Abstract
Photoimmunology evolved from experiments carried out in the 1970s on the immunology of cancer. In studying the antigenic properties of skin cancers induced in mice by UV radiation, I found that most of these tumors failed to grow when transplanted into normal, syngeneic mice but grew progressively in immunosuppressed mice. Thus, these UV-induced skin cancers were highly antigenic. The critical question was, how can these antigenic skin cancers escape immune rejection in their primary? The answer was that exposing their skin to UV radiation prevented mice from making an immune response against their tumors. The failure to reject these tumors was due to the development of UV tumor-specific regulatory T cells (Tregs) during the course of irradiation. In unraveling the mechanisms of this effect of UV, much has been learned about the immunology of the skin, including the function of Langerhans cells, the migration of immune cells in skin, the role of antigen-presenting cells in directing the immune response, and the role of keratinocytes as producers of immunological mediators. Thus, photoimmunology helped demonstrate that skin is an important immunologic organ and that the immune system can be influenced by the external environment via the skin.
Introduction
The theme of this meeting is how studies of the skin have led to advances in science and medicine. As you will hear on subsequent days, the use of skin models has taught us a great deal about the biology and pathogenesis of cancer. They have taught us about initiation and promotion and precancerous lesions, the role of inflammation in cancer progression, the importance of oncogenes and signaling pathways, and the role of DNA repair in carcinogenesis. My story tonight is no exception. It is the story of how studies of the skin taught us something about the immune system and about the role of the immune system in cancer development. But more than that, it is also a story about how studying the immunology of cancer led to new insights into skin biology. It is the story of the history of photoimmunology.
Photoimmunology is the study of the interactions between photons and the immune system. The photons of most significance for photoimmunology are those in the UV-B region of the solar spectrum. These are the wavelengths that benefit human health by catalyzing the production of vitamin D; they are also the primary wavelengths responsible for sunburn and skin cancer. In addition, it is this region of the spectrum that is most strongly affected by ozone depletion—decreased ozone in the upper atmosphere allows more UV-B radiation to reach the surface of the earth.
The fact that photons in this range have any effects at all on the immune system is both unexpected and remarkable. It is unexpected because UV radiation has little power of penetration through living tissues, and most is absorbed by the outer few millimeters of the skin. It is remarkable because life has evolved in an environment containing UV radiation, and there is no obvious reason why the immune system should be influenced by it. Before our studies on the immunology of skin cancer, no one would have dreamed that shining a light on the skin would have immunological consequences, much less that this would turn into an entire new field of investigation.
How did this happen? A long time ago, I was interested in tumor antigens, and I set out to investigate the antigenic properties of skin cancers induced in mice by UV radiation. The reason I was interested in this tumor system was because there was a suggestion in the literature that UV-induced tumors were more antigenic than other types of skin cancers, which was curious because they developed after a long latent period. The prevailing dogma was that cancers that arose quickly following carcinogen application were more antigenic than those that appeared after a long latent period, because the latter were subject to immune selection. So I set out to take a closer look at the antigens on these tumors.
Results and Discussion
In those days, tumor antigens were detected and defined by transplantation experiments in syngeneic animals. What I found was quite unexpected and initially quite puzzling: the first 10 tumors I transplanted into syngeneic mice failed to grow. I was about to give up when a colleague suggested that I try transplanting the tumors into immunosuppressed mice. That was the key! I then demonstrated formally that the failure of the tumors to grow in the normal mice was indeed due to immunological rejection: The addition of lymphocytes to immunosuppressed mice rendered them resistant to tumor growth, and rejection of tumors by normal mice primed them for a secondary response, which was tumor specific (1). But that raised the interesting question of how the tumors were able to grow in the original host, without succumbing to immunological rejection. The answer to this question was equally unexpected.
The first experiment tested whether the primary host had an age-related reduction in the immune response, which rendered it incapable of rejecting its tumor, or whether the skin in which the tumor arose constituted an immunologically privileged site. Neither of these possibilities was correct. Mice of the same age as the primary hosts were capable of rejecting subcutaneous implants of the tumors, and the primary hosts were unable to reject the tumors regardless of the site of implantation. We then investigated when during tumor induction the mice lost their ability to reject tumors by implanting tumors after various periods of UV irradiation. Surprisingly, long before the appearance of primary tumors, the mice became unable to reject tumors (2). However, the alteration was specific for UV-induced tumors because the mice were able to reject allogeneic and other syngeneic tumors (2,3). The systemic nature of the alteration was demonstrated by injecting tumor cells i.v. and examining the formation of lung metastases. Few or no metastases developed in normal mice, whereas many were present in UV-irradiated animals (4).
The next question we addressed was the nature of the systemic alteration. Was the UV-irradiated host lacking something required for tumor rejection, or did it contain something that inhibited tumor rejection? Our first approach was to perform a technique called parabiosis, in which the skin of two mice is sutured together. Such mice establish a common circulation within about a week. After parabiosing normal and UV-irradiated mice, as well as control groups in which normal mice were paired with other normal mice or immunosuppressed mice, and UV-irradiated mice were paired with each other, we challenged each member of the pair with tumor implants to ask who influenced whom? Most of the UV-irradiated pairs failed to reject their implants; most of the normal pairs rejected their tumor implants, as did the pairs of immunosuppressed and normal mice; whereas the majority of the normal mice parabiosed to UV-irradiated mice failed to reject the tumor challenge (Table 1). This indicated that UV irradiation induced a circulating factor that suppressed tumor rejection. Transfer of lymphoid cells or plasma components demonstrated that lymphoid cells from UV-irradiated mice had the ability to suppress the rejection of transplanted tumors by normal animals (5). The suppressive lymphoid cells were later determined to be T lymphocytes, or Tregs (6).
Table 1.
PARABIOSIS EXPERIMENT
| Recipientsa | Tumor Incidenceb | % |
|---|---|---|
| Normal: Normal | 1/16 : 1/16 | 6.3 |
| UV:UV | 9/20 : 18/20 | 93 |
| UV: Normal | 28/38 : 30/38 | 76 |
| Normal: ATXc | 1/14 : 0/14 | 3.6 |
Pairs of mice were parabiosed for 10 days and then each member of the pair was challenged with a fragment of a UV-induced tumor subcutaneously.
Number of implants that grew in each member of the pair/number of mice challenged.
ATX = mice immunosuppressed by adult thymectomy and sublethal X irradiation.
Further studies demonstrated that these cells were important in tumor induction in the primary host, as well. For example, mice were given a lethal dose of X-irradiation and reconstituted with lymphoid cells from normal or UV-irradiated donors and then grafted with UV-irradiated, precancerous skin. Mice whose lymphoid cells were derived from UV-irradiated donors developed many more tumors than those given normal cells. Also, transferring T lymphocytes from UV-irradiated mice to normal mice 3 times at the beginning of a course of chronic UV irradiation markedly accelerated tumor development compared to mice injected with normal T lymphocytes. In fact the latent period for tumor formation was reduced by half (7). These experiments showed that Tregs could influence the induction of primary skin cancers.
Overall, these studies indicated that UV irradiation had not only carcinogenic effects on the skin, but also caused antigenic changes, manifesting as strong tumor antigens, and had immunologic effects, as well. From this model of skin carcinogenesis, we learned several things about tumor immunology. First, we learned that transplanting tumors into normal recipients is not necessarily the same as transplanting them into the primary host, or even a carcinogen-treated animal. It is rather disheartening to note that after 40 years, investigators are still trying to develop cancer vaccines by immunizing normal recipients against tumor challenge, without regard for the fact that their approach may not succeed when applied to animals (or humans) in which cancer has developed. Second, these studies demonstrate clearly, that under the right circumstances, the immune system can be remarkably effective in destroying cancer cells as they arise, at least in the skin. Finally, they highlight the importance of Tregs in thwarting the immune response against cancer, a fact that is currently being taken into consideration in some approaches to immunotherapy in humans.
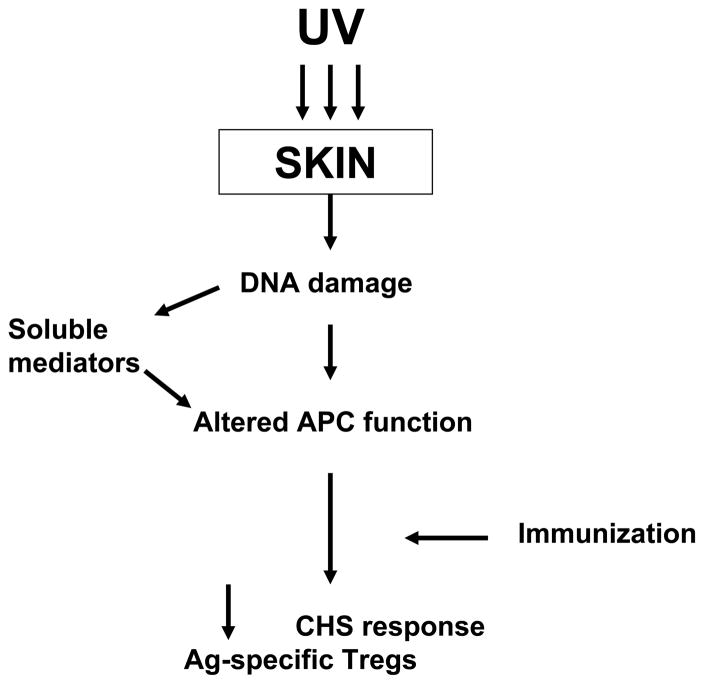
The next question addressed by us and by many others was, how does UV alter the immune response? A major clue came from characterizing the immunological profile of UV-irradiated mice (8). Both contact and delayed hypersensitivity (CHS, DHS) responses were found to be impaired in UV-irradiated mice, although at much lower doses than those required to induce the Tregs that suppressed tumor rejection. Furthermore, sensitization of UV-irradiated mice induced Tregs specific for the antigen used for sensitization. Thus, exposure to UV radiation modifies the immune response, such that introduction of certain antigens during a limited window of time after exposure results in activation of the suppressor pathway of the immune response, instead of the effector pathway. This led to various studies on the ability of UV to modify the course of infectious diseases in animal models and also to studies of the immunosuppressive effects of UV radiation in humans (9). Regarding the mechanism, many investigators showed that UV-irradiation of keratinocytes in culture released soluble mediators that mimicked effects of UVR. Among the many mediators implicated are interleukin-10, tumor necrosis factor, prostaglandin E2, platelet activating factor, and urocanic acid, just to name a few (10). Other studies showed that splenic antigen-presenting cells from UV-irradiated mice were deficient in their ability to induce an immune response, and instead induced Tregs (11). In terms of the initiating event, both DNA damage (12) and isomerization of urocanic acid (13) by UV radiation have been implicated. In the local suppression model in which a contact sensitizer is applied at the site of UV irradiation, the cellular target seems to be antigen-presenting cells in the skin. Alteration or redistribution of these cells, perhaps in conjunction with keratinocyte-derived soluble mediators, triggers the induction of Tregs, rather than effector cells (Figure 1). In the systemic suppression model in which a contact sensitizer or DHS-inducing antigen is applied to a distant, unirradiated site, a higher dose of UV radiation is required to cause immune suppression. Skin-derived soluble mediators are thought to alter antigen-presenting cells at distant sites, leading to induction of Tregs (Figure 2). The nature and mechanism of the alteration in antigen presentation leading to induction of Tregs is unknown, as are many other details in the pathways leading to immune suppression.
Figure 1.
Scheme for how UV irradiation causes systemic suppression of CHS and DHS response to antigens applied at distant, non-irradiated sites.
Figure 2.
Scheme for how UV irradiation suppresses CHS responses to antigen applied to the site of UV irradiation.
An intriguing question remaining to be answered is why UV irradiation leads to immune suppression. Clearly, life has evolved in an environment containing UV radiation, so it is likely that UV-induced immune suppression represents an unwanted consequence of a beneficial effect of UV exposure. One hypothesis is that since UV induces antigenic changes in the skin, UV-induced immune suppression evolved as a protective mechanism that prevents rejection of sun-damaged skin. Alternatively, the outpouring of cytokines that occurs following exposure to UV may function to repair UV-induced tissue damage, but coincidentally, some of these factors also cause the unwanted side effect of immune suppression. Recently, it has been hypothesized that this phenomenon serves as a means of suppressing UV-induced inflammatory reactions in the skin, which could be highly detrimental (14).
Regardless of the reasons for the existence of this phenomenon, we have learned much about the skin from these studies. First, the skin is full of immune cells that serve to direct the immune response down the suppressive or the effector pathway, depending on the circumstances. Also, there is still much to be learned about how this occurs. Second, keratinocytes are full of immunologically active mediators whose release can be triggered by DNA damage, and perhaps a variety of other stimuli as well. Finally, these studies have led to the realization that even something as superficial as shining a light on the skin can have serious immunologic consequences. Furthermore, they suggest that exposing the skin to a variety of substances—environmental chemicals, skin care products, etc.— may profoundly alter the immune response because of the intimate relationship between skin and the immune system.
Abbreviations
- ATX
adult thymectomy and sublethal X irradiation
- CHS
contact hypersensitivity
- DHS
delayed type hypersensitivity
References
- 1.Kripke M. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–36. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 2.Kripke M, Fisher M. Immunologic parameters of ultraviolet carcinogenesis. J Natl Cancer Inst. 1976;57:211–15. doi: 10.1093/jnci/57.1.211. [DOI] [PubMed] [Google Scholar]
- 3.Kripke M, Thorn R, Lill P, et al. Further characterization of immunologic unresponsiveness induced in mice by UV radiation: Growth and induction of non-UV-induced tumors in UV-irradiated mice. Transplantation. 1979;28:212–217. doi: 10.1097/00007890-197909000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Kripke M, Fidler I. Enhanced experimental metastasis of ultraviolet light-induced fibrosarcomas in ultraviolet light-irradiated syngeneic mice. Cancer Res. 1980;40:625–629. [PubMed] [Google Scholar]
- 5.Fisher M, Kripke M. Systemic alteration induced in mice by ltraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA. 1977;74:14823–1487. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher M, Kripke M. Further studies on the tumor-specific suppressor cells induced by ultraviolet radiation. J Immunol. 1978;121:1139–1144. [PubMed] [Google Scholar]
- 7.Fisher M, Kripke M. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 8.Kripke M. Immunological unresponsiveness induced by ultraviolet radiation. Imm Rev. 1984;80:87–102. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 9.Kripke M. Ultraviolet radiation and immunology: Something new under the sun. Presidential Address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- 10.Norval M, McLoone P, Lesiak A, et al. The effect of chronic ultraviolet radiation on the human immune system (Review) Photochem Photobiol. 2008;84:19–28. doi: 10.1111/j.1751-1097.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 11.Greene M, Sy M, Kripke M, et al. Impairment of antigen-presenting cell function by ultraviolet radiation. Proc Natl Acad Sci USA. 1979;76:6591–6595. doi: 10.1073/pnas.76.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kripke M, Cox P, Alas L, et al. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFabo E, Noonan F. Mechanism of immune suppression by ultraviolet radiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;158:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz T. The dark and the sunny sides of UVR-induced immunosuppression: Photoimmunology revisited. J Invest Dermatol. 2010;130:49–54. doi: 10.1038/jid.2009.217. [DOI] [PubMed] [Google Scholar]