Abstract
Sleep, which is evolutionarily conserved across species, is a biological imperative that cannot be ignored or replaced. However, the percentage of habitually sleep-restricted adults has increased in recent decades. Extended work hours and commutes, shift work schedules, and television viewing are particularly potent social factors that influence sleep duration. Chronic partial sleep restriction, a product of these social expediencies, leads to the accumulation of sleep debt over time and consequently increases sleep propensity, decreases alertness, and impairs critical aspects of cognitive functioning. Significant interindividual variability in the neurobehavioral responses to sleep restriction exists—this variability is stable and phenotypic—suggesting a genetic basis. Identifying vulnerability to sleep loss is essential as many adults cannot accurately judge their level of impairment in response to sleep restriction. Indeed, the consequences of impaired performance and the lack of insight due to sleep loss can be catastrophic. In order to cope with the effects of social expediencies on biological imperatives, identification of biological (including genetic) and behavioral markers of sleep loss vulnerability as well as development of technological approaches for fatigue management are critical.
Keywords: sleep deprivation, sleep duration, neurobehavioral functions, fatigue management, individual differences, genetics, biomarkers
Introduction
“Sleep persists in predators and prey, carnivores and vegetarians, on the land and in the water, in most mammals as they lie down relaxed, in ruminants while they stand, in birds while they perch, and in dolphins which constantly swim … in the smartest and in the dumbest of all mammalian species” (Rechtschaffen, 1998). Although an organism cannot procreate, gather food, or protect itself or offspring during sleep, sleep has been evolutionarily conserved from the sloth to the human. Indeed, some evidence suggests that sleep is vital to life. A number of studies from 1894 to 1962 reported lethal outcomes from total sleep deprivation in dogs, rabbits, and rats (Kleitman, 1963). However, these studies lacked proper controls for the procedures implemented to keep the animals awake. This methodological failure was corrected in 1983 when Rechtschaffen and colleagues developed a novel disk-over-water methodology with yoked control procedures (Rechtschaffen et al., 1983). Using this paradigm, mortality was more likely in the sleep-deprived rats (death occurred after 5–33 days) compared with yoked control rats that experienced the same forced ambulation but were allowed to rest. Research using Drosophila also showed that prolonged sleep deprivation could be deadly (Shaw et al., 2002). The results obtained from these more recent studies as well as earlier studies strongly suggest prolonged sleep deprivation can be lethal and can cause death even more rapidly than food deprivation (Everson et al., 1989).
Sleeping, like eating, is a biological imperative that consumes approximately one-third of human life. Just as missing a day’s worth of meals produces strong feelings of hunger, being deprived of a night’s sleep leads to overwhelming feelings of fatigue. Sleep resists being deprived; if exhausted enough, humans can fall asleep under even dangerous circumstances (Nansen, 1999). When sleep is denied, there is an increase in the frequency and duration of sleep episodes and an elevation in the intensity of sleep (Banks et al., 2010; Borbély and Tobler, 1996; Brunner et al., 1993; Goel et al., 2009a, 2010; Van Dongen et al., 2003). These properties of sleep and its ubiquitous manifestation across species indicate that sleep must serve an adaptive function. Despite our understanding that sleep plays a necessary and adaptive evolutionary purpose, the universal function of sleep, if one exists, remains elusive (Rechtschaffen, 1998; Siegel, 2005; Tobler, 1995). Our lack of knowledge regarding sleep’s fundamental function has led a select few to assume that humans need only a small “core” amount of sleep to function normally (Horne, 1985, 1988); however, a large body of research indicates that most humans who undergo chronic partial sleep deprivation exhibit profound neurobiological and physiological changes that can impede performance and negatively impact health (Banks et al., 2010; Dement and Greenber, 1966; Dinges, 2004; Goel et al., 2009a; Leproult and Van Cauter, 2010).
Healthy human sleep
Normal human sleep comprises two states—rapid eye movement (REM) and non-rapid eye movement (NREM)—that alternate cyclically during a sleep episode. Using electroencephalography (EEG), characteristics of these sleep states have been well defined (Rechtschaffen and Kales, 1968; Silber et al., 2007). NREM sleep shows synchronous cortical EEG (sleep spindles, K-complexes, and slow waves) and is associated with low muscle tone and minimal psychological activity whereas REM sleep shows desynchronized EEG and is associated with muscle atonia and dreaming (Kryger et al., 2011). In healthy humans, the timing, intensity, and duration of sleep are primarily regulated by two processes: homeostatic regulation and circadian timing (Borbély, 1982, 1998).
Sleep homeostasis
Sleep homeostasis describes the drive for sleep that increases progressively during wakefulness and decreases progressively during NREM sleep (Borbély, 1994). Organisms strive to maintain sleep at a constant level; therefore, the propensity for sleep is increased after prolonged wakefulness and decreased after prolonged sleep. Similarly, sleep restriction leads to an increase in the intensity and duration of subsequent sleep, whereas excess sleep leads to decreased sleep intensity and duration (Borbély, 1982). Researchers have found that slow waves, low-frequency EEG, likely represent a measure of sleep intensity—extended waking leads to increases in slow wave energy (SWE) during the subsequent recovery night and the extent of this SWE increase is a function of prior wake duration (Åkerstedt et al., 2009; Brunner et al., 1990). In addition to SWE, other biological markers of sleep homeostasis have been identified including extracellular adenosine, central nitrous oxide levels, and salivary amylase; levels of these markers increase with prolonged sleep–wakefulness and thus may reflect an increased sleep drive (Kalinchuk et al., 2006; Porkka-Heiskanen and Kalinchuk, 2011; Scharf et al., 2008; Seugnet et al., 2006). The homeostatic process of sleep–wake regulation interacts with but is independent from circadian control (Dijk et al., 1989).
Sleep as a circadian process
The circadian process that controls sleep is described as the 24-h oscillatory variation in the propensity for sleep. This 24-h period is the time it takes the Earth to rotate about its own axis which generates daily environmental cycles of ambient temperature and illumination. The alternation of light and darkness directly entrains an organism’s circadian rhythms and thus influences its life patterns, creating species that are active primarily during the light (diurnal), the dark (nocturnal), twilight periods (crepuscular), or during the light and the dark (cathemeral) (Pittendrigh, 1981). Environmental light is transmitted from the retina to the suprachiasmatic nuclei in the anterior hypothalamus which then transmit this information to, among others, the pineal gland via a multisynaptic pathway; the pineal gland secretes melatonin, a hormone that regulates various biological functions (Czeisler, 1995; Lucas et al., 1999; Ralph et al., 1990; Sadun et al., 1984; Watts, 1991). In humans, melatonin increases during the dark cycle which coincides with a period of inactivity and sleepiness and decreases during the light cycle which coincides with a period of activity and wakefulness (Aschoff and Wever, 1981; Shanahan and Czeisler, 1991). This circadian process interacts with but is independent from sleep homeostasis (Åkerstedt and Froberg, 1978).
Scientific evidence shows that human sleep is naturally regulated by these two processes, with sunrise and sunset providing the photic signals necessary to entrain the sleep–wake cycle (Wehr et al., 2001). However, with the introduction of artificial light and other technologies, television and alarm clocks have replaced these natural signals and thereby may not allow optimal sleep duration. Additionally, industries that operate 24 h a day create light and noise that can interfere with sleep (Basner et al., 2011) and require employees to work during hours usually devoted to sleep. Finally, with the increase in international business and air travel, many adults are frequently traveling across time zones which affects the circadian timing system. Thus, many humans are challenging basic biological pressures in order to accommodate social norms and obligations.
Human sleep duration
For most healthy adults, physiological sleep duration appears to range between 7.0 and 8.5 h; however, habitual sleep duration among adults is determined by a variety of factors and shows considerable variance within and between individuals (Van Dongen et al., 2005). Data from the 2005–2008 National Health and Nutrition Examination Survey suggest that self-reported sleep duration is distributed approximately normally and 2004–2007 National Health Interview Survey-Sample Adult data reveal that 7.8% of adults report sleeping less than 5 h per night, 20.5% report sleeping 6 h per night, 30.8% report sleeping 7 h per night, 32.5% report sleeping 8 h per night, and 8.5% of adults report sleeping more than 9 h per night (Krueger and Friedman, 2009). One limitation of large epidemiological studies is that sleep duration is examined using self-report which can be subject to inherent biases and thereby inaccurate.
Indeed, when comparing self-reported sleep duration to objectively measured sleep duration using actigraphy (an unobtrusive measure of gross motor activity which is analyzed to objectively identify sleep periods), subjectively reported sleep averaged 0.80 h longer than objectively measured sleep (Bradshaw et al., 2007; Lauderdale et al., 2008). Although wrist actigraphy provides an objective measure of inactivity, it still overestimates polysomnography (PSG)-measured sleep duration, which is the gold standard for determining physiological sleep. One study showed this overestimation was approximately 18 min on average (Blackwell et al., 2008). Therefore, although epidemiological studies are valuable in that they include data based on a large number of subjects, the actual amount of physiological sleep that adults receive is typically less than what is self-reported in these studies.
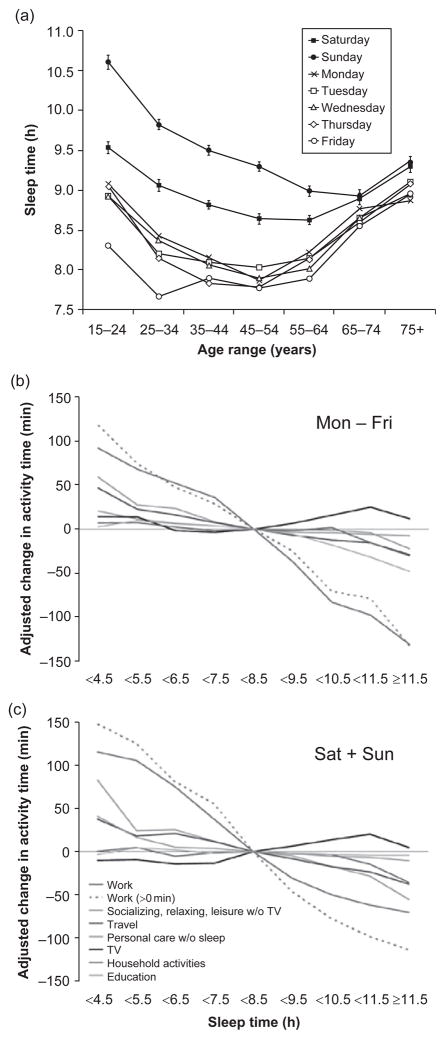
There is considerable debate as to whether or not sleep duration has been decreasing among adults in recent decades and if so, whether this reduction is resulting in higher rates of chronic sleep restriction or sleep debt (Dinges, 2004; Horne, 2004). However, according to the Center for Disease Control Morbidity and Mortality Weekly Report (2008), the percentage of adults who reported an average of less than or equal to 6 h of sleep within a 24-h period significantly increased from 1985 to 2004 (in both females and males and among all age groups 18–75). Among employed American adults, two epidemiological studies found that the prevalence of being a short sleeper (either ≤6 h/day (Knutson et al., 2010) or <6 h/day (Luckhaupt et al., 2010)) has increased significantly in the past few decades. Studies have suggested that habitual short sleepers do not require less sleep than other adults; rather, these individuals gradually accrue sleep debt over time (Aeschbach et al., 1996; Bradshaw et al., 2007; Klerman and Dijk, 2005). Evidence from the American Time Use Survey indicates that adult sleep duration is significantly shorter during weekdays compared to weekends, suggesting that adults attempt to recover sleep debt by extending sleep when it is presumably more convenient and when schedules are likely more flexible (see Fig. 1a; Basner et al., 2007).
Fig. 1.
(a) Average sleep duration with respect to age and day of the week. Average sleep time across age groups was longest on Sunday followed by Saturday and was markedly shorter during the week, gradually decreasing from Monday to Friday for most age groups. (b and c) Average change in weekday (b) (Mon–Fri) and weekend (c) (Sat+Sun) waking activity time depending on sleep time category (N = 23,325) based on multiple linear regression models adjusting for age, gender, ethnicity, educational attainment, income, presence of partner, and presence of children. The 7.5 h to <8.5 h sleep time category served as a reference. For work time, separate models were run for the whole group (Work) and those only who worked on the interview day (Work>0 min). The largest reciprocal relationship to sleep was found for work time, followed by commute/travel time. Short sleepers spent more time socializing, relaxing, and engaging in leisure activities, while both short and long sleepers watched more television than the average sleeper. (Reprinted with permission from Basner et al., 2007.)
Causes of chronic partial sleep restriction
Paid work
Compensated work time may be the most potent determinant of sleep duration (see Fig. 1b and c; Basner et al., 2007). There is a higher prevalence of short sleep duration among full-time employed adults when compared to part-time workers, students, retired individuals, homemakers, or unemployed adults (Knutson et al., 2010; Luckhaupt et al., 2010), and longer work hours are associated with shorter sleep duration (Hale, 2005; Nakashima et al., 2010; Virtanen et al., 2009). Adults working more than 8 h/day have the same bedtime compared to adults who do not work more than 8 h/day, but they wake up much earlier (Basner and Dinges, 2009). Using a prospective study design, Virtanen et al. (2009) found that working more than 55 h/week is a risk factor for the development of shortened sleep and for difficulty falling asleep. Although the average number of hours spent working has remained relatively stable during the past few decades, the prevalence of individuals who work greater than 48 h/week has increased (Rones et al., 1997). The duration of required working hours varies by occupation, with managerial, professional, manufacturing, and transportation industries typically involving longer work hours; interestingly, the prevalence of short sleep duration is also highest in these occupations (Luckhaupt et al., 2010). By contrast, the lowest prevalence of short sleep duration is observed in the “agriculture, forestry, fishing, and hunting industry” (Luckhaupt et al., 2010). Although workers within this category (such as farmers) often work long hours and begin work early in the morning, their work schedules are more in sync with the natural light–dark cycle compared to other occupations, and thus their work hours presumably interfere less with sleep quality and duration.
In contrast to the schedules of agriculture, forestry, fishing, or hunting industries, shift work schedules often require work to occur during the night when the circadian system is promoting sleep and require sleep to occur during the day when the circadian system is promoting wakefulness. This work schedule creates a conflict between the worker’s internal circadian time and his/her required sleep–wake schedule, leading to impaired wakefulness and disturbed sleep (Åkerstedt, 2005; Kolla and Auger, 2011). According to the Bureau of Labor Statistics (2005), approximately 15% of full-time wage and salary earners work shifts that are not during the daytime. Night workers sleep 2–4 h less per day than day workers (Åkerstedt, 2003) and workers either permanently on a night shift or on a rotation including the night shift are significantly more likely to sleep less than 6 h and experience excessive sleepiness during situations requiring a high degree of attention (Ohayon et al., 2010). Although some countermeasures, such as strategic light exposure (Burgess et al., 2002), or the use of melatonin, stimulants, and other pharmacological agents related to sleep–wake regulation can promote wakefulness or improve sleep among shift workers (Kolla and Auger, 2011), currently there is no treatment to completely normalize a shift worker’s sleep duration that can be widely used in real-world situations (Åkerstedt and Wright, 2009).
Commute/travel
According to the Census American Community Survey Report (2011), American workers spend nearly an hour commuting to and from work each day and 33.5% of workers have commutes that are greater than 30 min each way. Travel time (composed of traveling to work [commute], stores, schools, and social events) is negatively associated with sleep time (see Fig. 1b and c; Basner et al., 2007). A majority of Americans (55.4%) leave their homes between 06:00 and 08:30 in order to arrive at work on time (Census American Community Survey Report, 2011), and they do not arrive home until late in the evening in part due to traffic; the result is an increase in the length of the workday and a decrease in time for other activities such as socializing, relaxing, and sleeping.
Leisure
Socializing, relaxing, and engaging in other leisure activities are also negatively related to sleep duration (see Fig. 1b and c; Basner et al., 2007). For many adults, this time usually involves sitting in front of some type of screen, such as a television, computer, or smartphone. For example, watching television was the most common activity during the 2-h period before bedtime, suggesting that nighttime television viewing and sleep onset are tightly correlated (Basner and Dinges, 2009; Basner et al., 2007). Adults are now able to access e-mail, engage in social networking and online gaming, and use the web 24 h a day; devices have become so portable that they can easily be brought into bed and used right before going to sleep. Among adults, computer and mobile phone use in the bedroom is associated with greater variability in sleep–wake schedules and poorer sleep habits (Brunborg et al., 2011). In addition to the time used engaging in these activities, exposure to light emitted from these screens may also impede sleep. Blue (short wavelength) light represents the most potent portion of the light spectrum for suppressing melatonin and thus promoting wakefulness (Brainard et al., 2001; Lockley et al., 2003); moreover, blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin (West et al., 2011). Exposure to blue light immediately prior to bedtime (via flat screen televisions, smartphones, and tablet computers) may cause circadian phase delays and disrupt sleep (Cajochen et al., 2006, 2011).
Chronotype
Although humans are diurnal, some individuals prefer activity in the morning (larks) whereas others prefer activity in the evening (owls)—this preference influences the timing of sleep–wake cycles. Morning-type and evening-type individuals differ endogenously in the circadian phase of their biological clocks (Baehr et al., 2000; Kerkhof and VanDongen, 1996), which is partially determined by genetic polymorphisms (Goel, 2011). In addition to genetic factors, age, and gender also influence morningness–eveningness (Roenneberg et al., 2007). The Earth’s light/dark cycle and the work schedules of industrial societies complement individuals who function best in the morning rather than in the evening. Owls experience heightened alertness in the late evening and typically stay awake longer and have a delayed bedtime compared to larks; however, due to typical work schedules owls often use an alarm clock to wake up early in the morning, which produces chronic sleep restriction, extended wakefulness, and accumulated sleep debt during the work week (Korczak et al., 2008; Roenneberg et al., 2003; Roepke and Duffy, 2010; Taillard et al., 2003).
Social jetlag, a term coined by Roenneberg and colleagues (Wittmann et al., 2006), describes the misalignment of biological and social time caused by social activities such as work, commuting, and television viewing. For many adults, social jetlag and/or a preference for eveningness can lead to the accumulation of sleep debt during the work week due to repeated episodes of shortened sleep. This repeated and chronic partial sleep deprivation has been shown to have serious neurobehavioral and physiological consequences unlikely to be reversed by increasing sleep time on weekends and days off (Banks et al., 2010; Goel et al., 2009a; Van Dongen et al., 2003).
Consequences of chronic partial sleep restriction
According to the National Transportation Safety Board (1989, 1995), 30–40% of all U.S. truck accidents are fatigue related and several catastrophes including the Exxon Valdez accident, Chernobyl and Three Mile Island nuclear plant meltdowns, and Space Shuttle Challenger tragedy were partially due to human error resulting from sleepiness and fatigue (Mitler et al., 1988). Sleepiness due to long work hours has also been implicated in medical errors: interns, surgeons, physicians, and residents make significantly more errors, including those that are injurious or fatal, after working for an extended period of time and/or after chronic and acute sleep loss (Czeisler, 2009; Lockley et al., 2004). As such, gaining a greater understanding of the relationship between sleep and waking performance is an important focus of ongoing research.
Regulation of human performance
Similar to the regulation of sleep duration and intensity, distinct sleep/wake-related physiological processes regulate alertness and neurobehavioral performance. The homeostatic sleep-dependent process (process S) balances sleep propensity by keeping track of recent sleep history (Daan et al., 1984). As hours of wakefulness increase, homeostatic drive increases the propensity for sleep. The endogenous circadian process (process C) tracks changes in light exposure (as well as other zeitgebers or synchronizers) and entrains sleep propensity to the light–dark cycle; when sleep propensity is increased (during the night) waking performance is often degraded (Czeisler et al., 1999). Sleep inertia, a transient process that occurs immediately after awakening, causes deficits in waking performance; its effects depend on prior sleep history, circadian phase, and the depth of sleep at the time of awakening (Dinges, 1990). Finally, a second homeostatic process (process U) influences waking performance by monitoring sleep–wake on a longer time scale (nightly sleep duration) and interacting with the other processes (McCauley et al., 2009). Process U builds up over several days of prolonged wakefulness, when sleep debt is accruing and the need for sleep is increasing, and dissipates during sleep. The status of process U, similar to a sleep reservoir being full or empty, codetermines the rate by which the original homeostatic process (process S) increases sleep propensity and thus decreases waking performance (McCauley et al., 2009).
All of these processes interact to form a dynamic relationship between sleep propensity and waking performance (Raslear et al., 2011; Van Dongen and Dinges, 2005). During a 24-h period, the interaction of these processes leads to a period in the early morning when waking performance is particularly vulnerable to sleep restriction; neurobehavioral deficits are largest at 08:00 and become progressively smaller during the day, especially between 16:00 and 20:00 (Mollicone et al., 2010). The incidence of single-vehicle automobile accidents, human error, and accidents related to work performance and several industrial/engineering disasters also reveal early morning hours as particularly vulnerable to sleep restriction (Mitler et al., 1988). However, when an individual lacks a sufficient amount of sleep, either as a result of acute total sleep deprivation or chronic partial sleep restriction, homeostatic pressure can increase to the point whereby waking cognitive functions will be degraded even during periods of time when the circadian drive for wakefulness peaks (Doran et al., 2001).
Sleep propensity
Individuals who report sleeping less than 7.5 h/day on average are more likely to exhibit a stronger sleep propensity (the tendency to fall asleep)—they report unintentionally falling asleep during the day and nodding off or falling asleep while driving (McKnight-Eily et al., 2011; Punjabi et al., 2003). In the laboratory, sleep propensity—operationalized as the speed of falling asleep in both sleep-conducive and non-conducive conditions—is among the most well-validated measures of sleepiness (Roehrs et al., 2005). The effects of chronic sleep restriction on daytime physiological sleep propensity have been evaluated using the Multiple Sleep Latency Test (MSLT) (Carskadon and Dement, 1981) and the Maintenance of Wakefulness Test (MWT) (Mitler et al., 1982). During the MSLT, the subject is instructed to close his/her eyes and try to fall asleep while lying in a supine position, whereas during the MWT, the subject is instructed to look straight ahead and try to stay awake while seated upright. In both tests, PSG recordings are made (including EEG, electrooculogram and electromyogram) and the time taken to fall asleep is a measure of sleep propensity.
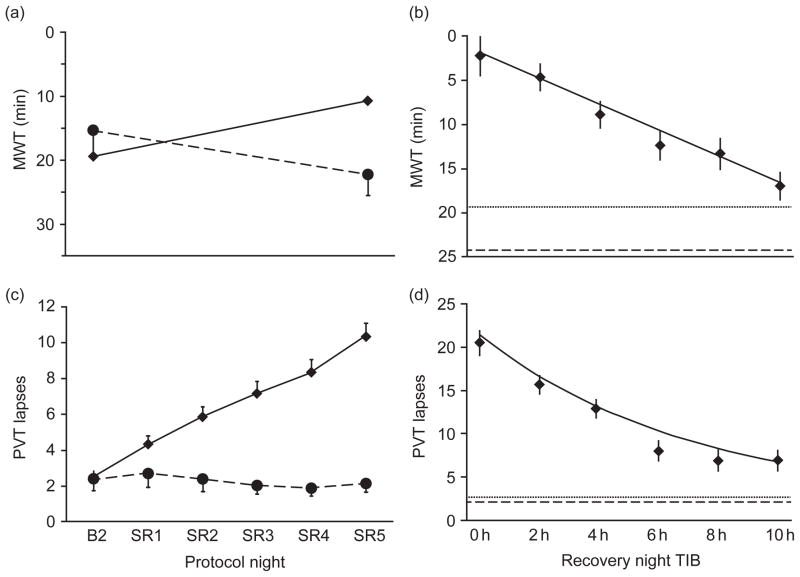
Whether attempting to fall asleep or resist sleep, the latency from waking to sleeping is reduced by chronic partial sleep restriction (see Fig. 2a; Banks et al., 2010; Belenky et al., 2003; Carskadon and Dement, 1981; Dinges et al., 1997; Guilleminault et al., 2003; Rupp et al., 2009). As the number of days of sleep restriction increases or the amount of sleep available during each night of sleep restriction decreases, the latency to fall asleep decreases (Belenky et al., 2003; Carskadon and Dement, 1981; Devoto et al., 1999; Guilleminault et al., 2003; Rupp et al., 2009). Spending 10 h in bed daily for 1 week prior to experiencing a week of sleep restriction may attenuate the effects of sleep restriction on sleep propensity as measured by MWT (Rupp et al., 2009). By contrast, after experiencing partial sleep restriction, one recovery night of up to 10 h time in bed (TIB) is insufficient to return sleep propensity back to presleep restriction levels (see Fig. 2b; Banks et al., 2010). MWT sleep latencies appear normalized after two 8 h TIB nights of recovery sleep (Rupp et al., 2009); however, due to the small sample size used in this study, replication of this finding is needed.
Fig. 2.
(a and c) Daily means (±SEM) of (a) MWT sleep onset latency and (c) PVT performance in the sleep restriction group (n = 142, 4 h TIB for five nights [SR1–SR5], solid line), and the control group (n = 17, 10 h TIB on all nights, dashed line). All subjects had 10 h TIB (22:00–08:00) on baseline day 2 (B2). Sleep restriction on SR1 to SR5 was from 04:00 to 08:00. Data are plotted to show deficits in neurobehavioral functions increasing (upward) on the ordinate. Relative to the control condition, sleep restriction degraded neurobehavioral function over days (decreased MWT sleep onset latency and increased PVT lapses [>500 ms reaction times]). (b and d) Daily means (±SEM) of (b) MWT sleep onset latency and (d) PVT performance following the acute recovery (REC) night as a function of increasing TIB dose (0–10 h, solid line), controlling for covariates (i.e., baseline, cumulative deficits during sleep restriction, age, and sex). Sleep-restricted subjects (n = 142) were randomized to either 0 h TIB (n = 13), 2 h TIB (n = 27), 4 h TIB (n = 29), 6 h TIB (n = 25), 8 h TIB (n = 21), or 10 h TIB (n = 27). For comparison, horizontal dotted lines show baseline night (B2, 10 h TIB) values, and horizontal dashed lines show control group (n = 17) means on day 8 (10 h TIB), which is the study day equivalent to REC. These neurobehavioral outcomes showed improvement as recovery sleep doses increased (MWT sleep onset latencies increased and PVT lapses decreased); however, even at the highest sleep recovery dose, functioning did not return to baseline or control condition levels. (Reprinted with permission from Banks et al., 2010.)
Behavioral alertness: Microsleeps and wake-state instability
The increased propensity for sleep due to restricted sleep time can lead to the occurrence of “microsleeps,” very brief sleep episodes that intrude into wakefulness despite an individual’s best effort to stay awake (Åkerstedt et al., 1987; Bjerner, 1949; Torsvall and Åkerstedt, 1987). Wake-state instability refers to the moment-to-moment shifts in the neurobiological systems mediating the motivated desire to sustain waking alertness and those mediating the involuntary homeostatic drive to fall asleep (Doran et al., 2001; Saper et al., 2005). This type of interaction between drives results in unpredictable behavior, including increased variability in cognitive performance and lapsing (i.e., brief periods of half a second to many seconds of no response) (Doran et al., 2001). Although individuals may not realize they are experiencing microsleeps and performance decrements, over time this instability can progress into full blown sleep attacks—when individuals will not spontaneously wake without additional stimulation (Dinges and Kribbs, 1991; Harrison and Horne, 1996; Kleitman, 1963). Sleep restriction can increase the incidence of microsleeps and produce decreased behavioral alertness, even during goal-directed behaviors (e.g., motor vehicle operation). The rapid intrusion of sleep into wakefulness and consequent wake-state instability can have profound adverse consequences, especially if experienced by those in safety-sensitive occupations, such as police officers, firefighters, health care providers, and motor vehicle operators (Barger et al., 2009; Lombardi et al., 2010; Trew et al., 2011). Unfortunately, workers in these professions are often subjected to chronic sleep restriction due to shift work schedules and long work hours and are thus particularly vulnerable to microsleeps and wake-state instability.
Behavioral alertness, measured using sustained attention tasks in the laboratory, has been shown to be sensitive to sleep restriction. According to the state instability hypothesis, sleep drive escalates instability in attention which creates increasingly variable neurobehavioral performance (periods of accurate responding are interrupted by errors of omission [lapses] comingled with errors of commission) (Doran et al., 2001). Studies support this hypothesis; cognitive performance variability is influenced by prior wakeful-ness, circadian phase, and the amount of time spent on the task (Doran et al., 2001; Graw et al., 2004; Mollicone et al., 2010; Zhou et al., 2011b). When sleep is chronically restricted, sleep debt accumulates across each night leading to greater impairment over time (Banks et al., 2010; Belenky et al., 2003; Dinges et al., 1997; Goel et al., 2009a; Rupp et al., 2009; Van Dongen et al., 2003; Wu et al., 2010). Thus, as the need for sleep increases, the brain’s ability to maintain alertness becomes progressively more overwhelmed by the activation of sleep processes which leads to microsleeps and consequent neurobehavioral instability (Doran et al., 2001). Even when highly motivated, an individual’s attempt to compensate for excessive sleepiness by engaging in various behaviors ultimately fails to prevent intrusions of microsleeps and impaired neurobehavioral performance (Horne and Pettitt, 1985; Hsieh et al., 2010).
Driving performance
Driving a vehicle requires sustained attention and is sensitive to the reduced alertness associated with sleepiness. Every year, thousands of automobile crashes, injuries, and fatalities are due to drivers falling asleep (Strohl et al., 1997). Adults who are employed, work more than 60 h/week, work irregular hours, or work at night, as well as those who are sleep deprived or chronically sleep restricted are more likely to have a car accident (Abe et al., 2010; Åkerstedt et al., 2005; Brown, 1994; Gander et al., 2005; Horne and Reyner, 1999; Philip et al., 2003, 2005; Scott et al., 2007; Stutts et al., 2003). In the laboratory, studies have primarily focused on short-term sleep restriction and have found that driving performance on simulators decreases (resulting in more crashes) after short sleep duration (4–6 h TIB) (De Valck and Cluydts, 2003; Lenne et al., 2004; Macchi et al., 2002; Otmani et al., 2005; Vakulin et al., 2007). Similarly, the less sleep obtained during chronic partial sleep restriction, the more driving performance (increase in number of driving simulator accidents) is impaired (Russo et al., 2003).
Cognitive performance
In addition to its negative effects on alertness, sleep deprivation degrades aspects of cognitive performance (Harrison and Horne, 2000; Kleitman, 1963; McCoy and Strecker, 2011). There are hundreds of published studies on the effects of total sleep deprivation on cognitive performance (Alhola and Polo-Kantola, 2007; Goel et al., 2009b; Lim and Dinges, 2010), but far fewer studies on the cognitive-impairing effects of chronic partial sleep restriction.
Sleep deprivation induces a wide range of effects on cognitive functions (Killgore, 2010; Poe et al., 2010); however, cognitive tasks vary considerably in their sensitivity to sleep loss. In general, regardless of the task, cognitive performance becomes progressively worse when time on task is extended; this is the classic “fatigue” effect that is exacerbated by sleep loss (Bjerner, 1949; Wilkinson, 1969). However, performance on brief cognitive tasks that measure speed of cognitive “throughput,” working memory, and other aspects of attention are also sensitive to sleep deprivation (Dinges, 1992). Two confounding factors that can obscure the effects of sleep loss on many cognitive tasks are intersubject variability and intrasubject variability (Dorrian et al., 2005). The intersubject confound describes differences in aptitude: one individual’s poorest performance during sleep deprivation may be superior to the best performance of a non-sleep-deprived individual (Goel et al., 2009b). The intrasubject confound describes a learning effect: a person may be cognitively diminished by sleep loss, but continue to improve on a repeated task due to the effects of learning (Goel et al., 2009b). The nature of the dependent variables selected for examining the cognitive effects of sleep deprivation can also be problematic. Sleep deprivation increases variability within subjects (i.e., state instability) and between subjects (i.e., differential vulnerability to the effects of sleep deprivation); therefore, the effects of sleep loss on cognitive measures may be missed due to insensitive metrics or data analyses (Olofsen et al., 2004; Van Dongen et al., 2004a; Whitney and Hinson, 2010). To provide an accurate and useful measure of performance during sleep loss as well as the dynamic expression of waking neurobehavioral integrity as it changes over time, cognitive assessments must be valid and reliable reflections of fundamental waking functions altered by sleep deprivation (Goel et al., 2009b). As such, measures of attention, vigilance, and declarative memory are often used, with reaction time as the dependent variable.
The Psychomotor Vigilance Test (PVT), a measure of sustained attention, is free of aptitude and learning effects and is widely used in sleep studies due to its sensitivity to sleep loss, sleep pathology, and functioning at an adverse circadian phase (Balkin et al., 2004; Dorrian et al., 2005; Lim and Dinges, 2008). The primary outcome measures of the PVT are reaction time and errors, including omissions (lapses) and commissions (false responses). In 2003, two large-scale experimental studies found that when sleep is reduced to 3, 4, 5, or 6 h TIB for several nights, sustained attention performance (PVT) as well as working memory and cognitive throughput (Serial Addition/Subtraction Task, Digit Symbol Substitution Task) decreases in a dose-dependent manner (see Fig. 2c; Belenky et al., 2003; Van Dongen et al., 2003). As sleep debt accumulates across days, performance becomes progressively worse over time; 14 days of chronic sleep restriction (4 or 6 h TIB each night) produced comparable cognitive deficits to those produced by 24–48 h of total sleep deprivation (Van Dongen et al., 2003). This finding has been replicated several times (Axelsson et al., 2008; Banks et al., 2010; Bliese et al., 2006; Cote et al., 2008; Dinges et al., 1997; Fafrowicz et al., 2010; Goel et al., 2009a; Rupp et al., 2009; Wu et al., 2010).
Research has also shown that the recovery rate from chronic sleep restriction may be slower than from acute total sleep loss (Banks et al., 2010; Rupp et al., 2009). In a large-scale experimental study examining dose–response effects of one night of recovery sleep after five nights of sleep restriction on PVT performance, recovery to either a subject’s own baseline values or values recorded for the sleep-satiated control group was not achieved at any of the doses examined, including the highest dose of 10 h TIB (see Fig. 2d; Banks et al., 2010). Due to circadian limitations on sleep duration, it is unlikely that recovery from 5 days of chronic sleep restriction can occur in one night of more than 10 h TIB; therefore, residual attentional deficits still present after one night of recovery may potentiate the effects of a subsequent sleep restriction period (Banks et al., 2010). Thus, attempting to recover lost sleep from a work week by extending sleep on a weekend night is likely insufficient for recuperating impaired alertness and sustained attention. Other studies examining recovery after chronic sleep restriction have found that PVT performance remains substandard even after 3, 5, or 7 recovery nights of 8 h TIB (Axelsson et al., 2008; Belenky et al., 2003; Rupp et al., 2009). However, similar to results for sleep propensity, prophylactic napping prior to sleep deprivation significantly improved reaction time performance and spending 10 h TIB daily for 1 week prior to experiencing chronic partial sleep restriction attenuated the decrement in PVT performance during sleep loss and facilitated improvement of PVT performance during recovery (Dinges et al., 1987; Rupp et al., 2009). Thus, chronic sleep restriction induces slow changes in neural processes mediating alertness and attention that cause performance to become progressively worse over time, producing the accumulation of severe performance decrements. Similarly, the slow recovery rate from chronic sleep restriction suggests that this type of sleep loss may induce long-term neuromodulatory changes in brain physiology (Banks et al., 2010; Rupp et al., 2009).
Researchers have proposed that attention and working memory, considered basic cognitive actions, are essential to virtually all other cognitive processes; therefore, sufficient attention and memory performance are necessary for optimal functioning of neural circuits that mediate higher cognitive functions, such as executive function (Balkin et al., 2008). Interestingly, there is a positive relationship between the level of sustained attention required to perform a task and the degree to which task performance is impaired by sleep loss (Jennings et al., 2003). Although there is evidence that sleep deprivation adversely affects prefrontal cortex-related executive attention and working memory abilities, these cognitive effects are often not as prominent or easy to measure as those involving basic processes such as cognitive and psychomotor speed (Goel et al., 2009b). Therefore, more complex cognitive tasks involving higher cognitive functions have been regarded as insensitive to sleep deprivation by some researchers (Harrison and Horne, 2000).
Sleep restriction affects mental flexibility, attention shifting, and the inhibition of automatic responses, all reflective of executive function (McCoy and Strecker, 2011). Executive function can be defined as “the ability to plan and coordinate a willful action in the face of alternatives, to monitor and update action as necessary and suppress distracting material by focusing attention on the task at hand” (Jones and Harrison, 2001). Many tasks believed to engage different aspects of executive function have been used in sleep deprivation studies. Examples of such tasks include the Brixton Spatial Anticipation Test, Controlled Oral Word Association Test (COWAT), Hayling Sentence Completion Task, Torrance Tests of Creative Thinking, Tower of London, Thurstone’s Verbal Learning Task, and Wisconsin Card Sorting Task (Harrison and Horne, 2000; Jones and Harrison, 2001). In experimental studies, individuals who are sleep restricted exhibit deficits on the Wisconsin Card Sorting Task (Herscovitch et al., 1980) and Stroop test (Stenuit and Kerkhofs, 2008) but do not exhibit deficits on the Hayling, Brixton, COWAT, or Tower of London Tasks (Goel et al., 2009a). Although subjects are able to accurately perform many cognitive assessments, compensatory mechanisms become engaged in response to chronic sleep restriction which impedes the ability to think flexibly and increases the use of automatic processing, impulsivity, and rigid rule adherence to complete tasks (Stenuit and Kerkhofs, 2008; Swann et al., 2006).
Plessow and colleagues studied new parents, a population of healthy adults who are often subjected to chronic sleep restriction for several months due to nightly feedings and duties related to caring for a new infant. Parents were administered an explicit-cueing version of the task-switching paradigm to assess their ability to flexibly adapt to changing environmental demands. Sleep-deprived new parents exhibited significantly slower reaction times during task switches compared to new parents reporting adequate sleep; correlational analyses showed that greater self-reported sleep debt was associated with greater impairment on the task (Plessow et al., 2010). These results indicate that chronic sleep restriction can impair an individual’s ability to implement task goals in order to switch tasks in a fast-changing environment—factors important for doctors, pilots, and individuals in the armed forces, who constantly rely on fast and flexible goal shifting and are likely to experience chronic sleep restriction.
Interindividual differences in response to sleep restriction
As stated previously, average sleep duration is normally distributed and widely differs depending on the individual (Van Dongen et al., 2005). In addition, although humans are diurnal mammals, there are individual differences in the timing of their behaviors; some prefer to be active in the morning whereas others prefer to be active in the late evening (Kleitman, 1963; Roenneberg et al., 2003). Similarly, studies of total sleep deprivation as well as chronic sleep restriction have reported substantial interindividual differences in the magnitude of sensitivity/resilience to the effects of sleep loss on neurobehavioral functions (see Fig. 3a; Goel et al., 2009a, 2010; Van Dongen et al., 2004b). Prior sleep history and endogenous circadian rhythms likely play an important role in an individual’s response to sleep loss; however, laboratory studies that carefully control for these factors still find marked individual differences in neurobehavioral responses to sleep loss (Leproult et al., 2003; Van Dongen et al., 2004b). In the studies described earlier, when sleep duration was limited to less than 7 h per night for several consecutive nights, performance on sustained attention, working memory, and cognitive throughput was significantly impaired; however, not all subjects were affected to the same degree (Axelsson et al., 2008; Belenky et al., 2003; Bliese et al., 2006; Goel et al., 2009a, 2010; Van Dongen et al., 2003). Individuals are highly vulnerable, somewhat vulnerable, or highly resistant to the neurobehavioral effects of sleep restriction (Goel and Dinges, 2011). It remains unknown whether the same individuals are vulnerable to the adverse effects of both acute total sleep deprivation and chronic sleep restriction.
Fig. 3.
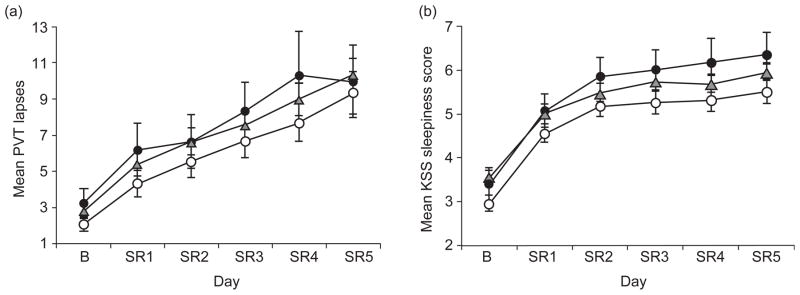
Neurobehavioral performance at baseline and during chronic partial sleep deprivation for the PER3 genotype groups. Mean (±SEM) (a) PVT lapses (>500 ms reaction times) per trial and (b) Karolinska Sleepiness Scale (KSS) scores at baseline (b) for each partial sleep restriction night (SR1–SR5) for PER34/4 (open circles), PER34/5 (gray triangles), and PER35/5 (closed circles) subjects. All genotypes demonstrated large but equivalent cumulative decreases in cognitive performance (PVT) and increases in sleepiness (KSS) across sleep restriction nights. As illustrated by increasing standard error bars in (a), interindividual differences in PVT lapses increased across sleep restriction nights in the entire study group (from Goel et al., 2009a).
A few studies have found that these interindividual differences may be task dependent; one individual’s sustained attention may be most affected by sleep loss whereas another individual’s working memory may be most affected (Frey et al., 2004; Van Dongen et al., 2004b). In addition, interindividual sensitivity to sleep loss is trait like; when neurobehavioral performance was assessed repeatedly (during separate laboratory visits), the same individuals remained particularly vulnerable or resistant to the effects of sleep loss even when previously sleep satiated or sleep restricted (Van Dongen et al., 2004b). Finally, these stable interindividual differences account for a substantial proportion of variance in cognitive performance decrements induced by sleep loss. Demographic factors (age, sex, IQ), baseline functioning, various aspects of habitual sleep timing, and circadian chronotype have not accounted for the robust and stable differences in neurobehavioral responses to sleep restriction (Doran et al., 2001; Goel and Dinges, 2011; Van Dongen and Dinges, 2003; Van Dongen et al., 2004b).
Due to the trait like, or phenotypic, nature of these interindividual differences, research has focused on understanding which genetic polymorphisms may underlie vulnerability to sleep loss. Several genetic mechanisms have been examined in relation to the effects of chronic sleep restriction including the PERIOD3 (PER3) variable number tandem repeat (VNTR) polymorphism and the DQB1*0602 allele. Although the VNTR polymorphism of the circadian gene PER3 (PER34/4, PER34/5, and PER35/5) has been shown to influence executive function deficits in response to total sleep loss (Groeger et al., 2008; Vandewalle et al., 2009; Viola et al., 2007), it does not appear to influence neurobehavioral effects of chronic sleep restriction. A large-scale experimental study examining individuals with different PER3 genotypes found that PER34/4, PER34/5, and PER35/5 genotypes exhibited similar baseline performance on neurobehavioral assessments and demonstrated large but equivalent cumulative increases in sleepiness and sleep propensity and cumulative decreases in cognitive performance and physiologic alertness across five nights of sleep restricted to 4 h per night (see Fig. 3; Goel et al., 2009a). Daily intersubject variability also increased across sleep restriction days similarly in all genotypes (Goel et al., 2009a). Although PER3 genotypes did not differ at baseline in habitual sleep, physiological sleep structure, or sleepiness, during sleep restriction, PER35/5 subjects had elevated sleep homeostatic pressure, measured physiologically by EEG SWE during NREM compared with PER34/4 subjects (Goel et al., 2009a). Goel and colleagues also examined the human leukocyte antigen DQB1*0602 allele which is closely associated with narcolepsy. Subjects who were either positive or negative for the DQB1*0602 allele differed in levels of sleep homeostatic pressure, baseline sleepiness, and sleep physiology but showed comparable cumulative decreases in cognitive performance and increases in sleepiness in response to chronic sleep restriction (Goel et al., 2010). Additional investigations are underway to identify other genetic polymorphisms that may mediate an individual’s neurobehavioral vulnerability to sleep restriction and acute total sleep deprivation (Goel, 2011).
Identifying correlates of interindividual differences in neurobehavioral vulnerability to chronic sleep restriction, such as biomarkers (including genetic polymorphisms as well as adenosine and salivary amylase levels) and behavioral predictors, will provide a viable means to determine those individuals in the general population who need longer habitual sleep durations and/or who require effective interventions and countermeasures for unavoidable sleep loss (Goel and Dinges, 2011). This is a particularly important area of research since adults are not adept at accurately judging how affected they are by chronic sleep restriction (see Figs. 3 and 4; Banks and Dinges, 2007; Van Dongen et al., 2003; Zhou et al., 2011b). Subjective sleepiness is measured using two widely used and well-validated questionnaires: the Stanford Sleepiness Scale (SSS) which requires subjects to rate how sleepy they feel on a scale of 1–7 (Hoddes et al., 1973) and the Karolinska Sleepiness Scale (KSS) which requires subjects to rate how sleepy they feel on a scale of 1–9 (Åkerstedt and Gillberg, 1990). Although subjects are able to detect the rapid and severe changes in levels of alertness and sleepiness in response to total sleep deprivation, subjects are much less sensitive in detecting the smaller changes in levels of alertness and sleepiness that accumulate during each day with sleep restriction (see Figs. 3 and 4; Banks and Dinges, 2007; Goel et al., 2009a; Van Dongen et al., 2003; Zhou et al., 2011a). After a week (or two) of sleep restriction, subjects show marked cognitive impairment and severe decreases in alertness but rate themselves as only moderately sleepy (Belenky et al., 2003; Van Dongen et al., 2003). Consequently, a subject’s rating of subjective sleepiness does not accurately parallel the continuing accumulation of cognitive performance deficits associated with sleep loss. This finding suggests that people who are chronically sleep restricted underestimate the impact of sleep restriction and may overestimate their levels of alertness and ability to perform various cognitive tasks (Banks and Dinges, 2007; Czeisler, 2009; Van Dongen et al., 2003; Zhou et al., 2011a).
Fig. 4.
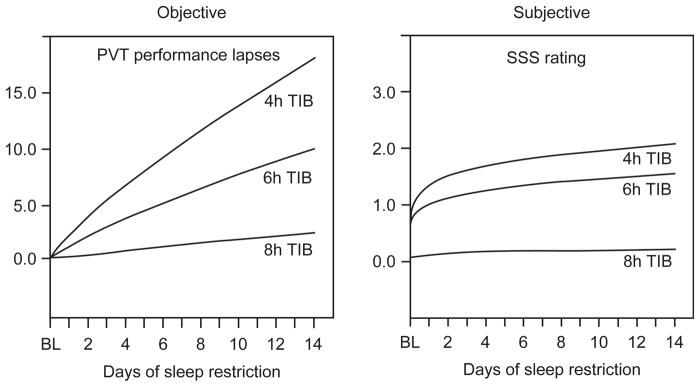
Restriction of nocturnal sleep in healthy adults resulted in near-linear increases in PVT lapses (>500 ms reaction times) of attention across 14 days (coefficients of change near 1.0), but subjective ratings of sleepiness, measured using the Stanford Sleepiness Scale (SSS), showed a nonlinear coefficient below 0.5 for change over days. As objective performance continued to decline near-linearly, there were only minor further increases in the subjective ratings of sleepiness. By the end of the 14 days of sleep restriction, when PVT performance was at its worst levels, subjects in the 4 and 6-h sleep period conditions reported feeling only slightly sleepy. Therefore, unlike performance measures, sleepiness ratings appeared to show adaptation to sleep restriction (N = 35 subjects; 8 h condition n = 9, 6 h condition n = 13, and 4 h condition n = 13). (Reprinted with permission from Van Dongen et al., 2003.)
Management of sleep restriction-induced neurobehavioral impairment
Due to the high prevalence of chronic partial sleep restriction in industrialized societies, the severe consequences of sleep loss on alertness and cognitive function, and our inability to accurately monitor our own sensitivity to chronic sleep loss, objective actions need to be employed to manage sleep debt. Currently, fatigue management within transportation, health professions, and other occupational settings are primarily based on policy; a regulatory organization imposes limits to daily/weekly shift duration as well as the number of consecutive shifts or rest hours (Czeisler, 2009; Gander et al., 2011). Regulatory organizations may also screen potential employers for sleep disorders when occupational duties pose safety risks (Czeisler, 2009; Gander et al., 2011). Although these policies can improve workplace safety and productivity, many regulations are not strictly enforced or adhered to and it is nearly impossible for an employer to ensure that workers are actually obtaining an appropriate amount of sleep each night (Czeisler, 2009; Gander et al., 2011).
Balkin et al. (2011) summarized various technological approaches to fatigue management that accurately predict and/or objectively monitor sleepiness and fatigue effects in real time and that allow for optimally timed administration of interventions or countermeasures (such as the use of properly dosed pharmaceuticals or napping). When discussing the ideal monitoring system, the authors state that technological approaches should be valid, reliable, sensitive, specific, and generalizable. In order to adhere to these criteria, the approach would have (a) the ability to predict fatigue, based on the factors that produce it (sleep history, hours of wakefulness, circadian rhythms, time of day, and biomarkers or behavioral correlates of sensitivity to sleep loss); (b) the ability to measure and monitor fatigue/performance online in the operational environment in order to detect downward trends in alertness or performance before such trends reach the threshold at which operational performance is actually impacted; and (c) the ability to effectively intervene when potential deficits are identified or anticipated, with interventions calibrated so as to restore and sustain alertness/performance as long as needed (e.g., until the operator returns home after the work shift and can safely obtain adequate, recuperative sleep) (Balkin et al., 2011). Examples of instruments currently being examined for such use include EEG measurement of brain wave activity, ocular measures, video-based monitoring systems and portable assessments of attention, and reaction time. Fatigue detection and prediction instruments, implemented in addition to fatigue-related regulations, will help ensure work-place and road safety and adequate levels of alertness in workers while on duty (Balkin et al., 2011).
Conclusion
Despite being treated as a commodity that can be traded for other activities, sleep is essential for optimal neurobehavioral functioning. The 24/7 schedule of many occupations in current industrialized societies as well as other social- and work-related expediencies has produced a high percentage of habitually sleep-restricted adults, a trend that will to continue in the decades ahead. Although chronic partial sleep restriction leads to increased sleep propensity and neurobehavioral deficits, adults are not adept at accurately judging how affected they are by this sleep loss. These effects can have serious adverse consequences including fatal medical errors, catastrophic oil spills, and vehicle crashes. Finding biological and behavioral markers of sleep loss vulnerability and developing technological approaches to fatigue management are two domains of research essential for offsetting the negative effects of societal factors on the biological need for sleep.
Acknowledgments
Supported by NIH NR004281; the National Space Biomedical Research Institute through NASA NCC 9-58; and the Department of the Navy, Office of Naval Research (Award No. N00014-11-1-0361).
Abbreviations
- COWAT
Controlled Oral Word Association Test
- EEG
electroencephalography
- KSS
Karolinska Sleepiness Scale
- MSLT
Multiple Sleep Latency Test
- MWT
Maintenance of Wakefulness Test
- NREM
non-rapid eye movement
- PSG
polysomnography
- PVT
Psychomotor Vigilance Test
- REM
rapid eye movement
- SSS
Stanford Sleepiness Scale
- SWE
slow wave energy
- TIB
time in bed
References
- Abe T, Komada Y, Nishida Y, Hayashida K, Inoue Y. Short sleep duration and long spells of driving are associated with the occurrence of Japanese drivers’ rear-end collisions and single-car accidents. Journal of Sleep Research. 2010;19:310–316. doi: 10.1111/j.1365-2869.2009.00806.x. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Cajochen C, Landolt H, Borbély AA. Homeostatic sleep regulation in habitual short sleepers and long sleepers. American Journal of Physiology. 1996;270:R41–R53. doi: 10.1152/ajpregu.1996.270.1.R41. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T. Shift work and disturbed sleep/wakefulness. Occupational Medicine (Oxford) 2003;53:89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T. Shift work and sleep disorders. Sleep. 2005;28:9–11. [PubMed] [Google Scholar]
- Åkerstedt T, Froberg JE. Persistence of circadian-rhythms in phenomenological and physiological arousal under conditions of continuous activity without sleep. Ergonomics. 1978;21:866. [Google Scholar]
- Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. International Journal of Neuroscience. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: Support from EEG dynamics. Sleep. 2009;32:217–222. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerstedt T, Peters B, Anund A, Kecklund G. Impaired alertness and performance driving home from the night shift: A driving simulator study. Journal of Sleep Research. 2005;14:17–20. doi: 10.1111/j.1365-2869.2004.00437.x. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Torsvall L, Gillberg M. Sleepiness in shiftwork. A review with emphasis on continuous monitoring of EEG and EOG. Chronobiology International. 1987;4:129–140. doi: 10.3109/07420528709078519. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Wright KP., Jr Sleep loss and fatigue in shift work and shift work disorder. Sleep Medicine Clinics. 2009;4:257–271. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatric Disease and Treatment. 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- Aschoff J, Wever R. The circadian system of man. In: Aschoff J, editor. Handbook of behavioral neurobiology. New York: Plenum Press; 1981. pp. 311–331. [Google Scholar]
- Axelsson J, Kecklund G, Åkerstedt T, Donofrio P, Lekander M, Ingre M. Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiology International. 2008;25:297–308. doi: 10.1080/07420520802107031. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: With an emphasis on morningness-eveningness. Journal of Sleep Research. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, Horrey WJ, Graeber RC, Czeisler CA, Dinges DF. The challenges and opportunities of technological approaches to fatigue management. Accident Analysis and Prevention. 2011;43:565–572. doi: 10.1016/j.aap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, Kamimori GH, Redmond DP, Vigneulle RM, Thorne DR, Belenky G, et al. On the importance of countermeasures in sleep and performance models. Aviation, Space, and Environmental Medicine. 2004;75:A155–A157. [PubMed] [Google Scholar]
- Balkin TJ, Rupp T, Picchioni D, Wesensten NJ. Sleep loss and sleepiness—Current issues. Chest. 2008;134:653–660. doi: 10.1378/chest.08-1064. [DOI] [PubMed] [Google Scholar]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. Journal of Clinical Sleep Medicine. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Banks S, Van Dongen HPA, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: Dose-response effects of one night for recovery. Sleep. 2010;33:1013–1026. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger LK, Lockley SW, Rajaratnam SMW, Landrigan CP. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Current Neurology and Neuroscience Reports. 2009;9:155–164. doi: 10.1007/s11910-009-0024-7. [DOI] [PubMed] [Google Scholar]
- Basner M, Dinges DF. Dubious bargain: Trading sleep for Leno and Letterman. Sleep. 2009;32:747–752. doi: 10.1093/sleep/32.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, et al. American time use survey: Sleep time and its relationship to waking activities. Sleep. 2007;30:1085–1095. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Muller U, Elmenhorst EM. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep. 2011;34:11–23. doi: 10.1093/sleep/34.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. Journal of Sleep Research. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Bjerner B. Alpha depression and lowered pulse rate during delayed actions in a serial reaction test. Acta Physiologica Scandinavica. 1949;19:1–93. [Google Scholar]
- Blackwell T, Redline S, Ancoli-Israel S, Schneider JL, Surovec S, Johnson NL, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: The SOF study. Sleep. 2008;31:283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliese PD, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. Journal of Sleep Research. 2006;15:376–385. doi: 10.1111/j.1365-2869.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- Borbély A. Endogenous sleep-promoting substances. Trends in Pharmacological Sciences. 1982;3:350. [Google Scholar]
- Borbély A. Sleep homeostasis and models of sleep regulation. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. Philadelphia: W. B. Saunders; 1994. [Google Scholar]
- Borbély AA. Processes underlying sleep regulation. Hormone Research. 1998;49:114–117. doi: 10.1159/000023156. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Tobler I. Sleep regulation: Relation to photoperiod, sleep duration, waking activity, and torpor. Progress in Brain Research. 1996;111:343–348. doi: 10.1016/s0079-6123(08)60417-3. [DOI] [PubMed] [Google Scholar]
- Bradshaw DA, Yanagi MA, Pak ES, Peery TS, Ruff GA. Nightly sleep duration in the 2-week period preceding multiple sleep latency testing. Journal of Clinical Sleep Medicine. 2007;3:613–619. [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Rollag MD, Greeson J, Byrne B, Glickman G, et al. Human melatonin regulation is not mediated by the three cone photopic visual system. Journal of Clinical Endocrinology and Metabolism. 2001;86:433–436. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- Brown ID. Driver fatigue. Human Factors. 1994;36:298–314. doi: 10.1177/001872089403600210. [DOI] [PubMed] [Google Scholar]
- Brunborg GS, Mentzoni RA, Molde H, Myrseth H, Skouverøe KJ, Bjorvatn B, et al. The relationship between media use in the bedroom, sleep habits and symptoms of insomnia. Journal of Sleep Research. 2011;4:569–575. doi: 10.1111/j.1365-2869.2011.00913.x. [DOI] [PubMed] [Google Scholar]
- Brunner DP, Dijk DJ, Borbély AA. Repeated partial sleep-deprivation progressively changes the EEG during sleep and wakefulness. Sleep. 1993;16:100–113. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- Brunner DP, Dijk DJ, Tobler I, Borbély AA. Effect of partial sleep deprivation on sleep stages and EEG power spectra: Evidence for non-REM and REM sleep homeostasis. Electroencephalography and Clinical Neurophysiology. 1990;75:492–499. doi: 10.1016/0013-4694(90)90136-8. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. Workers on Flexible and Shift Schedules in 2004 Summary. Washington, DC: U.S. Department of Labor Economic News Release; 2005. [Google Scholar]
- Burgess HL, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Medicine Reviews. 2002;6:407–420. [PubMed] [Google Scholar]
- Cajochen C, Frey S, Anders D, Spati J, Bues M, Pross A, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. Journal of Applied Physiology. 2011;110:1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Jud C, Munch M, Kobialka S, Wirz-Justice A, Albrecht U. Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. European Journal of Neuroscience. 2006;23:1082–1086. doi: 10.1111/j.1460-9568.2006.04613.x. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. QuickStats: Percentage of adults aged > 18 years who reported an average of < 6 hours of sleep per 24-Hour period, by sex and age group - National Health Interview Survey, United States, 1985 and 2006. Morbidity and Mortality Weekly Report. 2008;57:209. [Google Scholar]
- Census American Community Survey Report. Workers on Flexible and Shift Schedules in 2004 Summary. Washington, DC: U.S. Department of Commerce American Community Survey Reports; 2011. [Google Scholar]
- Cote KA, Milner CE, Osip SL, Baker ML, Cuthbert BP. Physiological arousal and attention during a week of continuous sleep restriction. Physiology and Behavior. 2008;95:353–364. doi: 10.1016/j.physbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Czeisler CA. The effect of light on the human circadian pacemaker. Circadian Clocks and Their Adjustment. 1995;183:254–290. doi: 10.1002/9780470514597.ch14. [DOI] [PubMed] [Google Scholar]
- Czeisler CA. Medical and genetic differences in the adverse impact of sleep loss on performance: Ethical considerations for the medical profession. Transactions of the American Clinical and Climatological Association. 2009;120:249–285. [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DGM, Borbély AA. Timing of human sleep—Recovery process gated by a circadian pacemaker. American Journal of Physiology. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- De Valck E, Cluydts R. Sleepiness as a state-trait phenomenon, comprising both a sleep drive and a wake drive. Medical Hypotheses. 2003;60:509–512. doi: 10.1016/s0306-9877(02)00444-9. [DOI] [PubMed] [Google Scholar]
- Dement W, Greenber S. Changes in total amount of stage 4 sleep as a function of partial sleep deprivation. Electroencephalography and Clinical Neurophysiology. 1966;20:523–526. doi: 10.1016/0013-4694(66)90110-6. [DOI] [PubMed] [Google Scholar]
- Devoto A, Lucidi F, Violani C, Bertini M. Effects of different sleep reductions on daytime sleepiness. Sleep. 1999;22:336–343. doi: 10.1093/sleep/22.3.336. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, Daan S, Lewy AJ. Bright morning light advances the human circadian system without affecting NREM sleep homeostasis. American Journal of Physiology. 1989;256:R106–R111. doi: 10.1152/ajpregu.1989.256.1.R106. [DOI] [PubMed] [Google Scholar]
- Dinges DF. Are you awake? In: Bootzin R, Kihlstrom J, Schacter D, editors. Sleep and cognition. Washington, DC: American Psychological Association; 1990. pp. 159–175. [Google Scholar]
- Dinges DF. Probing the limits of functional capability: The effects of sleep loss on short-duration tasks. In: Broughton RJ, Oglivie RD, editors. Sleep, arousal, and performance. Boston: Birkhauser; 1992. pp. 176–188. [Google Scholar]
- Dinges DF. Sleep debt and scientific evidence. Sleep. 2004;27:1050–1052. [PubMed] [Google Scholar]
- Dinges DF, Kribbs NB. Performing while sleepy: Effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, sleepiness and performance. Chister: Wiley; 1991. [Google Scholar]
- Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: Contributions of circadian phase and prior wakefulness. Sleep. 1987;10:313–329. [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: Evidence of state instability. Archives Italiennes de Biologie. 2001;139:253–267. [PubMed] [Google Scholar]
- Dorrian J, Rogers N, Dinges D. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida C, editor. Sleep deprivation. New York: Marcel Dekker; 2005. [Google Scholar]
- Everson CA, Bergmann BM, Rechtschaffen A. Sleep-deprivation in the rat. 3. Total sleep-deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- Fafrowicz M, Oginska H, Mojsa-Kaja J, Marek T, Golonka K, Tucholska K. Chronic sleep deficit and performance of a sustained attention task-an electrooculography study. Chronobiology International. 2010;27:934–944. doi: 10.3109/07420528.2010.488981. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Badia P, Wright KP. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. Journal of Sleep Research. 2004;13:305–315. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- Gander P, Hartley L, Powell D, Cabon P, Hitchcock E, Mills A, et al. Fatigue risk management: Organizational factors at the regulatory and industry/company level. Accident Analysis and Prevention. 2011;43:573–590. doi: 10.1016/j.aap.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Gander PH, Marshall NS, Harris RB, Reid P. Sleep, sleepiness and motor vehicle accidents: A national survey. Australian and New Zealand Journal of Public Health. 2005;29:16–21. doi: 10.1111/j.1467-842x.2005.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Goel N. Genetics of sleep timing, duration, and homeostasis in humans. Sleep Medicine Clinics. 2011;6:171–182. doi: 10.1016/j.jsmc.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. http://dx.doi.org/10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75:1509–1519. doi: 10.1212/WNL.0b013e3181f9615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Dinges DF. Behavioral and genetic markers of sleepiness. Journal of Clinical Sleep Medicine. 2011;7:S19–S21. doi: 10.5664/JCSM.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiology and Behavior. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Groeger JA, Viola AU, Lo JCY, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Powell NB, Martinez S, Kushida C, Raffray T, Palombini L, et al. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Medicine. 2003;4:177–184. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Hale L. Who has time to sleep? Journal of Public Health Medicine. 2005;27:205–211. doi: 10.1093/pubmed/fdi004. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. Occurrence of ‘microsleeps’ during daytime sleep onset in normal subjects. Electroencephalography and Clinical Neurophysiology. 1996;98:411–416. doi: 10.1016/0013-4694(96)95612-6. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. The impact of sleep deprivation on decision making: A review. Journal of Experimental Psychology: Applied. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- Herscovitch J, Stuss D, Broughton R. Changes in cognitive processing following shortterm cumulative partial sleep deprivation and recovery oversleeping. Journal of Clinical and Experimental Neuropsychology. 1980;2:301–319. [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA. Sleep function, with particular reference to sleep-deprivation. Annals of Clinical Research. 1985;17:199–208. [PubMed] [Google Scholar]
- Horne J. The substance of sleep. New Scientist. 1988;117:60–62. [Google Scholar]
- Horne J. Is there a sleep debt? Sleep. 2004;27:1047–1049. [PubMed] [Google Scholar]
- Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep-deprivation. Acta Psychologica. 1985;58:123–139. doi: 10.1016/0001-6918(85)90003-4. [DOI] [PubMed] [Google Scholar]
- Horne J, Reyner L. Vehicle accidents related to sleep: A review. Occupational and Environmental Medicine. 1999;56:289–294. doi: 10.1136/oem.56.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SL, Li TH, Tsai LL. Impact of monetary incentives on cognitive performance and error monitoring following sleep deprivation. Sleep. 2010;33:499–507. doi: 10.1093/sleep/33.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychological Science. 2003;14:473–479. doi: 10.1111/1467-9280.02456. [DOI] [PubMed] [Google Scholar]
- Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Medicine Reviews. 2001;5:463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. Journal of Neurochemistry. 2006;99:483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, VanDongen HPA. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neuroscience Letters. 1996;218:153–156. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- Killgore WD. Effects of sleep deprivation on cognition. Progress in Brain Research. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Sleep and wakefulness. Chicago: University of Chicago; 1963. [Google Scholar]
- Klerman EB, Dijk DJ. Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep. 2005;28:1253–1259. doi: 10.1093/sleep/28.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla BP, Auger RR. Jet lag and shift work sleep disorders: How to help reset the internal clock. Cleveland Clinic Journal of Medicine. 2011;78:675–684. doi: 10.3949/ccjm.78a.10083. [DOI] [PubMed] [Google Scholar]
- Korczak AL, Martynhak BJ, Pedrazzoli M, Brito AF, Louzada FM. Influence of chronotype and social zeitgebers on sleep/wake patterns. Brazilian Journal of Medical and Biological Research. 2008;41:914–919. doi: 10.1590/s0100-879x2008005000047. [DOI] [PubMed] [Google Scholar]
- Krueger PM, Friedman EM. Sleep duration in the United States: A cross-sectional population-based study. American Journal of Epidemiology. 2009;169:1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. St Louis: Elsevier Saunders; 2011. [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne MG, Dwyer F, Triggs TJ, Rajaratnam S, Redman JR. The effects of a nap opportunity in quiet and noisy environments on driving performance. Chronobiology International. 2004;21:991–1001. doi: 10.1081/cbi-200035956. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2003;284:280–290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocrine Development. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Annals of the New York Academy of Sciences. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological Bulletin. 2010;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. Journal of Clinical Endocrinology and Metabolism. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Cronin JW, Evans EE, Cade BE, Lee CJ, Landrigan CP, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. The New England Journal of Medicine. 2004;351:1829–1837. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- Lombardi DA, Folkard S, Willetts JL, Smith GS. Daily sleep, weekly working hours, and risk of work-related injury: US National Health Interview Survey (2004–2008) Chronobiology International. 2010;27:1013–1030. doi: 10.3109/07420528.2010.489466. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Stirland JA, Darrow JM, Menaker M, Loudon ASI. Free running circadian rhythms of melatonin, luteinizing hormone, and cortisol in Syrian hamsters bearing the circadian tau mutation. Endocrinology. 1999;140:758–764. doi: 10.1210/endo.140.2.6538. [DOI] [PubMed] [Google Scholar]
- Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33:149–159. doi: 10.1093/sleep/33.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi MM, Boulos Z, Ranney T, Simmons L, Campbell SS. Effects of an afternoon nap on nighttime alertness and performance in long-haul drivers. Accident Analysis and Prevention. 2002;34:825–834. doi: 10.1016/s0001-4575(01)00089-6. [DOI] [PubMed] [Google Scholar]
- McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HPA. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. Journal of Theoretical Biology. 2009;256:227–239. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy J, Strecker R. The cognitive cost of sleep lost. Neurobiology of Learning and Memory. 2011 doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily LR, Liu Y, Croft JB, Perry GS, Strine T. Unhealthy sleep-related behaviors —12 States, 2009. Atlanta, GA: U.S. Department of Health and Human Services Centers for Disease Control and Prevention; 2011. pp. 223–238. [PubMed] [Google Scholar]
- Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC. Catastrophes, sleep, and public-policy—Consensus report. Sleep. 1988;11:100–109. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Gujavarty KS, Browman CP. Maintenance of Wakefulness Test—A polysomnographic technique for evaluating treatment efficacy in patients with excessive somnolence. Electroencephalography and Clinical Neurophysiology. 1982;53:658–661. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollicone DJ, Van Dongen HPA, Rogers NL, Banks S, Dinges DF. Time of day effects on neurobehavioral performance during chronic sleep restriction. Aviation, Space, and Environmental Medicine. 2010;81:735–744. doi: 10.3357/asem.2756.2010. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Morikawa Y, Sakurai M, Nakamura K, Miura K, Ishizaki M, et al. Association between long working hours and sleep problems in white-collar workers. Journal of Sleep Research. 2010;20:110–116. doi: 10.1111/j.1365-2869.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- Nansen F, editor. Farthest north. New York: The Modern Library; 1999. [Google Scholar]
- National Transportation Safety Board. Factors that affect fatigue in heavy truck accidents. Washington, DC: National Transportation Safety Board; 1995. [Google Scholar]
- Ohayon MM, Smolensky MH, Roth T. Consequences of shiftworking on sleep duration, sleepiness, and sleep attacks. Chronobiology International. 2010;27:575–589. doi: 10.3109/07420521003749956. [DOI] [PubMed] [Google Scholar]
- Olofsen E, Dinges DF, Van Dongen HP. Nonlinear mixed-effects modeling: Individualization and prediction. Aviation, Space, and Environmental Medicine. 2004;75:A134–A140. [PubMed] [Google Scholar]
- Otmani S, Pebayle T, Roge J, Muzet A. Effect of driving duration and partial sleep deprivation on subsequent alertness and performance of car drivers. Physiology and Behavior. 2005;84:715–724. doi: 10.1016/j.physbeh.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Philip P, Sagaspe P, Taillard J, Moore N, Guilleminault C, Sanchez-Ortuno M, et al. Fatigue, sleep restriction, and performance in automobile drivers: A controlled study in a natural environment. Sleep. 2003;26:277–280. doi: 10.1093/sleep/26.3.277. [DOI] [PubMed] [Google Scholar]
- Philip P, Sagaspe P, Taillard J, Valtat C, Moore N, Åkerstedt T, et al. Fatigue, sleepiness, and performance in simulated versus real driving conditions. Sleep. 2005;28:1511–1516. doi: 10.1093/sleep/28.12.1511. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian systems: Entrainment. In: Aschoff J, editor. Handbook of behavioral neurobiology. New York: Plenum Press; 1981. pp. 95–124. [Google Scholar]
- Plessow F, Kiesel A, Petzold A, Kirschbaum C. Chronic sleep curtailment impairs the flexible implementation of task goals in new parents. Journal of Sleep Research. 2010;20:279–287. doi: 10.1111/j.1365-2869.2010.00878.x. [DOI] [PubMed] [Google Scholar]
- Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Progress in Brain Research. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Medicine Reviews. 2011;15:123–135. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Punjabi NM, Bandeen-Roche K, Young T. Predictors of objective sleep tendency in the general population. Sleep. 2003;26:678–683. doi: 10.1093/sleep/26.6.678. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Raslear TG, Hursh SR, Van Dongen HP. Predicting cognitive impairment and accident risk. Progress in Brain Research. 2011;190:155–167. doi: 10.1016/B978-0-444-53817-8.00010-4. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A. Current perspectives on the function of sleep. Perspectives in Biology and Medicine. 1998;41:359–390. doi: 10.1353/pbm.1998.0051. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep-deprivation in rats. Science. 1983;221:182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- Roehrs T, Carskadon MA, Dement WC, Roth T. Daytime sleepiness and alertness. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. New York: Saunders Company; 2005. [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Medicine Reviews. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Roepke SE, Duffy JF. Differential impact of chronotype on weekday and weekend sleep timing and duration. Nature and Science of Sleep. 2010;2010:213–220. doi: 10.2147/NSS.S12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rones PL, Iig RE, Gardner JM. Trends in hours of work since the mid-1970s. Monthly Labor Review. 1997;120:3–14. [Google Scholar]
- Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: Realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–321. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Thomas A, Thorne D, Sing H, Redmond D, Rowland L, et al. Oculomotor impairment during chronic partial sleep deprivation. Clinical Neurophysiology. 2003;114:723–736. doi: 10.1016/s1388-2457(03)00008-7. [DOI] [PubMed] [Google Scholar]
- Sadun AA, Schaechter JD, Smith LEH. A retinohypothalamic pathway in man—Light mediation of circadian-rhythms. Brain Research. 1984;302:371–377. doi: 10.1016/0006-8993(84)90252-x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Progress in Neurobiology. 2008;86:264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LD, Hwang WT, Rogers AE, Nysse T, Dean GE, Dinges DF. The relationship between nurse work schedules, sleep duration, and drowsy driving. Sleep. 2007;30:1801–1807. doi: 10.1093/sleep/30.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Boero J, Gottschalk L, Duntley SP, Shaw PJ. Identification of a biomarker for sleep drive in flies and humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19913–19918. doi: 10.1073/pnas.0609463104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan TL, Czeisler CA. Light exposure induces equivalent phase-shifts of the endogenous circadian-rhythms of circulating plasma melatonin and core body-temperature in men. Journal of Clinical Endocrinology and Metabolism. 1991;73:227–235. doi: 10.1210/jcem-73-2-227. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, et al. The visual scoring of sleep in adults. Journal of Clinical Sleep Medicine. 2007;3:121–131. [PubMed] [Google Scholar]
- Stenuit P, Kerkhofs M. Effects of sleep restriction on cognition in women. Biological Psychology. 2008;77:81–88. doi: 10.1016/j.biopsycho.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Stenuit KP, Blatt J, Forrest C, Georges K, Kiley J, Kurrus R, et al. NCSDR/NHTSA Expert Panel on Driver Fatigue and Sleepiness 1997 [Google Scholar]
- Stutts JC, Wilkins JW, Scott Osberg J, Vaughn BV. Driver risk factors for sleep-related crashes. Accident Analysis and Prevention. 2003;35:321–331. doi: 10.1016/s0001-4575(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Swann CE, Yelland GW, Redman JR, Rajaratnam SMW. Chronic partial sleep loss increases the facilitatory role of a masked prime in a word recognition task. Journal of Sleep Research. 2006;15:23–29. doi: 10.1111/j.1365-2869.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. Journal of Sleep Research. 2003;12:275–282. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- Tobler I. Is sleep fundamentally different between mammalian species? Behavioural Brain Research. 1995;69:35–41. doi: 10.1016/0166-4328(95)00025-o. [DOI] [PubMed] [Google Scholar]
- Torsvall L, Åkerstedt T. Sleepiness on the job—Continuously measured EEG changes in train drivers. Electroencephalography and Clinical Neurophysiology. 1987;66:502–511. doi: 10.1016/0013-4694(87)90096-4. [DOI] [PubMed] [Google Scholar]
- National Transportation Safety Board. Prince William Sound near Valdez, Alaska. Washington, DC: National Transportation Safety Board; 1989. Marine Accident Report: Grounding of the U.S. Tankship EXXON VALDEZ on Bligh Reef. [Google Scholar]
- Trew A, Searles B, Smith T, Darling EM. Fatigue and extended work hours among cardiovascular perfusionists: 2010 Survey. Perfusion. 2011;26:361–370. doi: 10.1177/0267659111409278. [DOI] [PubMed] [Google Scholar]
- Vakulin A, Baulk SD, Catcheside PG, Anderson R, van den Heuvel CJ, Banks S, et al. Effects of moderate sleep deprivation and low-dose alcohol on driving simulator performance and perception in young men. Sleep. 2007;30:1327–1333. doi: 10.1093/sleep/30.10.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- Van Dongen HP, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. Journal of Sleep Research. 2003;12:181–187. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clinics in Sports Medicine. 2005;24:237–249. vii–viii. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Van Dongen H, Dinges DF, Olofsen E. Bayesian individualization of biomathematical predictions for cognitive performance impairment from sleep loss. Sleep. 2004;27:144. [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Archer SN, Wuillaume C, Balteau E, Degueldre C, Luxen A, et al. Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. Journal of Neuroscience. 2009;29:7948–7956. doi: 10.1523/JNEUROSCI.0229-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ, et al. PER3 polymorphism predicts sleep structure and waking performance. Current Biology. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- Virtanen M, Ferrie JE, Gimeno D, Vahtera J, Elovainio M, Singh-Manoux A, et al. Long working hours and sleep disturbances: The Whitehall II prospective cohort study. Sleep. 2009;32:737–745. doi: 10.1093/sleep/32.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG. The efferent projections of the suprachiasmatic nucleus. Anatomical insights into the control of circadian rhythms. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic nucleus: The mind’s clock. New York: Oxford University Press; 1991. pp. 77–106. [Google Scholar]
- Wehr TA, Aeschbach D, Duncan WC., Jr Evidence for a biological dawn and dusk in the human circadian timing system. The Journal of Physiology. 2001;535:937–951. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KE, Jablonski MR, Warfield B, Cecil KS, James M, Ayers MA, et al. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. Journal of Applied Physiology. 2011;110:619–626. doi: 10.1152/japplphysiol.01413.2009. [DOI] [PubMed] [Google Scholar]
- Whitney P, Hinson JM. Measurement of cognition in studies of sleep deprivation. Progress in Brain Research. 2010;185:37–48. doi: 10.1016/B978-0-444-53702-7.00003-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson RT. Loss of sleep. Proceedings of the Royal Society of Medicine. 1969;62:903–904. [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wu H, Stone WS, Hsi X, Zhuang J, Huang L, Yin Y, et al. Effects of different sleep restriction protocols on sleep architecture and daytime vigilance in healthy men. Physiological Research. 2010;59:821–829. doi: 10.33549/physiolres.931895. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, et al. Sleep, wake and phase dependent changes in neurobehavioral function under forced desynchrony. Sleep. 2011a;34:931–941. doi: 10.5665/SLEEP.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XA, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, et al. Dynamics of neurobehavioral performance variability under forced desynchrony: Evidence of state instability. Sleep. 2011b;34:57–61. doi: 10.1093/sleep/34.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]