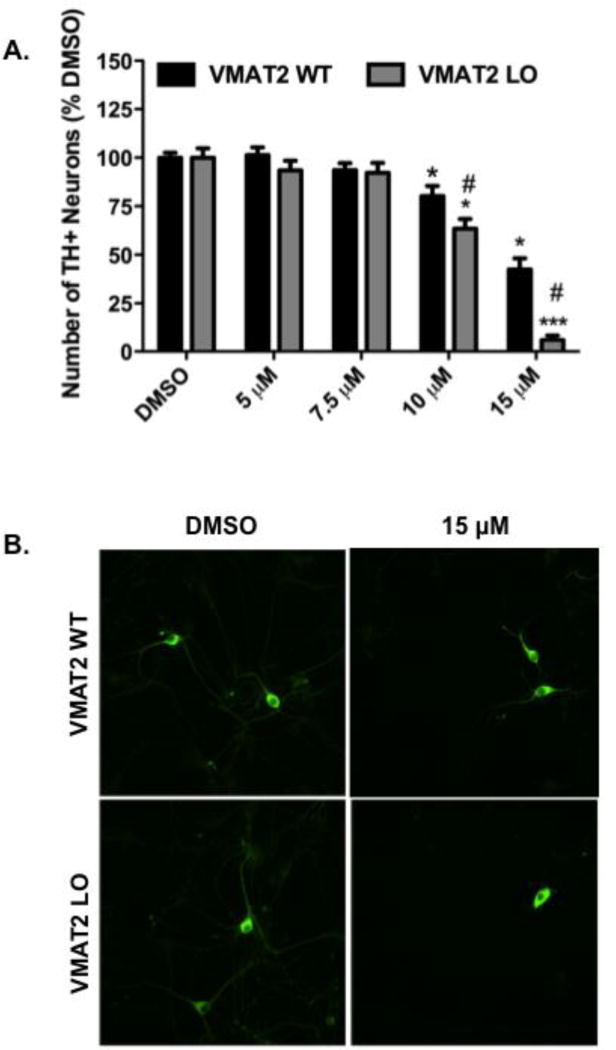
We then sought to elaborate the dopaminergic effects of DE-71 by assessing its neurotoxicity on primary cultured neurons isolated from the dopamine-rich mesencephalic region of neonatal VMAT2 WT and LO mice. A dose-dependent reduction in TH+ neurons was observed in both the WT and LO cultures, with 10 and 15 μM demonstrating a significant loss of TH+ neurons in the WT cultures (Figure 3A and B). Additionally, cultures isolated from VMAT2 LO mice demonstrated an even greater reduction in TH+ at 10 and 15 μM, compared with the WT cultures (Figure 3A and B). Indeed, a significant difference in the loss of TH+ neurons from the VMAT2 LO cultures was observed at both the 10 and 15 μM DE-71 concentrations compared to WT. This suggests that reductions in the expression and function of VMAT2 influence the dopaminergic neurotoxicity following exposure to DE-71.
Figure 3.
Exposure of mesencephalic primary cultures from VMAT2 WT and LO to DE-71 shows a reduction in the number of TH+ neurons. (A) Treatment of mesencephalic cultures from VMAT2 WT animals caused a significant reduction in the number of TH+ neurons at 10 and 15 μM DE-71. A further reduction in TH+ neurons was observed at 10 and 15 μM when VMAT2 LO cultures were treated with DE-71. Columns represent the percent change from DMSO control for each genotype. Data represent the mean ± SEM of 21 experimental replicates per treatment group performed across 3 separate experiments. *Values for treatments that are significantly different from their respective genotype DMSO control (p < 0.05). ***Values that are significantly different from their respective genotype DMSO control (p < 0.001). #Values that are significantly different from VMAT2 WT at the same DE-71 concentration (p < 0.05). (B) Representative mesencephalic cultures stained for TH+ from VMAT2 WT and LO mice and treated with DMSO or 15 μM DE-71.
Prior studies have demonstrated that the PBDE congeners 47, 99, 100, and 154 accumulate to the greatest degree in the body and brain of animals as well as humans (Darnerud, 2003). Thus, using GC-ECD methods, we determined the concentrations of these congeners, as well as others, in the brains of VMAT2 WT and LO mice treated with 30 mg/kg of DE-71 for 30 days (Table 1). Upon analysis PBDE congeners 28, 47, 66, 85, 99, 100, 153, and 154 accounted for 99% of all congeners recovered in the brain, with 47, 99, 100, and 154 being the most predominant congeners. While an obvious difference in concentration of specific congeners was observed between treated and untreated animals, there was not a statistically significant difference in congener concentration between the VMAT2 WT and LO mice treated with DE-71.
Table 1.
VMAT2 WT and LO mice exposed to 30 mg/kg DE-71 for 30 days show an increase in brain concentrations of PBDE congeners.
| BDE-28 | BDE-47 | BDE-66 | BDE-85 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | |
|---|---|---|---|---|---|---|---|---|
| WTCTL | 0+0 | 0.004+0.004 | 0+0 | 0+0 | 0.01+0.003 | 0+0 | 0+0 | 0+0 |
| LOCTL | 0+0 | 0.01+0.01 | 0+0 | 0+0 | 0+0 | 0+0 | 0+0 | 0+0 |
| WT DE-71 | 0+0 | 1.00+0.52 | 0.01+0.01 | 0.07+0.03 | 1.70+0.63 | 0.41+0.17 | 1.78+0.16 | 0.10+0.04 |
| LO DE-71 | 0+0 | 0.19+0.08 | 0+0 | 0.03+.002 | 0.67+0.12 | 0.17+0.07 | 1.52+0.16 | 0.05+0.01 |
Data are expressed as mean ± SEM (4–6 animals per treatment group). Data are presented as mean PBDE congener levels (μg/g wet weight).
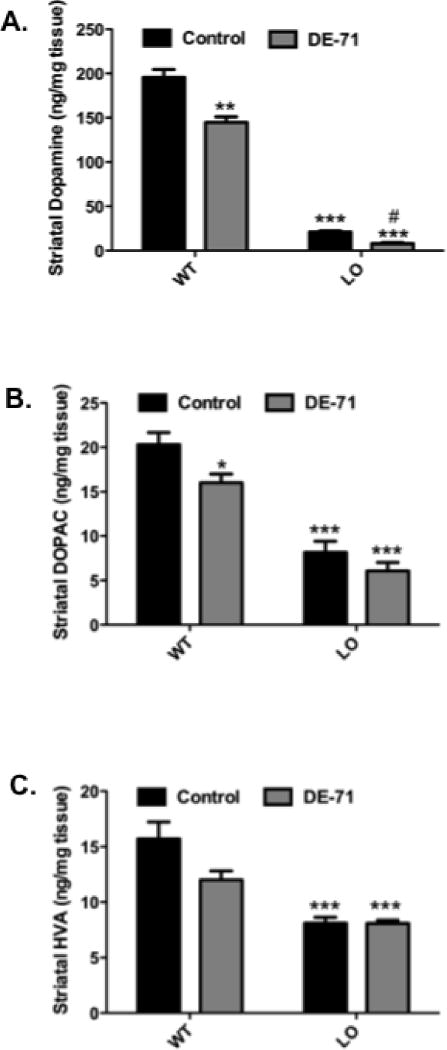
We next assessed the impact of DE-71 exposure on dopamine and its metabolites in the striatum of VMAT2 WT and LO mice. As seen in Figure 4A, a significant reduction in striatal dopamine is shown between the untreated VMAT2 WT and LO mice (~90%), as previously observed (Caudle, et al., 2007, Taylor, et al., 2009). Evaluation of the effects of DE-71 exposure on VMAT2 WT mice showed an approximately 25% decrease in striatal dopamine in these animals. Additionally, exposure of the VMAT2 LO mice to DE-71 caused an approximately 60% additional reduction in striatal dopamine, compared to the untreated VMAT2 LO mice (Figure 4A). A statistically significant decrease (~25%) in levels of the dopamine metabolite DOPAC were also observed in the VMAT2 WT mice treated with DE-71 when compared with untreated WT controls. However, a similar reduction was not observed between the treated and untreated VMAT2 LO mice (Figure 4B). Levels of HVA also remained unchanged in all treated mice (Figure 4C), although a trend for reduction in HVA levels between the treated and untreated VMAT2 WT mice is observed (p=0.0574).
Figure 4.
Striatal DA, DOPAC, and HVA levels in VMAT2 WT and LO mice following 30 days of exposure to 30 mg/kg DE-71. (A) Exposure of VMAT2 WT mice to DE-71 caused a significant reduction in striatal dopamine. While VMAT2 LO mice endogenously show an ~90% reduction in striatal dopamine, treatment of VMAT2 LO mice with DE-71 caused a further loss of striatal dopamine. (B) Exposure of VMAT2 WT mice to DE-71 also resulted in a reduction of striatal DOPAC. No change was observed in DOPAC levels in VMAT2 LO mice treated with DE-71. (C) No change was observed in striatal HVA levels in VMAT2 WT or LO mice treated with DE-71. Columns represent raw values (ng/mg tissue) ± SEM (4–6 animals per group). *Values that are significantly different from control (p < 0.05). **Values that are significantly different from control (p < 0.01). ***Values that are significantly different from control (p < 0.001). #Values that are different from untreated VMAT2 LO control (p < 0.05).
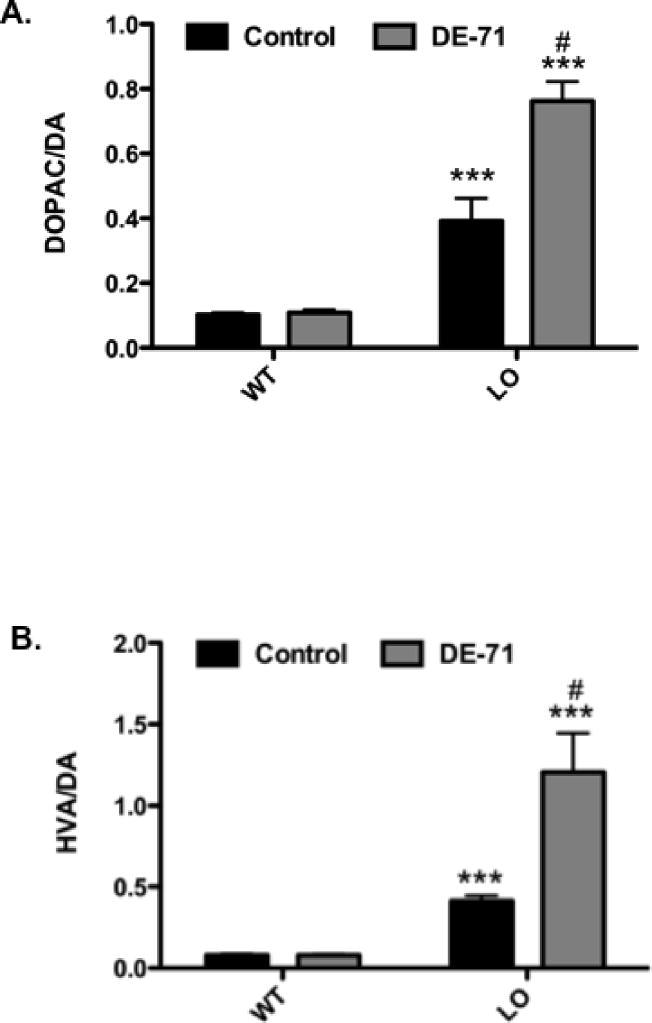
Further assessment of dopamine handling also demonstrated an increase in the ratio of DOPAC/DA and HVA/DA in the striatum. While no difference was observed between treated and untreated VMAT2 WT animals, an approximately 300 and 400% increase in DOPAC/DA and HVA/DA, respectively was seen between untreated VMAT2 WT and LO mice (Figure 5A and B). In contrast to the VMAT2 WT animals, VMAT2 LO mice treated with DE-71 exhibited an even greater increase in both DOPAC/DA (50%) and HVA/DA (200%), compared with untreated LO animals (Figure 5A and B). Taken in concert, these data suggest that exposure to DE-71 reduces levels of striatal dopamine and that deficits in VMAT2 expression and function exacerbate these reductions, leading to further indices of dopamine mishandling in the striatum.
Figure 5.
Ratio of DOPAC/DA and HVA/DA in the striatum of VMAT2 WT and LO mice treated for 30 days with 30 mg/kg DE-71. (A) Exposure to DE-71 did not alter the ratio of DOPAC/DA in VMAT2 WT mice. However, the ratio of DOPAC/DA was elevated in untreated VMAT2 LO mice. This ratio was further increased in VMAT2 LO mice exposed to DE-71. (B) Exposure to DE-71 did not alter the ratio of HVA/DA in VMAT2 WT mice. However, the ratio of HVA/DA was elevated in untreated VMAT2 LO mice. This ratio was further increased in VMAT2 LO mice exposed to DE-71. Results represent mean ratios of raw values (ng/mg tissue) ± SEM (4–6 animals per treatment group). ***Values that significantly differ from control (p < 0.001). #Value that is significantly different from untreated VMAT2 LO control (p < 0.01).
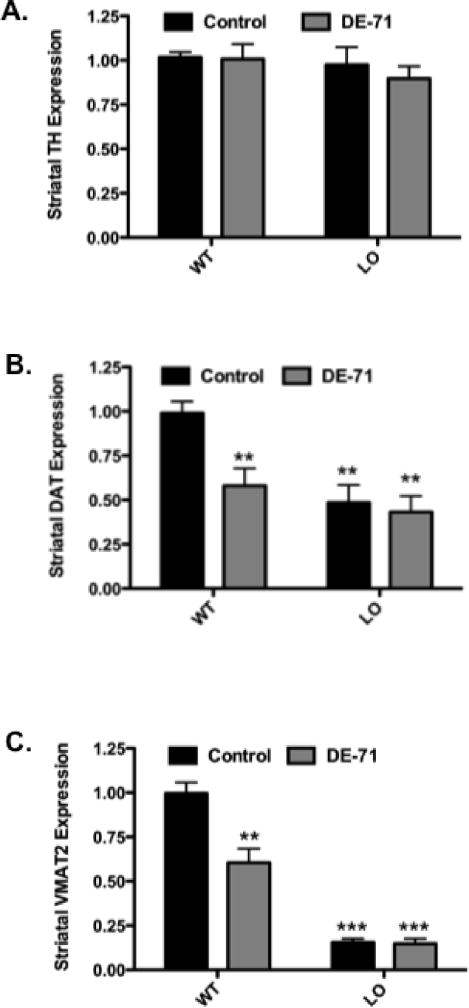
To further assess the effects of DE-71 on the nigrostriatal dopamine system and the influence of VMAT2 expression on its neurotoxicity, we measured expression of specific striatal proteins involved in dopamine homeostasis. VMAT2 WT and LO control mice, as well as mice that received DE-71, did not show a reduction in striatal TH expression as demonstrated by immunoblotting techniques (Figure 6A). We next evaluated the effects of DE-71 exposure on DAT expression in VMAT2 WT and LO mice. In contrast to TH, a significant reduction in DAT (~50%) was seen between untreated VMAT2 WT and LO mice. Additionally, exposure of VMAT2 WT animals to DE-71 resulted in a 40% reduction in DAT levels, similar to that seen in the untreated VMAT2 LO mice (Figure 6B). In contrast, exposure of VMAT2 LO mice to DE-71 did not cause a further reduction in striatal DAT levels beyond that observed in the VMAT2 LO controls. Interestingly, these reductions in DAT appear to be specific to the striatum as DAT expression was unaltered in other brain regions that receive dopaminergic innervation, such as the frontal cortex and hypothalamus (data not shown). As previously demonstrated VMAT2 LO mice exhibit an approximately 90% reduction in VMAT2 levels (Caudle, et al., 2007, Taylor, et al., 2009). Interestingly, exposure of VMAT2 WT mice to DE-71 caused a 40% decrease in VMAT2 expression in the striatum. However, a further reduction in VMAT2 expression was not observed when VMAT2 LO mice were treated with DE-71 (Figure 6C). Alterations in transporter expression appear to be specific to the dopamine system within the striatum as no change was observed in the expression of transporters relevant to the cholinergic, GABAergic or glutamatergic neurotransmitter systems (data not shown). Additionally, changes in expression levels of α-synuclein in the striatum did not show a difference following exposure to DE-71 (data not shown).
Figure 6.
Exposure of VMAT2 WT and LO mice to 30 mg/kg DE-71 for 30 days show a reduction in the expression of DAT and VMAT2, but not TH. (A) Striatal TH expression was not altered in VMAT2 WT and LO mice treated with DE-71. (B) Treatment of VMAT2 WT mice with DE-71 resulted in a reduction in striatal DAT expression compared with untreated WT animals. Untreated VMAT2 LO mice exhibited a significant decrease in striatal DAT that was not exacerbated by treatment with DE-71. (C) Similarly, exposure of VMAT2 WT mice to DE-71 caused a reduction in striatal VMAT2 expression, compared with untreated VMAT2 WT mice. While untreated VMAT2 LO mice display a significant reduction in VMAT2, treatment with DE-71 did not cause a further reduction in VMAT2. Data represent mean ± SEM (4–6 animals per treatment group). **Values for animals that are significantly different from controls (p < 0.01). ***Values for animals that are significantly different from controls (p < 0.001).
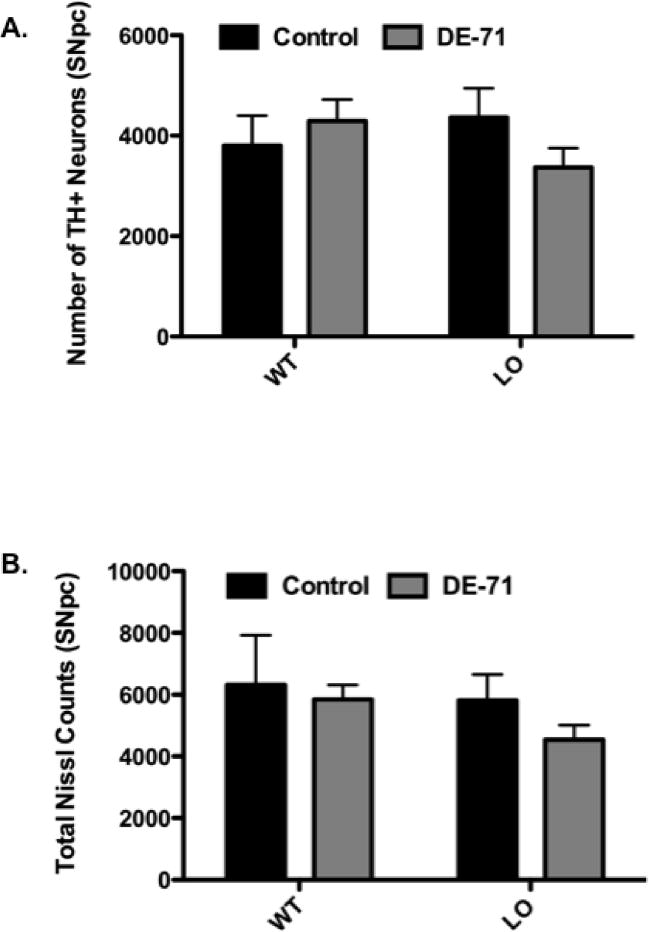
In order to determine whether the reductions in dopamine as well as DAT and VMAT2 in the striatum were due to loss of dopaminergic cell bodies that reside within the SNpc, we employed an unbiased stereological technique to quantify the numbers of TH-positive (TH+) neurons in this region. As seen in Figure 7A and B, no change was detected in the number of TH+ neurons or total number of neurons in the SNpc of mice exposed to DE-71. These results suggest that alterations to striatal dopamine, as well as DAT and VMAT2, occurred independently of overt damage to the dopaminergic neurons in the SNpc and their projections to the striatum.
Figure 7.
Exposure of VMAT2 WT and LO mice to 30 mg/kg DE-71 for 30 days did not cause a reduction in dopamine neurons or total neurons in the SNpc. (A) Number of TH+ neurons in the SNpc of treated and untreated VMAT2 WT and LO mice. (B) Number of total neurons in the SNpc of treated and untreated VMAT2 WT and LO mice. Data represent mean ± SEM (3–5 animals per treatment group.
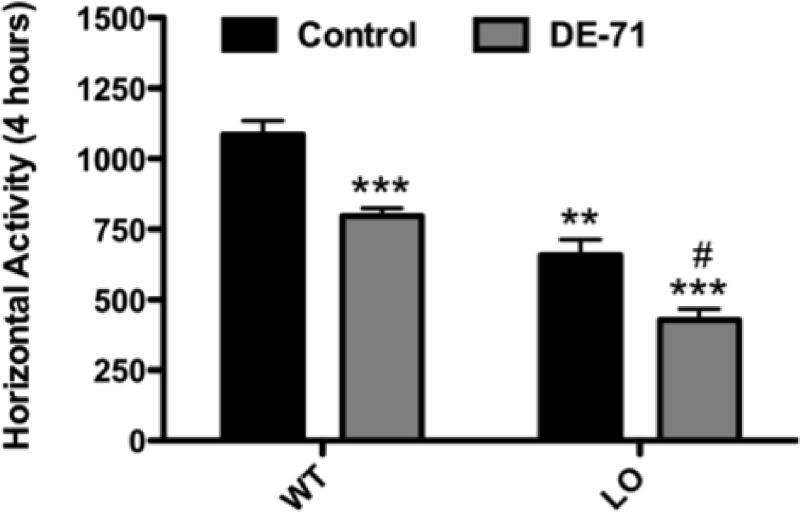
Finally, to evaluate the behavioral effects of DE-71 we assessed the total locomotor activity of VMAT2 WT and LO mice following exposure to DE-71 or vehicle. As previously seen, reductions in the expression and function of VMAT2 resulted in a significant decrease in locomotion (~40%) in the vehicle treated VMAT2 LO mice (Figure 8) (Caudle, et al., 2007). Using this paradigm we were able to detect an ~25% reduction in locomotor activity in VMAT2 WT mice treated with DE-71 compared with VMAT2 WT controls. Additionally, exposure of VMAT2 LO mice to DE-71 appears to exacerbate the locomotor deficits already present in the VMAT2 LO mice, causing a further (~35%) decrease in activity. Thus, exposure to DE-71 for 30 d induces significant locomotor deficits in VMAT2 WT mice, whereas these behavioral alterations are substantially increased following exposure of VMAT2 LO mice to DE-71.
Figure 8.
Exposure of VMAT2 WT and LO mice to 30 mg/kg DE-71 for 30 days caused a reduction in locomotor behavior. VMAT2 WT mice exposed to DE-71 show a significant reduction in locomotor activity, compared with untreated VMAT2 WT mice. Untreated VMAT2 LO mice exhibit a reduction in locomotor behavior that is exacerbated following exposure to DE-71. Data represent mean ± SEM (8–10 mice per treatment group). **Values for animals that are significantly different from controls (p < 0.01). ***Values for animals that are significantly different from controls (p < 0.001). #Values for animals that are significantly different from untreated VMAT2 LO controls (p < 0.05).
DISCUSSION
As PBDEs exhibit the same or similar uses and physiochemical properties as that of PCBs, there is concern that similar health issues related to deleterious effects on the nigrostriatal dopamine system could be forthcoming. To date, these effects have not been evaluated. Thus, we sought to assess the impact of exposure of in vitro and in vivo models to the PBDE mixture, DE-71 on dopaminergic endpoints associated with PD and to examine the influence of altered dopamine handling in mediating these effects. In this study we demonstrate that exposure of in vitro models to DE-71 caused a dose-dependent inhibition of VMAT2 as well as neurotoxicity in SK-N-SH cells. Further exposure of DE-71 to VMAT2 WT and LO mesencephalic cultures showed specific loss of TH+ neurons that was more sensitive in cultures with reduced levels of VMAT2. Treatment of mice with DE-71 resulted in a significant accumulation in brain tissue of the most prominent PBDE congeners found in the environment and human tissue. These levels facilitated decreases in striatal dopamine and dopamine handling as well as reductions in striatal DAT and VMAT2 expression. These alterations could underlie observed deficits in locomotor activity in the treated animals. Interestingly, these changes appear to occur independent of overt damage to dopaminergic terminals in the striatum or dopamine cell bodies in the SNpc.
Since we were interested in evaluating the impact of DE-71 on dopaminergic neurons, we initially sought to determine the neurotoxic effects on the SK-N-SH neuroblastoma cell line. Similar to previous results using this cell line and this compound (Yu, et al., 2008), DE-71 effectively induced cell loss at low micromolar concentrations. Having established an effective dosing paradigm we next assessed the ability of DE-71 to influence the function of dopamine sequestration by VMAT2. In the dopamine neuron, VMAT2 serves to package newly synthesized and recycled dopamine from the cytosol into synaptic vesicles, preparing them for release by neurotransmission. HEK-hVMAT2 cells were acutely exposed to DE-71 resulting in a significant inhibition of VMAT2 function as determined by its dose-dependent deficiency in dopamine uptake into vesicles. Previously, Mariussen and Fonnum (2003) performed a similar experiment using synaptic vesicles isolated from rat brain followed by incubation with varying concentrations of DE-71. As with our study, they found DE-71 to be a potent inhibitor of VMAT2 with an IC50 of 3.0 μM, which is almost identical to our IC50 of ~2.8 μM. These results have important implications as VMAT2 has been shown to serve a critical function in mediating dopamine handling and alteration of its expression and function can have deleterious effects on the nigrostriatal dopamine system (Caudle, et al., 2007, Taylor, et al., 2009).
Upon extending our in vitro exposure model to primary cultures, we demonstrated that dopamine neurons isolated from the ventral mesencephalon of VMAT2 WT animals, as well as VMAT2 LO mice were sensitive to DE-71 with the VMAT2 LO neurons showing an enhanced vulnerability to DE-71-mediated neurotoxicity. Although other groups have investigated the effects of DE-71 as well as specific PBDE congeners on neurotoxicity in primary cultured neurons isolated from the cerebellum, hippocampus, and cortex (Giordano, et al., 2008, Kodavanti, et al., 2005, Reistad, et al., 2006), this is the first study that has examined the neurotoxicity of DE-71 on the ventral mesencephalon, and specifically, dopamine neurons. While the specific mechanism underlying the DE-71-mediated loss of TH+ neurons in our cultures is unclear, it appears that the reduced expression of VMAT2 influences the sensitivity of these neurons to DE-71. As reduction in VMAT2 expression and function has previously been shown to be detrimental to the survival and function of nigrostriatal dopamine neurons, the increased sensitivity of the VMAT2 LO TH+ neurons to DE-71 could result from the further inhibition of VMAT2 function by the compound, as demonstrated in our in vitro system.
Following assessment of dopaminergic neurotoxicity in several in vitro systems we treated VMAT2 WT and LO mice with 30 mg/kg of DE-71 via oral gavage daily for 30 days. Analysis of PBDE congener levels in the brain determined that significant concentrations of relevant PBDE congeners were being deposited. As with several other reports, the predominant congeners measured in our brain samples were PBDE-28, -47, -66, -85, -99, -100, -153, and -154. These results are in line with a developmental study in rats in which 30.6 mg/kg of DE-71 was given perinatally and congener levels were evaluated in the brains of offspring (Kodavanti, et al., 2010). Although it is difficult to make a concise extrapolation of PBDE levels across disparate in vitro and in vivo systems in our study, it appears that the levels of PBDE congeners identified in our mouse brains are similar to those used in our in vitro models. However, it should be noted that these concentrations are much higher than what has been recently observed in postmortem human brain samples in our lab (unpublished results) as well as others (Mitchell, et al., 2012). These discrepancies can be attributed to the different exposure scenarios in animal studies compared to the human population.
In addition to detecting significant deposition of PBDEs in the brain we were also able to demonstrate significant reductions in striatal dopamine in both the treated VMAT2 WT and LO mice. Although a similar study has not been performed, previous work has shown PBDEs to alter the levels of cortical DA following a single injection of PBDE-47 (Gee, et al., 2011). Additionally, an in vitro study by Dreiem et al., (2010) demonstrated a significant reduction in tissue DA levels measured in isolated synaptosomes that had been treated with DE-71. Interestingly, reductions in striatal dopamine have been repeatedly shown to be mediated by the expression and function of VMAT2 with increased VMAT2 facilitating an increase in tissue dopamine while inhibition or genetic deletion causes substantial deficits in dopamine (Caudle, et al., 2007, German, et al., 1981, Pothos, et al., 2000, Vergo, et al., 2007). This suggests that VMAT2 expression is imperative to regulating the tissue DA levels and maintaining DA homeostasis in the striatum. As we have demonstrated the ability of DE-71 to inhibit the ability of VMAT2 to sequester dopamine, it is possible that the decrease in striatal DA levels in the VMAT2 WT mice was a result of VMAT2 inhibition by DE-71. Furthermore, the exacerbated loss of striatal dopamine in the VMAT2 LO mice exposed to DE-71 could be explained by the inhibition of the remaining VMAT2 in these mice. Interestingly, although at 2 months of age VMAT2 LO mice exhibit an ~90% reduction in tissue dopamine, these levels continue to decline as the animals age, showing an additional ~40% and ~65% loss in striatal dopamine at 6 and 12 months of age, respectively (Caudle, et al., 2007). Thus, the exacerbated loss of striatal dopamine observed in VMAT2 LO mice treated with DE-71 could suggest an accelerated decline in the health of the dopamine neurons.
Further evidence that DE-71 disrupts dopamine handling was observed following assessment of the ratio of DOPAC/DA and HVA/DA in treated and untreated VMAT2 WT and LO mice. The increase in this ratio suggests an increase in DA turnover, which provides additional evidence for mishandling of DA by VMAT2 (Zigmond, et al., 2002). Previous studies of the VMAT2 LO mice demonstrated an increase in both the ratio DOPAC/DA and HVA/DA, again reaffirming the dopamine mishandling in these animals (Caudle, et al., 2007). However, exposure to DE-71 in the LO animals further increased these ratios suggesting that exposure to DE-71 is causing a greater disruption of DA homeostasis, possibly at the level of VMAT2. Interestingly, these ratios are most likely attributed to a reduction in striatal dopamine as levels of DOPAC and HVA did not appreciably change in the VMAT2 LO animals. In contrast, DOPAC and HVA did exhibit slight reductions in the DE-71 treated VMAT2 WT mice, which may have contributed to the lack of effect on these ratios in these animals. As previously reported the VMAT2 LO mice age these ratios increase, suggesting a progressive decrement to dopamine handling. Interestingly, the increase in the DOPAC/DA and HVA/DA in the 6-and 12-month old VMAT2 LO mice is similar in magnitude to the increase observed between untreated and treated VMAT2 LO. These similarities could imply that the dopamine system has potentially become more vulnerable following exposure to DE-71, which could potentially impact the health of the nigrostriatal dopamine system and influence the susceptibility of the dopamine system to future insults.
To date, evaluation of PBDE exposure on the expression of proteins involved in regulating the proper function of the dopamine neurons has not been performed. We found that exposure to DE-71 caused a significant reduction in the expression of striatal DAT in both the VMAT2 WT as well as the VMAT2 LO mice. Interestingly, the reduction in striatal DAT expression in the VMAT2 WT mice in our study is very similar to the reductions in striatal DAT that we previously observed following exposure of mice to PCBs (Caudle, et al., 2006). In addition, we have also shown a significant reduction in the expression of VMAT2 in our WT animals exposed to DE-71. A similar reduction in VMAT2 was also seen in animals that received 15 mg/kg Aroclor 1254:1260 for 30 days (Caudle, et al., 2006). However, in light of alterations to both DAT and VMAT2, exposure to DE-71 did not cause a change in the expression of striatal TH, similar to that seen with PCBs. As TH is considered to be a reliable marker of DA neuron terminal integrity and terminal loss, the loss of DAT and VMAT2 expression are likely not due to neuronal toxicity.
Our initial hypothesis was that reduction of VMAT2 expression and function as seen in the VMAT2 LO mice would serve to exacerbate the potential dopaminergic neurotoxicity of DE-71. However, exposure of VMAT2 LO mice to DE-71 did not elicit any further deficits in DAT, VMAT2, or TH, compared with untreated VMAT2 LO mice. Although the reasons for this lack of additional neurotoxicity is unknown, it can be speculated that perhaps we established a ceiling effect with our dosing paradigm and even under conditions of low VMAT2 expression reductions in expression of DAT and VMAT2 could not be driven further. Therefore, under similar conditions, a higher concentration of DE-71 or an extended exposure time line could be enough to push the system and elicit further deficits.
In addition to loss of striatal dopamine as well as markers of the dopamine neuron terminals in the disease, a key pathological feature of PD is loss of dopamine neurons from the SNpc. Unlike the dopaminergic deficits we were able to detect in the striatum DE-71 did not impact the number of TH+ neurons present in the SNpc. We have previously demonstrated the ability of VMAT2 reduction to elicit progressive dopaminergic neuron degeneration (Caudle, et al., 2007). However, these losses were age dependent as the animals that exhibited these losses were significantly older (~18 months) than the mice used in this study. These results suggest that the reduction in striatal dopamine as well as loss of DAT and VMAT2 expression in the striatum are not due to loss of dopaminergic neurons in the SNpc. These results coupled with the lack of change in TH expression in the striatum point to a relatively intact dopaminergic cell body and terminal in the SNpc and striatum, respectively, suggesting a yet to be known mechanism that is modulating the expression of DAT and VMAT2 as well as DA levels in the striatum.
One of the hallmark clinical features of PD is a deficit in motor function due to loss of dopamine in the striatum. Indeed, this dopamine-mediated loss of motor faculties is what accounts for the reduction in locomotor acitivity observed in the VMAT2 LO mice (Caudle, et al., 2007). In addition to a baseline reduction in activity in the untreated VMAT2 LO animals we found that exposure to DE-71 caused a significant reduction in locomotor behvavior in VMAT2 WT mice with an exacerbated deficit in locomotion observed in the VMAT2 LO mice. Previous work utilizing locomotor evaluation of mice following exposure to PBDEs has generally found no change in locomotor activity or a hyperactive phenotype. These studies have relied upon different dosing paradigms and concentrations of DE-71 or specific congeners than those used in our study. For example, Kodavanti et al., (2010) exposed mice to DE-71 throughout gestation and lactation and found no change in locomotor activity in the offspring. Additionally, assessment of developmental exposure to PBDE congeners 47, 99, and 209 appears to result in offspring that are hyperactive (Kuriyama, et al., 2005, Rice, et al., 2007, Suvorov, et al., 2009). Finally, a 90 day exposure of adult rats to 15 μg/kg PBDE 99 did not cause alterations in locomotor behavior (Daubie, et al., 2011). Thus, the alterations we found in our study could be attributed to both the dosing paradigm as well as the concentrations of DE-71 that were given. Additionally, these changes in locomotor activity can be attributed to deficits in the nigrostriatal dopamine system rather than a consequence of toxicity as they correlate well with alterations in several well established markers of nigrostriatal dopamine system integrity, as previously seen in the VMAT2 LO animals (Caudle, et al., 2007). With that said, rescue of the locomotor phenotype with L-DOPA administration would provide strong supportive evidence for the role of the dopamine system in mediating the DE-71 locomotive deficits.
In conclusion, exposure of in vitro and in vivo models of the nigrostriatal dopamine system to DE-71 demonstrates for the first time its neurotoxic effects on dopamine homeostasis and locomotor behvavior. These effects are influenced by the expression of VMAT2, which appears to be a putative target for DE-71. Thus, we suggest that inhibition of VMAT2 by DE-71 significantly alters the ability of VMAT2 to efficiently package cytosolic dopamine, resulting in the accumulation of neurotoxic oxidative species and damage to the dopamine neuron, including reductions in striatal dopamine, as well as DAT and VMAT2 expression, culminating in locomotor deficits. As DE-71 has been shown to target VMAT2, reduction of VMAT2 serves to challenge the nigrostriatal dopamine system and provides the opportunity to further evaluate the alterations in dopamine handling enacted by exposure to DE-71. These findings, coupled with the shared neurochemical effects with PCBs, provide significant implications for further consideration of PBDEs as dopaminergic neurotoxins and possible environmental risk factors for PD.
HIGHLIGHTS.
PBDEs share many physiochemical properties as the dopaminergic neurotoxicant, PCBs
PBDEs previously shown to inhibit vesicular dynamics in dopamine system
PBDEs disrupt the nigrostriatal dopamine system, in vitro and in vivo
Disruption is exacerbated in transgenic mice with a 95% reduction in VMAT2
These are the first data demonstrating a role of PBDEs in dopamine neurotoxicity
Acknowledgments
FUNDING SOURCES
This work was supported by National Institutes of Health grants [R00ES017477 to W.M.C.], [P01ES016731 to W.M.C., G.W.M., and K.D.P], [T32 ES012879 to G.W.M.], and [R01ES015991, P30ES005022, R21NS072097 to J.R.R.].
We would like to gratefully acknowledge Dr. Malu Tansey and her laboratory for assistance with developing the mesencepahlic primary culture protocol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joshua M. Bradner, Email: joshua.m.bradner@emory.edu.
Tiffany A. Suragh, Email: tiffany.ashley.suragh@emory.edu.
W. Wyatt Wilson, Email: wwwilso@emory.edu.
Carlos R. Lazo, Email: carlos.r.lazo@emory.edu.
Kristen A. Stout, Email: kristen.stout@gmail.com.
Hye Mi Kim, Email: hye.kim@emory.edu.
Min Z. Wang, Email: mzwang@emory.edu.
Douglas I. Walker, Email: douglas.walker@tufts.edu.
Kurt D. Pennell, Email: kurt.pennell@tufts.edu.
Jason R. Richardson, Email: jr891@eohsi.rutgers.edu.
Gary W. Miller, Email: gary.miller@emory.edu.
W. Michael Caudle, Email: william.m.caudle@emory.edu.
References
- 1.Ballschmiter K, Zell M. Baseline studies of the global pollution. I. Occurrence of organohalogens in pristine European and antarctic aquatic environments. Int J Environ Anal Chem. 1980;8:15–35. doi: 10.1080/03067318008071876. [DOI] [PubMed] [Google Scholar]
- 2.Caudle WM, Colebrooke RE, Emson PC, Miller GW. Altered vesicular dopamine storage in Parkinson’s disease: a premature demise. Trends Neurosci. 2008;31:303–308. doi: 10.1016/j.tins.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, Miller GW. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson’s disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- 4.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrigan FM, French M, Murray L. Organochlorine compounds in human brain. Hum Exp Toxicol. 1996;15:262–264. doi: 10.1177/096032719601500314. [DOI] [PubMed] [Google Scholar]
- 6.Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- 7.Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- 8.Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003;29:841–853. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 9.Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daubie S, Bisson JF, Lalonde R, Schroeder H, Rychen G. Neurobehavioral and physiological effects of low doses of polybrominated diphenyl ether (PBDE)-99 in male adult rats. Toxicol Lett. 2011;204:57–63. doi: 10.1016/j.toxlet.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 11.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 12.Dreiem A, Okoniewski RJ, Brosch KO, Miller VM, Seegal RF. Polychlorinated biphenyls and polybrominated diphenyl ethers alter striatal dopamine neurochemistry in synaptosomes from developing rats in an additive manner. Toxicol Sci. 2010;118:150–159. doi: 10.1093/toxsci/kfq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- 14.Gee JR, Moser VC, McDanie KL, Herr DW. Neurochemical changes following a single dose of polybrominated diphenyl ether 47 in mice. Drug Chem Toxicol. 2011;34:213–219. doi: 10.3109/01480545.2010.536768. [DOI] [PubMed] [Google Scholar]
- 15.German DC, McMillen BA, Sanghera MK, Saffer SI, Shore PA. Effects of severe dopamine depletion on dopamine neuronal impulse flow and on tyrosine hydroxylase regulation. Brain Res Bull. 1981;6:131–134. doi: 10.1016/s0361-9230(81)80037-8. [DOI] [PubMed] [Google Scholar]
- 16.Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 18.Hallgren S, Sinjari T, Hakansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol. 2001;75:200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- 19.Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidd SK, Schneider JS. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–178. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodavanti PR, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, Rayner JL, Kannan K, Birnbaum LS. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- 22.Kodavanti PR, Derr-Yellin EC. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on [3H]arachidonic acid release in rat cerebellar granule neurons. Toxicol Sci. 2002;68:451–457. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- 23.Kodavanti PR, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85:952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- 24.Kodavanti PR, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinov A, Arsenault G, Chittim B, McAlees A, McCrindle R, Potter D, Tashiro C, Yeo B. Identification of the minor components of Great Lakes DE-71 technical mix by means of 1H NMR and GC/MS. Chemosphere. 2008;73:S39–43. doi: 10.1016/j.chemosphere.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low dose PBDE 99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113:149–154. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JK, Chung J, McAlpine FE, Tansey MG. Regulator of G-protein signaling-10 negatively regulates NF-kappaB in microglia and neuroprotects dopaminergic neurons in hemiparkinsonian rats. J Neurosci. 2011;31:11879–11888. doi: 10.1523/JNEUROSCI.1002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem Int. 2003;43:533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagen. 2012;53:589–598. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norstrom RJ, Simon M, Moisey J, Wakeford B, Weseloh DV. Geographical distribution (2000) and temporal trends (1981–2000) of brominated diphenyl ethers in Great Lakes hewing gull eggs. Environ Sci Technol. 2002;36:4783–4789. doi: 10.1021/es025831e. [DOI] [PubMed] [Google Scholar]
- 32.Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch Toxicol. 2006;80:785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- 34.Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicol Sci. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- 35.Reistad T, Mariussen E, Ring A, Fonnum F. In vitro toxicity of tetrabromobisphenol-A on cerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol Sci. 2007;96:268–278. doi: 10.1093/toxsci/kfl198. [DOI] [PubMed] [Google Scholar]
- 36.Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Richards ML, Sadee W. Human neuroblastoma cell lines as models of catechol uptake. Brain Res. 1986;384:132–137. doi: 10.1016/0006-8993(86)91228-x. [DOI] [PubMed] [Google Scholar]
- 38.Seegal RF, Bush B, Brosch KO. Decreases in dopamine concentrations in adult, non-human primate brain persist following removal from polychlorinated biphenyls. Toxicology. 1994;86:71–87. doi: 10.1016/0300-483x(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 39.Seegal RF, Marek KL, Seibyl JP, Jennings DL, Molho ES, Higgins DS, Factor SA, Fitzgerald EF, Hills EA, Korrick SA, Wolff MS, Haase RF, Todd AC, Parsons P, McCaffrey RJ. Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: a beta-CIT imaging study. Neurobiol Dis. 2010;38:219–225. doi: 10.1016/j.nbd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steenland K, Hein MJ, Cassinelli RT, 2nd, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- 41.Suvorov A, Girard S, Lachapelle S, Abdelouahab N, Sebire G, Takser L. Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology. 2009;95:203–209. doi: 10.1159/000155651. [DOI] [PubMed] [Google Scholar]
- 42.Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009;29:8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 44.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 45.Yu K, He Y, Yeung LW, Lam PK, Wu RS, Zhou B. DE-71-induced apoptosis involving intracellular calcium and the Bax-mitochondria-caspase protease pathway in human neuroblastoma cells in vitro. Toxicol Sci. 2008;104:341–351. doi: 10.1093/toxsci/kfn088. [DOI] [PubMed] [Google Scholar]
- 46.Zigmond MJ, Hastings TG, Perez RG. Increased dopamine turnover after partial loss of dopaminergic neurons: compensation or toxicity? Parkinsonism Relat Disord. 2002;8:389–393. doi: 10.1016/s1353-8020(02)00019-6. [DOI] [PubMed] [Google Scholar]