Abstract
Lung failure is the most common organ failure seen in the intensive care unit. The pathogenesis of acute respiratory failure (ARF) can be classified as (1) neuromuscular in origin, (2) secondary to acute and chronic obstructive airway diseases, (3) alveolar processes such as cardiogenic and noncardiogenic pulmonary edema and pneumonia, and (4) vascular diseases such as acute or chronic pulmonary embolism. This article reviews the more common causes of ARF from each group, including the pathological mechanisms and the principles of critical care management, focusing on the supportive, specific, and adjunctive therapies for each condition.
Introduction
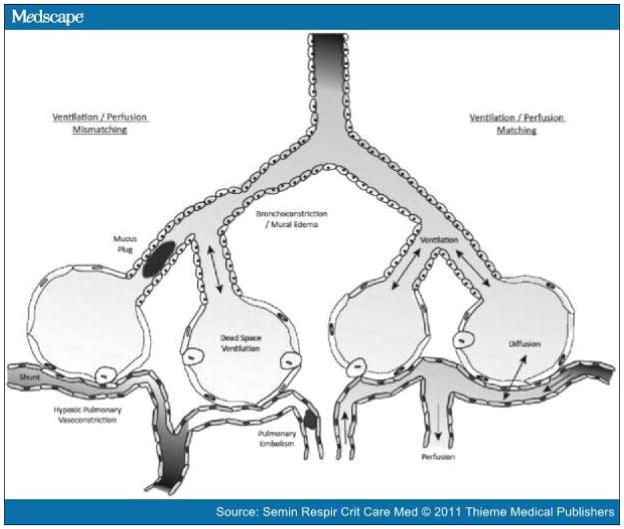
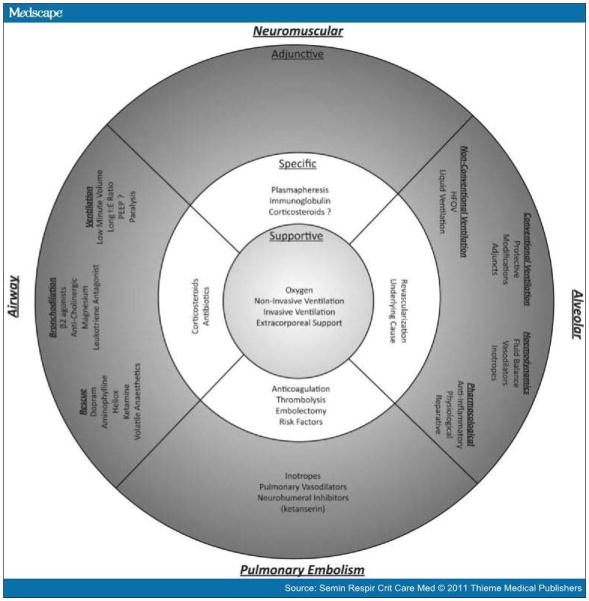
Lung failure is the most common organ failure in the intensive care unit (ICU).[1] Approximately 56% of ICU patients suffer from acute respiratory failure (ARF), with one third of those subsequently dying.[2] The pathogenesis of ARF can be classified as (1) primarily neuromuscular in origin, (2) secondary to acute and chronic obstructive airway diseases, (3) alveolar processes such as cardiogenic and noncardiogenic pulmonary edema and pneumonia, and (4) vascular diseases such as acute or chronic pulmonary embolism. All mechanisms of ARF ultimately impair the ability of the alveolus to match ventilation with perfusion and impair gas exchange, either oxygen uptake, carbon dioxide excretion, or both (Fig. 1). Regardless of the cause of ARF, the physiological responses are often similar. Compensatory mechanisms, such as tachypnea and the use of accessory muscles of respiration, may initially preserve adequate gas exchange. Eventually, the work of breathing becomes excessive, exhaustion develops, and life-threatening respiratory decompensation follows with the development of hypoxia and hypercarbia. This article reviews the management of the more common causes of ARF, considering the pathological mechanisms by which respiratory failure develops. The article also focuses on the appropriate treatments for the different causes of ARF (Fig. 2).
Figure 1.
The four primary alveolar processes: ventilation, diffusion, perfusion, and their regulatory mechanism hypoxic pulmonary vasoconstriction. All forms of lung failure result from a defect in one or more of these processes which culminates in V/Q mismatch, or its extreme variants shunt and dead space ventilation.
Figure 2.
Comparison of therapies for different modes of lung failure. See text for details.
Neuromuscular Causes of Acute Respiratory Failure
Guillain-Barré syndrome (GBS) describes a group of acute inflammatory polyneuropathies resulting from demyelination or axonal degeneration. The syndrome is probably autoimmune in origin, often occurring after a recent infection, and has a typical pathological finding of mononuclear cell infiltration of the peripheral nervous system.[4] The worldwide incidence is between 0.6 and 4 cases per 100,000 per year, rising with age, and there is a slight male preponderance.[4] GBS classically manifests as rapidly ascending symmetrical flaccid paralysis, either with or without sensory changes, and autonomic hyperreflexia.[5] It is most severe between 2 and 4 weeks after onset.[6] The diagnosis is dependent on typical clinical features, examination of cerebrospinal fluid, and neurophysiological studies.
Myasthenia gravis (MG) is an autoimmune disorder of the neuromuscular junction, with autoantibodies directed against the postsynaptic nicotinic acetylcholine receptor, or other junctional proteins, resulting in failure of motor end plate transmission.[7] Characteristic findings are weakness, worsening with exercise, and recovery after a period of rest. MG is uncommon, with a conservative worldwide incidence of 30 per million per year.[8] The American prevalence is 200 per million, giving an estimated 60,000 cases.[9] It is mainly a disease of young women and old men.[10] Diagnosis is based on clinical features and pharmacological, serological, and neurophysiological tests.[11] Myasthenic crisis is defined by the presence of respiratory failure requiring mechanical ventilation (MV).[12] Approximately 20% suffer a crisis, usually in the first 2 years after diagnosis.[12] Several risk factors have been associated with the development of a crisis, including infection, medication change, surgery, or trauma.[7] ARF may result from either a myasthenic crisis, due to therapeutic inadequacy, or from a cholinergic crisis, due to medication excess, with both crises being clinically indistinguishable.[7]
There are several mechanisms by which respiratory failure may develop in both GBS and MG.[13, 14] Bulbar dysfunction, recognizable by dysarthria, dysphonia, and dysphagia, leads to lingual, pharyngeal, and laryngeal muscle weakness. As a consequence, both upper airway patency and protective reflexes diminish, resulting in increasing airflow resistance and the vulnerability to aspiration.[15] The development of pneumonia may further compromise respiratory function. Inspiratory muscle weakness inhibits full lung expansion, predisposing to atelectasis, shunt, and hypoxia with compensatory rapid shallow breathing increasing the percentage of dead-space ventilation. Expiratory muscle weakness further limits coughing and secretion clearance, again inclining to lobar collapse, mechanical inefficiency, and ventilation/perfusion (V/Q) mismatch.
Subjective predictors of the need for MV include inadequate cough plus clinical signs of respiratory distress. Objective features include an arterial blood gas analysis and the memorable 20/30/40 rule, with MV likely to be required with a vital capacity <20 mL/kg, a maximum inspiratory pressure <30 cm H2O, and a maximal expiratory pressure <40 cm H2O, [13] although vital capacity measurements may be unreliable.[16] Predictive rules have also been developed.[17] The decision to implement MV is based on the rate of deterioration and ability to protect the airway. The requirement for MV in GBS varies between 25 and 50% of patients and lasts a median duration of 18 to 29 days.[14] All patients with myasthenic crisis are, by definition, in respiratory failure.[12]
MV may be either invasive or noninvasive (NIV). Few studies have addressed the use of NIV in acute neuromuscular respiratory failure. However, for NIV to be considered, the standard criteria for its implementation must be observed, including adequate level of consciousness and circulatory stability.[18] Bilevel positive airway pressure (BiPAP) has been used to avoid tracheal intubation in both GBS and MG, including four patients with bulbar dysfunction.[19–21] The presence of hypercapnea at baseline was predictive of NIV failure in MG. Failure of BiPAP in GBS has also been reported.[22]
No prospective evidence exists to guide tracheal intubation, but induction with the minimal amount of hemodynamic disturbance is required. The autonomic instability of GBS makes extremes of heart rate and blood pressure possible, rendering tracheal intubation hazardous.[13] The choice of intubation technique is dependent on the expertise of the clinician, with alternatives being fiberoptic intubation or formal rapid sequence intubation. Topical anesthesia may allow a further dose reduction in anesthetic agent. Etomidate can suppress adrenal function and has been associated with an increased mortality in sepsis in one report.[23, 24] It should be used with caution in the critically ill.[25] Although ketamine stimulates the circulatory system and is useful in hemodynamically unstable patients, it may not be a good choice in GBS.[26, 27] Suxamethonium has differing effects between the two conditions. Its use may result in hyperkalemia in GBS, whereas due to reduced acetylcholine receptor activity it may prove ineffective in MG.[28, 29] A larger dose may result in a phase II block, especially if prior plasmapheresis has induced a plasma esterase deficiency.[30] However, patients with both conditions may not require neuromuscular blockade due to their existing weakness. If paralysis is deemed necessary, a much reduced dose of a shorter-acting, nondepolarizing neuromuscular blocking agent is preferable.
Once tracheal intubation has been achieved the respiratory targets should be similar for most mechanically ventilated patients aiming for normal gas exchange. The use of sighs has been suggested, but the practice lacks prospective evidence.[31] Due to the potential requirement for tracheostomy, prediction systems have been developed to determine the likely duration of MV.[32, 33] Complications during prolonged MV are common, especially ventilator-associated pneumonia and bacteremia, but may be reduced by early tracheostomy.[34] Little evidence exists to guide weaning and extubation in these conditions. Reintubation is common, and preextubation blood gas values and pulmonary function tests do not correlate with extubation failure.[35]
Both plasmapheresis and intravenous immunoglobulin are established therapies for MG and GBS and are equally efficacious (Fig. 2).[36–39] Plasmapheresis has disadvantages, including its invasiveness, requirement for specialist personnel and equipment, and increased complication rate. Corticosteroids have no role in the acute management of GBS but may have a role in MG.[40, 41] Acetylcholinesterase inhibitors are not recommended during a myasthenic crisis due to the potential for both adverse effects, including bronchoconstriction, bradycardia, and asystole, and inaccurate diagnostic test results.[7, 42]
Although the outcome in GBS is subtype dependent, between 4 and 15% die, a further 7 to 20% remain disabled after a year, and many survivors are limited by fatigue and weakness.[4] The relapse rate is 3 to 5%.[43] For MG, the median duration of MV may be decreasing, from 13 days in the 1980s to 6 days in the late 1990s.[44] Between 27 and 44% fail extubation, with atelectasis the strongest predictor of this.[35, 45] A third of survivors will relapse.[46] Death rates from MG are lower than those for GBS at under 5%.[10]
Acute and Chronic Airway Obstruction Causes of Acute Respiratory Failure
Asthma and chronic obstructive pulmonary disease (COPD) share several pathological and therapeutic traits. They are, however, clearly distinguished by the chronic nature, poor reversibility, and associated comorbidities that characterize COPD.
Asthma is caused by reversible bronchial narrowing characterized by airway inflammation, smooth muscle contraction, and mucous plugging (Fig. 1).[47] Central airway narrowing, due to bronchoconstriction, may produce the spirometric features of severe asthma, whereas peripheral airway obstruction impedes local alveolar ventilation, with V/Q ratios falling to less than 0.1.[48] Dynamic hyperinflation causes both respiratory impairment (by placing the respiratory muscles at a mechanical disadvantage) and hypotension, via elevated intrathoracic pressure impeding venous return.[47, 49] Two fatal phenotypes have been described, one of slower onset with eosinophilic airway infiltration and luminal debris; the other, much less common, is characterized by rapid onset, neutrophilic bronchial infiltration without luminal debris.[50]
Asthma exerts an enormous health care burden. In the United States over 23 million suffer from asthma, with half having at least one attack per year, resulting in 3365 deaths in 2006.[51] Almost a quarter of asthmatic patients presenting to the emergency department (ED) have life-threatening asthma.[52] Ten percent of those admitted to hospital require ICU admission, and 2% receive invasive MV.[53] Three severe forms of asthma are currently described: acute severe, life threatening, and near fatal, each marking a progression from a state of physiological compensation to multisystem decompensation to emergent respiratory collapse ().[54]
Patients with life-threatening or near-fatal asthma should be managed in a critical care environment. The mainstay of asthma treatment is the use of pharmacological therapies aimed at bronchodilation and reducing bronchial inflammation (Fig. 2). Controlled humidified oxygen is provided, aiming for an oxygen saturation of 94 to 98%.[55] β2 agonists are the mainstay of therapy, with salbutamol and terbutaline being of equivalent efficacy.[54] Oxygen-driven nebulization is the route of administration of choice and may be repeat, back to back, or continuous.[54] Intravenous β2 therapy is not recommended due to a lack of superiority over inhaled administration plus increased complications and is reserved for refractory cases or where nebulized therapy is not possible.[56] To date, nebulized adrenaline has not been shown to offer any advantage over salbutamol.[57] The anticholinergic ipratropium bromide significantly improves bronchodilation and outcomes when given in conjunction with β2 agonists.[58] Corticosteroids reduce mortality, and given their delayed onset of action should be administered as soon as possible.[59] In severe cases, parenteral therapy may be easier to administer than inhaled therapy, with a dose of hydrocortisone 100 mg every 6 hours as efficacious as higher doses. Magnesium sulfate has an array of mechanisms through which it augments bronchodilation and reduces inflammation and is used if the initial therapeutic response is poor or features of life-threatening asthma are present.[55, 60] Aminophylline is not recommended because it is unlikely to increase bronchodilation over the aforementioned therapies and may induce unwanted side effects, including arrhythmias and vomiting.[61] Leukotriene receptor antagonists may improve outcomes in acute asthma, but further studies are warranted before widespread use.[62, 63] Oxygen-helium mixtures, in the form of heliox, have a density approximately one third that of air and may reduce the elevated work of breathing due to increased airway resistance.[64] At present, the evidence does not support heliox use in nonintubated patients, and little data exists for patients receiving MV.[65, 66]
NIV has been successfully used to avoid intubation, but further studies are required before it can be recommended.[67, 68] If invasive ventilation is required, the best pharmacological choice for tracheal intubation remains uncertain. Both ketamine and propofol possess bronchodilatory properties, whereas high-dose rocuronium and suxamethonium are rapid-onset neuromuscular blockers of similar efficacy.[69, 70] Suxamethonium suffers from many side effects and, with the advent of the reversal agent sugammadex, has arguably been superseded by rocuronium.[71] Atracurium is potentially associated with histamine release and is not recommended.[69, 72] Invasive MV can be complicated by dynamic hyperinflation and intrinsic positive end-expiratory pressure (PEEP).[69] Use of low tidal volume (Vt), slow respiratory rate, and increased expiratory period, perhaps in conjunction with an increased inspiratory flow rate, should assist expiration.[73] The use of extrinsic PEEP has been suggested for spontaneous breathing modes to reduce the work to trigger the ventilator, but it is not normally recommended.[73] The mechanically ventilated asthmatic patient is at risk of several complications, including myopathy, critical illness polyneuropathy, further bronchospasm and barotrauma, and hypotension.[69] Ongoing paralysis may be required to control airway pressures, especially if there are difficulties with bronchospasm, cough, and patient-ventilator dyssynchrony.[69] For those patients who fail conventional MV, additional treatment options are available. Refractory cases may be treated with either the intravenous anesthetic agent ketamine or a volatile anesthetic agent such as isoflurane, although special adaptions are required for the ICU ventilator, and mucous plugging may limit agent delivery.[74–77] The use of extracorporeal oxygenation (ECMO) has been successfully described for those failing conventional MV.[78] Approximately 10% of survivors die within 1 year of a period of MV.[79]
COPD is a syndrome of progressive airflow limitation caused by chronic inflammation and destruction of the lung. Although the specific mechanisms differ, COPD has similarities to asthma, including airway inflammation, edema, and luminal secretions occurring in response to long-term noxious stimuli such as cigarette smoke, air pollution, and occupational noxious particles.[80–82] Unlike asthma, parenchymal destruction also occurs.[83] Pulmonary pathology includes dynamic hyperinflation, airflow obstruction, V/Q mismatch, and respiratory muscle fatigue.[84] Approximately 10% of the world’s population are estimated to have COPD, with 14.8 million Americans diagnosed with this condition by 2009, and a further 12 million estimated to be undiagnosed.[51, 85] It is estimated that patients with COPD require 670,000 hospital admissions, form 2% of ICU admissions, and suffer 126,000 deaths annually in the United States.[51, 86, 87] Several factors have been implicated in the pathogenesis of acute exacerbations, including respiratory infection, air pollution, pulmonary emboli, and cardiac dysfunction, although in 30% no inciting event is identified.[88] Because the gross pathology of COPD overlaps with asthma, it follows that several of the same therapies are used.
Oxygen therapy is necessary to avoid the life-threatening effects of hypoxia and takes precedence over fears of hypercarbia.[89] However, uncontrolled oxygen administration may increase hypercarbia via increased hypoxic pulmonary vasoconstriction-induced dead space, the Haldane effect, and rarely the overly emphasized mechanism of diminished respiratory drive.[90] Therefore, a modest fraction of inspiratory O2 (FiO2) level to achieve safe oxygenation is suggested. Both salbutamol and ipratropium improve pulmonary mechanics, but whether they have an additive effect when administered concurrently is uncertain.[91–93] Corticosteroids also improve pulmonary function and hasten the resolution of exacerbations, with parenteral and oral administration proven to be equivalent.[94] Whether all exacerbations, or just those associated with purulent sputum, should be treated with antibiotics is uncertain. In a single study of patients requiring MV during an exacerbation, a course of antibiotics significantly reduced mortality, the duration of ventilation, and length of hospitalization.[95] If empirical antibiotics are utilized, they should treat the likely causative pathogens such as Moraxella catarrhalis, Haemophilus influenzae, and Streptococcus pneumoniae.[88] Current recommendations are that initial therapy should be with an aminopenicillin, a macrolide, or a tetracycline, depending on local microbiological flora.[96] Theophyllines are not recommended due to a consistent association with adverse effects and an inconsistent therapeutic effect.[97] Doxapram is effective as a respiratory stimulant but has been superseded by NIV and is only recommended if NIV is either unavailable or inappropriate.[96]
NIV should be implemented once clinical criteria have been met, although exclusion criteria, such as moderate acidemia, are important.[18] Numerous studies have proven the value of NIV in hypercapnic respiratory failure and demonstrated its superiority over invasive MV in terms of mortality, morbidity, and resource utilization.[98] NIV failure, however, occurs in 30% of patients and signifies a poor prognosis.[99] Early extubation to NIV has become a popular method of avoiding prolonged invasive ventilation and the need for tracheostomy.[100] Ventilatory practice is similar to that for asthma; however, the arterial target blood gas values should ideally be similar to the patient’s usual values when stable, striving for a pH near the normal range and not trying to normalize the PaCO2. Outcomes for COPD patients requiring MV are at least as good as for patients ventilated for other forms of ARF.[101] Indicators of worse outcome include increasing severity of the exacerbation, multiorgan dysfunction, and a poor premorbid state. Curiously, more patients with COPD die from cardiovascular disease, especially ischemic heart disease, than pulmonary disease.[102] This brings into focus the ongoing controversy over the potential increased mortality with β2 agonist therapy in acute exacerbations of COPD, and with long-acting β2 agonists in asthma. One study has reported that β antagonism was associated with improved outcomes in acute respiratory failure, possibly due to the cardioprotective effects of β2 blockers.[105]
Alveolar Causes of Acute Respiratory Failure
Acute lung injury (ALI) is a syndrome of increased permeability pulmonary edema characterized histologically by diffuse alveolar damage and clinically by severe hypoxemia refractory to oxygen therapy, decreased pulmonary compliance, and bilateral alveolar infiltrates on the chest radiograph.[106, 107] It is common, with an incidence of 86 per 100,000 person-years, and equates to over 190,000 cases and 74,500 fatalities annually in the United States.[108] Although mortality has declined, recent studies still report an approximate 25% death rate.[109–111] Several conditions, both pulmonary and extrapulmonary, are associated with the development of ALI, with sepsis and liver failure-related ALI having particularly poor outcomes.[112, 113] Genetic predispositions for the development of ALI have been proposed, but no firm associations have yet been established.[114]
ALI has been described as passing through three pathological phases.[115] An initial exudative phase begins with neutrophil-mediated damage to the alveolus, causing epithelial and endothelial damage, leading to airspace flooding with protein-rich edema fluid and immune cells. A proliferative phase begins 3 to 5 days later, enabling epithelial regeneration via type II pneumocyte propagation and differentiation into type I pneumocytes. A third fibrotic phase may develop and is marked by transformation to a fibrosing alveolitis.
The diagnosis of ALI remains limited to the fulfillment of the American-European Consensus Conference Criteria but is not without uncertainties ().[116, 117] The definition does not take into account the ventilatory settings, with alterations in PEEP potentially changing the PaO2:FiO2 ratio.[118] Also, interpretation of the chest radiograph can be challenging, with mild infiltrates often being a source of confusion.[119] An elevated pulmonary artery occlusion pressure does not exclude a diagnosis of ALI because hydrostatic and increased permeability pulmonary edema may coexist, a fact compounded by a limited understanding of the pulmonary artery catheter.[120–122] Interestingly, the histological appearance of the lung at autopsy frequently does not show the diffuse alveolar damage that is pathognomonic of ALI.[123] Despite initial promising investigations, attempts to identify a biomarker for ALI have been not been successful thus far.[124]
Supportive therapy is indicated when endogenous respiratory function is, or is predicted to become, insufficient to maintain life. Once the pathological effects of reduced compliance, V/Q mismatch and hypoxia, and an increase in pulmonary dead space combine to increase the work of breathing above an achievable level, exogenous ventilation is required to support gas exchange (Fig. 1).[125] NIV has been investigated in ALI to a limited degree, with the evidence presently showing a high failure rate of 50%, particularly in the sickest patients.[126]
No one mode of invasive MV has been shown to be superior to another for respiratory support in ALI, although assist control makes it very straightforward to control the tidal volume.[127] The older modes, such as synchronized intermittent mandatory ventilation, are limited by a single control variable, either pressure or flow (or its surrogate volume).[128] These modes either guarantee minute volume, as in volume control, at the potential expense of airway pressure, or airway pressure, as in pressure control ventilation, at the potential expense of minute volume. Newer dual-control modes seek to combine the superiority of the descending flow waveform used in pressure control, with the guaranteed Vt of volume control.[129] This can occur in either a breath-to-breath fashion, such as in pressure-regulated volume control, or within a breath, such as volume-assured pressure control.[130, 131] Further novel methods of conventional ventilation have been developed and include airway pressure release ventilation, neurally adjusted ventilatory assist, and proportional-assist ventilation.[132–134] Future knowledge-based systems and other artificial intelligence designs may further improve our ventilatory ability.[128]
Although MV provides lifesaving respiratory support, if incorrectly employed, it also has the potential to cause further lung injury. Excessive airway pressure (barotrauma), excessive volume (volutrauma), and repetitive alveolar opening and closing (atelectrauma) can damage the lung both directly and indirectly via the generation of systemic proinflammatory cytokines (biotrauma).[135–138] Biotrauma can progress to multiorgan failure.[139] Ventilator-associated diaphragmatic dysfunction and oxygen toxicity complete the spectrum of ventilator-associated lung injury.[140–142] Using computed tomography (CT), the inhomogeneity of pulmonary consolidation in ALI has been demonstrated, suggesting Vt is delivered to a much smaller unit of lung than anticipated, a concept termed baby lung.[143]
The landmark National Heart, Lung, and Blood Institute ARDS Network ARMA study, published in 2000, reported a 9% absolute risk reduction in mortality in ARDS using reductions in Vt from a traditional 12 mL/kg to 6 mL/kg predicted body weight, and plateau airway pressures from 50 cm H2O to 30 cm H2O. [144] Analysis of several studies using similar lung protective ventilatory strategies reveals no safe upper airway pressure limit.[145] The ‘open-lung approach’ uses PEEP to minimize atelectrauma.[146] At present there is no clear advantage with the use of either a standard higher or lower PEEP, although there is the suggestion that higher PEEP may be beneficial in the most severely ill.[147] Despite retrospective studies showing an association between larger Vt ventilation and the development of ALI in those initially without ALI, there remained widespread resistance to the implementation of protective ventilation for all patients.[148] A very recent prospective, randomized, controlled trial has confirmed a fivefold increase in the development of ALI in those ventilated with Vt 10 mL/kg compared with 6 mL/kg.[149] In addition to using low Vt and low airway pressures, ventilated patients should also be positioned in a semirecumbent position to minimize the risk of ventilator-associated pneumonia.[150] The duration of MV is significantly reduced with both interrupted sedation and daily spontaneous breathing trials.[151, 152] For sedation, propofol may be superior to midazolam in facilitating earlier extubation.[153] While most patients spend two thirds of their ventilated period weaning, it remains difficult to predict successful extubation, with predictors such as the rapid shallow breathing index being imperfect.[101, 154, 155]
Modifications to MV include the use of permissive hypercapnea, which can be a central element of protective ventilation, but it is limited by its lack of applicability in conditions requiring a normal PaCO2, such as brain injuries. [156] Inverse-ratio ventilation allows a longer inspiratory time and may alleviate high peak airway pressures but requires deep sedation and paralysis and should not be routinely used.[157]
Several adjunctive therapies have been used in ALI (Fig. 2). Neuromuscular blockade can reduce airway pressure by eliminating patient effort but can be associated with myopathy. One recent clinical trial (the ACURASYS study) reported improved clinical outcomes with the use of early paralysis, without any increase in weakness.[109] The investigators used muscle paralysis early in the course of severe ALI (PaO2:FiO2 <150). The beneficial effect may have been explained by more effective implementation of a lung protective ventilation strategy. Prone positioning works through different mechanisms to improve V/Q matching.[158] Like many therapies, such as recruitment maneuvers, it may increase oxygenation but has no clear overall impact on mortality.[159, 160] It may, however, be beneficial in the more severely hypoxic patients.[161] By insufflating the distal trachea with either an intermittent or a continuous flow of oxygen, dead-space gas containing carbon dioxide (CO2) may be eliminated, allowing a reduction in ventilatory requirements. [162] Rotational bed therapy is occasionally used to increase functional residual capacity and improve secretion clearance. Although it may reduce the incidence of ventilator-associated pneumonia, it has no effect on duration of ventilation or mortality.[163]
Modifying fluid balance has been moderately successful in reducing the duration of MV.[164] Diuresis to reduce both central venous and pulmonary capillary wedge pressures may also be helpful.[164, 165] Consistent with this finding, positive fluid balance is associated with increased mortality.[166]
Several pharmacological agents have been tested for the treatment of ALI. However, none has been shown to reduce mortality in a convincing phase 3 clinical trial, except for the use of neuromuscular blockade as already discussed. Pulmonary vasodilation has been tried with inhaled nitric oxide and intravenous prostacyclin, and pulmonary vasoconstriction attempted with almitrine.[167–170] Activated protein C has been tested without evidence of benefit, although the results of the current trial of activated protein C for very severe sepsis (PROWESS SHOCK) will include some patients with ALI.[171] Antiinflammatory effects have been tested with glucocorticoids, ketoconazole, pentoxyfylline, lisofylline, and fish oils;[172–176] miscellaneous agents include surfactant and β2 agonists.[177, 178] All these trials have been negative, with the possible exception of corticosteroids, although their role remains uncertain at best.[179, 180] A newer therapeutic target is the regeneration of the injured alveolus, via either mesenchymal stem cells or growth factors, such as keratinocyte growth factor.[181, 182]
Nonconventional modes of ventilation have also been tried. High-frequency oscillatory ventilation (HFOV) utilizes a column of gas oscillated at high frequencies (180 to 1800/min) to achieve a narrower spectrum of airway pressures and avoid injuries associated with the extremes of airway pressure.[183] Alveolar ventilation is dependent on various novel mechanisms, including cardiogenic mixing, Taylor dispersion, asymmetric velocity profiling, and molecular diffusion.[184] Initial results are encouraging, and further large studies are ongoing.[185] Liquid ventilation utilizes perflurocarbons, which are highly soluble for oxygen and CO2. By instilling such a carrier liquid into the lungs, air-liquid interfaces, which cause much of the damage of conventional MV, may theoretically be avoided.[186] However, studies of liquid ventilation have been disappointing to date.[187]
The recent H1N1 influenza epidemic, with its tranche of severely hypoxic ARDS patients, highlighted the major limitation of all forms of MV in very severe ARDS.[188] Extracorporeal gas exchange devices are based on cardiopulmonary bypass circuit technology and permit varying degrees of CO2 removal and oxygenation.[189] Early studies utilizing this technology are now merely of historical interest because conventional MV has changed to such a degree.[190,191] Most recently, the CESAR study, albeit with some methodological limitations, reported a survival benefit of ECMO in patients with severe respiratory failure, although the clinical trial primarily demonstrated that, in the United Kingdom, referral of very ill patients with severe ARDS to a major tertiary center was beneficial.[192,193] Despite a small evidence base, worldwide over 2500 adults (and over 36,000 children) have received extracorporeal support, either for severe respiratory failure that has failed MV, severe cardiac failure, or both.[194]
One of the interesting features of ALI is that few patients die from refractory hypoxia; the underlying illness, sepsis, severity of acute organ dysfunction, and multiorgan failure cause most deaths.[195–197] Survivors suffer from pulmonary and neuromuscular disorders, and only 50% return to work.[108]
In contrast to ALI, cardiogenic pulmonary edema is characterized by no substantial structural damage to the alveolus and an edema fluid with a lower protein concentration.[198] Increased pulmonary capillary hydrostatic pressure, often aided by a low plasma oncotic pressure, produces an imbalance in transcapillary fluid movement, as predicted by the Starling equation.[199] Substantial alveolar interstitial edema overwhelms the intrinsic safety factors, such as epithelial membrane impermeability, leading to airspace flooding.[200] Such increases in pulmonary hydrostatic pressure may occur via either a generalized increase in fluid volume or an acute redistribution of fluid, as occurs with acute heart failure.[201] Interestingly, the majority of patients with acute heart failure are normo- or hypertensive, and just 3% develop flash pulmonary edema.[202] Acute heart failure is the commonest cause of increased hydrostatic pressure and is very prevalent, with almost 658,000 emergency department visits in the United States per year.[203] Mortality from acute cardiogenic pulmonary edema ranges from 12 to 15% and is significantly worse than the overall 5% rate for acute heart failure.[204–206]
Diagnosis of cardiogenic pulmonary edema is dependent on the demonstration that the pulmonary edema has developed from cardiac dysfunction. Important prognostic clinical signs, such as raised jugular venous pressure or a third heart sound, may be difficult to appreciate in the critically ill.[199,207] Respiratory distress, exhibiting wheeze and widespread crepitations, occurs in any form of pulmonary edema, limiting their diagnostic value.[199] Chest radiography often shows a representative pattern but is limited in its ability to differentiate forms of pulmonary edema.[208,209] Approximately 20% of patients with acute heart failure lack radiological evidence of pulmonary congestion, possibly because a 30% increase in lung water is required for edema to show radiographically.[210,211] Elevated plasma levels of b-type natriuretic peptide (BNP) and N-terminal- proBNP occur in response to ventricular dilatation and pressure overload and have some value in determining the cardiac etiology of acute dyspnea.[212] A normal electrocardiogram is unlikely in the presence of heart failure, whereas an abnormal electrocardiogram can reveal an ischemic myocardium.[213,214] Ultrasonography can determine the presence or absence of cardiac dysfunction and may also distinguish between cardiogenic and noncardiogenic pulmonary edema.[215,216] Pulmonary artery catheterization may provide direct evidence of an elevated left atrial pressure by the measurement of an elevated pulmonary arterial wedge pressure and can also provide evidence regarding the cardiac output and systemic vascular resistance. However, routine use of the pulmonary arterial catheter does not improve outcome in most critically ill patients.[120,217] Measurement of the concentration of protein in pulmonary edema fluid along with the protein concentration in plasma is a noninvasive method to determine whether the pulmonary edema is hydrostatic or from increased permeability.[218]
Supportive therapy for acute cardiogenic pulmonary edema focuses on reducing pulmonary capillary hydrostatic pressure, with specific therapy aimed at the underlying cause. Supplemental oxygen is administered to target normoxia because hyperoxia may be associated with numerous unwanted effects in acute heart failure.[89,219] NIV provides several useful mechanisms, including increasing functional residual capacity, reducing myocardial preload and afterload, reducing oxygen consumption via a reduction in the work of breathing, and improving gas exchange permitting increased coronary oxygen supply.[220] A meta-analysis published in 2010, including the new large Three Interventions in Cardiogenic Pulmonary Oedema study (3CPO), has shown that continuous positive airway pressure (CPAP) and BiPAP are equally efficacious in reducing the need for invasive ventilation; however, only CPAP had a beneficial effect on mortality.[204,221] Morphine has numerous beneficial effects such as vasodilation, analgesia, and anxiolysis, but evidence of its benefit is lacking, and it may even be detrimental.[222] Cardiac preload may also be reduced with selective venodilators such as nitrates, and they are internationally recommended, although the development of hypotension can limit their usefulness.[223] Prospective, placebo-controlled evidence is lacking, although nitrates may be superior to diuretics.[223,224]
Diuretics are traditionally given for acute cardiogenic pulmonary edema, with their rapid relieving effect due to vasodilation rather than the later diuresis originally intended.[225] Although much accumulated evidence shows a beneficial short-term effect, again prospective placebo-controlled evidence is lacking.[223] Because diuretics are frequently used in nonhypervolemic patients with acute heart failure, due to multiple unwanted effects their place has been questioned.[226] Nesiritide is not recommended because it is associated with increased mortality when used in those with acutely decompensated heart failure.[227] Angiotensin-converting enzyme inhibition may be beneficial in acute cardiogenic pulmonary edema.[228,229] Further studies are awaited. Pharmacological support is required for the hypotensive or shocked patient, but the evidence base remains poor and does not support a clear superiority between catecholamines, phosphodiesterase inhibitors, and levosimendan.[230] An intraaortic balloon pump may be required to allow vital specific therapy such as coronary revascularization, or as a bridge to further supportive therapy such as a left ventricular (LV) assist device.[231–233] Extracorporeal support devices may similarly provide rescue therapy.[234]
Pulmonary Vascular Causes of Acute Respiratory Failure
Pulmonary embolism (PE) presents a severe impediment to arterial flow through the pulmonary vasculature, via mechanical obstruction, hypoxic pulmonary vasoconstriction, and the release of vasoactive neurohumeral factors, and it affects both cardiac and pulmonary function.[235] Although circulatory effects occur when 30 to 50% of the pulmonary vascular bed is occluded with thrombus, the size of the embolic burden is not the major determinant of outcome but, rather, whether right ventricular (RV) failure occurs.[236] As right-sided afterload increases acutely, the unconditioned thin-walled RV is unable to generate a pressure above 40 mm Hg; right heart output decreases, leading to decreased LV output and diminishing coronary blood flow.[236] The imbalance between increased RV workload with decreased coronary perfusion creates RV ischemia and predisposes to pulseless electrical activity and sudden cardiac arrest.[237,238] Respiratory effects result largely from V/Q mismatch, with vascular occlusion leading to dead space ventilation and also diverting excessive blood flow through patent vessels leading to shunt (Fig. 1).[235] Also, extreme right-sided pressures can open the foramen ovale, allowing intracardiac right to left shunting, further worsening oxygenation, and predisposing to paradoxical emboli.[239] Oxygenation suffers more with the reduced systemic oxygen delivery producing relatively greater peripheral oxygen uptake leading to more desaturated venous blood entering the pulmonary vasculature.[235]
It has been estimated that 600,000 cases of PE occur annually in the United States and contribute either directly or indirectly to some 200,000 deaths.[240] Grading the severity of PE has changed recently from the size of the embolism (massive, submassive, nonmassive) to the risk of death (high, intermediate, low) for an individual.[241] An episode is considered to be high risk if shock or hypotension is present and occurs initially in 5% or less.[242] In contrast, non-high-risk PE is subdivided into intermediate-risk and low-risk PE, which are differentiated by the presence of RV dysfunction or myocardial injury. The mortality from an episode of PE is 8 to 15% if hemodynamically stable, 25 to 58% if hemodynamically unstable, and as high as 65% if cardiopulmonary arrests occurs.[242,243] Idiopathic PE is common, and 20% of persons with PE do not have an identifiable risk factor.[242] The most significant risk factors are trauma, orthopedic surgery, and general surgery, with odds ratios greater than 10.[244] PE represents one end of a spectrum of venous thromboembolism, highlighted by the fact that 70% of patients with PE have lower-limb deep venous thrombosis.[245]
Several rule systems for predicting the likelihood of PE have been developed, although simple clinical intuition may be just as good.[246–249] The mode of diagnosis depends on the severity of the syndrome.[241] Unstable patients with shock or arterial hypotension, in whom PE is considered likely, should undergo either CT, preferably multidetector CT (MDCT), or if too unstable for transfer, echocardiography (echo) in the ICU.[241,250] Echo signs of PE are mainly those of RV dysfunction and include RV dilatation and hypokinesis, increased RV:LV diameter ratio, increased velocity of the tricuspid regurgitation jet, and right-to-left shunt through the foramen ovale.[251,252] The presence of an RV thrombus virtually confirms the diagnosis and indicates an increased mortality.[253] Additionally, a normal RV on echo scan effectively excludes PE as the cause of circulatory compromise and allows a search for an alternative diagnosis such as pericardial tamponade.[241] If a multidetector CT scan is undertaken, thrombi may possibly be visualized as far as the fifth-order subsegmental pulmonary arteries.[254] Ventricular septal bowing and an RV:LV diameter ratio of greater than 1 are CT evidence of RV dysfunction. If the MDCT scan is negative the patient should have lower limb ultrasonography.[241,255,256] Other investigations are not considered here because they are inappropriate for the emergent management of high-risk PE.
Basic supportive therapy includes supplemental oxygen therapy to correct hypoxia and cautious fluid expansion to optimize RV filling without inducing RV ischemia.[257,258] Oxygen consumption can be reduced via the institution of MV to reduce the work of breathing, and sedation to diminish agitation.[259,260] Extreme care must be taken with the institution of anesthesia for tracheal intubation in a hemodynamically unstable patient. Ketamine’s hemodynamic profile makes it an obvious, if evidence-light, choice.[27] Various pharmacological agents are available to support the circulation, either as vasodilators (isoprenaline, dobutamine, levosimendan, phosphodiesterase inhibitors), vasoconstrictors (dopamine, noradrenaline, adrenaline), or pulmonary vasodilators.[261–263] Because no randomized, controlled studies have evaluated these therapies, the evidence to guide practice is limited, with just a few physiological studies and case reports existing.[258] Two small case series investigating dobutamine in PE have shown increases in cardiac index and oxygen transport.[264,265] However, given its systemic vasodilatory properties it is uncertain if this is the ideal means of circulatory support. Sildenafil and both inhaled nitric oxide and prostacyclin may reduce pulmonary artery pressures but require larger evaluation before they can be recommended.[266–269] Ketanserin is a 5-HT blocker and in a small study slightly lowered pulmonary pressures in acute PE.[270]
Presently, specific therapy has two aims: the dissolution of the embolism and prevention of further embolism (Fig. 2). The embolism may be targeted by anticoagulation, thrombolysis, or embolectomy, either surgical or percutaneous. Prevention of further embolization requires anticoagulation or a vena caval filter, plus therapy for any risk factors. Unfractionated heparin is the anticoagulant of choice if thrombolysis is considered.[241,271] If there are not specific contraindications, high-risk PE should be treated with thrombolysis, with catheter-directed thrombolysis not being superior to systemic thrombolysis.[241,271,272] Over 90% of individuals treated with thrombolysis demonstrate clinical and echocardiographic improvements.[273] While a general mortality benefit has yet to be demonstrated with thrombolysis, it may be present in those with high-risk PE.[274,275] The optimal thrombolytic agent or regime is yet to be determined, but a short infusion period may achieve quicker thrombolysis with less bleeding complications.[271] If thrombolysis fails or is contraindicated, then surgical embolectomy or percutaneous embolectomy, via defragmentation and aspiration, may be considered and may have comparable outcomes.[276–278] ECMO may prove lifesaving until clot resolution is possible.[279]
After an acute episode, long-term anticoagulation is required, with the duration and depth determined by the causative factors specific to the case.[250] Chronic thrombotic pulmonary hypertension complicates up to 3.8% of patients at 2 years post-PE.[280]
Future Directions
Just as Ibsen heralded in a new era in respiratory support with the application of exogenous ventilation to replace failed endogenous ventilation during the Copenhagen polio epidemic of 1952, the emerging technology of extracorporeal lung support may herald a new era allowing severe respiratory failure to be assisted with an exogenous means of gas exchange in some patients with ARF. Management of lung failure now mirrors that of other organ failures, such as renal, cardiac, and, to a lesser degree, hepatic, in which the failed functional unit may be replaced at least temporarily. The greatest progress in the last 2 decades in the treatment of ARF has been the evidence that lung protective ventilation with smaller tidal volume and reduced plateau airway pressure markedly reduces mortality in patients with ALI and ARDS. With the rapid progression of growth factor and stem cell research, management of ALI and ARDS, and perhaps other causes of ARF, may become more directly treatable with the ability to improve healing of the injured organ.
Table 1.
Features of Severe Asthma54
| Near-Fatal Asthma | Life-Threatening Asthma | Acute-Severe Asthma |
|---|---|---|
| RESPIRATORY | RESPIRATORY | RESPIRATORY |
| Hypercapnea | Normocapnia | Inability to complete sentences |
| Need for ventilation with raised airway pressures | Hypoxia | Respiratory rate ≥ 25 |
| Cyanosis | Peak expiratory flow rate 30–50% best or predicted | |
| Silent chest | CIRCULATORY | |
| PEF (peak expiratory flow) 33% best or predicted CIRCULATORY | Heart rate ≥ 110 | |
| Arrhythmia | ||
| Hypotension | ||
| NEUROMUSCULAR | ||
| Altered consciousness | ||
| Exhaustion |
Table 2.
Diagnostic Criteria for Acute Lung Injury116
| Acute Onset |
| Bilateral infiltrates on chest radiograph |
| Pulmonary artery pressure <18 mm Hg or no evidence of left atrial hypertension |
| PaO2:FiO2 ≤ 300 mm Hg (ARDS 200 mm Hg) |
Acknowledgments
RMS is funded by the Northern Ireland Public Health Agency (NI PHA) Research and Development Division (RDD) and DFM by the NI PHA RDD Translational Research Group for Critical Care. MAM is funded in part by the NHBLI HL51856.
References
- 1.Vincent J-L, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Vincent J-L, Akça S, De Mendonça A, et al. SOFA Working Group. Sequntial organ failure assessment. The epidemiology of acute respiratory failure in critically ill patients(*) Chest. 2002;121(5):1602–1609. doi: 10.1378/chest.121.5.1602. [DOI] [PubMed] [Google Scholar]
- 3.Hadden RDM, Cornblath DR, Hughes RAC, et al. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. Ann Neurol. 1998;44(5):780–788. doi: 10.1002/ana.410440512. [DOI] [PubMed] [Google Scholar]
- 4.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366(9497):1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 5.Winer JB. Guillain-Barré syndrome. BMJ. 2008;337:a671. doi: 10.1136/bmj.a671. doi: 10:1136/bmj.a671. Review. No abstract available 227–231. [DOI] [PubMed] [Google Scholar]
- 6.The prognosis and main prognostic indicators of Guillain-Barré syndrome: a multicentre prospective study of 297 patients. The Italian Guillain-Barré Study Group. Brain. 1996;119(Pt 6):2053–2061. [PubMed] [Google Scholar]
- 7.Chaudhuri A, Behan PO. Myasthenic crisis. QJM. 2009;102(2):97–107. doi: 10.1093/qjmed/hcn152. [DOI] [PubMed] [Google Scholar]
- 8.McGrogan A, Sneddon S, de Vries CS. The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology. 2010;34(3):171–183. doi: 10.1159/000279334. [DOI] [PubMed] [Google Scholar]
- 9.Phillips LH., II The epidemiology of myasthenia gravis. Ann N Y Acad Sci. 2003;998:407–412. doi: 10.1196/annals.1254.053. [DOI] [PubMed] [Google Scholar]
- 10.Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. 2009;72(18):1548–1554. doi: 10.1212/WNL.0b013e3181a41211. [DOI] [PubMed] [Google Scholar]
- 11.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(Suppl):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 12.Bershad EM, Feen ES, Suarez JI. Myasthenia gravis crisis. South Med J. 2008;101(1):63–69. doi: 10.1097/SMJ.0b013e31815d4398. [DOI] [PubMed] [Google Scholar]
- 13.Racca F, Del Sorbo L, Mongini T, Vianello A, Ranieri VM. Respiratory management of acute respiratory failure in neuromuscular diseases. Minerva Anestesiol. 2010;76(1):51–62. [PubMed] [Google Scholar]
- 14.Mehta S. Neuromuscular disease causing acute respiratory failure. Respir Care. 2006;51(9):1016–1021. discussion 1021–1023. [PubMed] [Google Scholar]
- 15.Putman MT, Wise RA. Myasthenia gravis and upper airway obstruction. Chest. 1996;109(2):400–404. doi: 10.1378/chest.109.2.400. [DOI] [PubMed] [Google Scholar]
- 16.Rieder P, Louis M, Jolliet P, Chevrolet JC. The repeated measurement of vital capacity is a poor predictor of the need for mechanical ventilation in myasthenia gravis. Intensive Care Med. 1995;21(8):663–668. doi: 10.1007/BF01711545. [DOI] [PubMed] [Google Scholar]
- 17.Sharshar T, Chevret S, Bourdain F, Raphaël J-C French Cooperative Group on Plasma Exchange in Guillain-Barré Syndrome. Early predictors of mechanical ventilation in Guillain-Barré syndrome. Crit Care Med. 2003;31(1):278–283. doi: 10.1097/00003246-200301000-00044. [DOI] [PubMed] [Google Scholar]
- 18.Garpestad E, Brennan J, Hill NS. Noninvasive ventilation for critical care. Chest. 2007;132(2):711–720. doi: 10.1378/chest.06-2643. [DOI] [PubMed] [Google Scholar]
- 19.Pearse RM, Draper A, Grounds RM. Non-invasive ventilation to avoid tracheal intubation in a patient with Guillain-Barré syndrome. Br J Anaesth. 2003;91(6):913–916. doi: 10.1093/bja/aeg252. [DOI] [PubMed] [Google Scholar]
- 20.Rabinstein A, Wijdicks EFM. BiPAP in acute respiratory failure due to myasthenic crisis may prevent intubation. Neurology. 2002;59(10):1647–1649. doi: 10.1212/01.wnl.0000033797.79530.16. [DOI] [PubMed] [Google Scholar]
- 21.Seneviratne J, Mandrekar J, Wijdicks EFM, Rabinstein AA. Noninvasive ventilation in myasthenic crisis. Arch Neurol. 2008;65(1):54–58. doi: 10.1001/archneurol.2007.1. [DOI] [PubMed] [Google Scholar]
- 22.Wijdicks EFM, Roy TK. BiPAP in early Guillain-Barré syndrome may fail. Can J Neurol Sci. 2006;33(1):105–106. doi: 10.1017/s0317167100004790. [DOI] [PubMed] [Google Scholar]
- 23.Allolio B, Stuttmann R, Leonhard U, Fischer H, Winkelmann W. Adrenocortical suppression by a single induction dose of etomidate. Klin Wochenschr. 1984;62(21):1014–1017. doi: 10.1007/BF01711723. [DOI] [PubMed] [Google Scholar]
- 24.Sprung CL, Annane D, Keh D, et al. CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 25.Bloomfield R, Noble DW. Etomidate, pharmacological adrenalectomy and the critically ill: a matter of vital importance. Crit Care. 2006;10(4):161. doi: 10.1186/cc5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craven R. Ketamine. Anaesthesia. 2007;62(Suppl 1):48–53. doi: 10.1111/j.1365-2044.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 27.Morris C, Perris A, Klein J, Mahoney P. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009;64(5):532–539. doi: 10.1111/j.1365-2044.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 28.Reilly M, Hutchinson M. Suxamethonium is contraindicated in the Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 1991;54(11):1018–1019. doi: 10.1136/jnnp.54.11.1018-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenkraft JB, Book WJ, Mann SM, Papatestas AE, Hubbard M. Resistance to succinylcholine in myasthenia gravis: a doseresponse study. Anesthesiology. 1988;69(5):760–763. doi: 10.1097/00000542-198811000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Baraka A, Baroody M, Yazbeck V. Repeated doses of suxamethonium in the myasthenic patient. Anaesthesia. 1993;48(9):782–784. doi: 10.1111/j.1365-2044.1993.tb07590.x. [DOI] [PubMed] [Google Scholar]
- 31.Murthy JM, Meena AK, Chowdary GV, Naryanan JT. Myasthenic crisis: clinical features, complications and mortality. Neurol India. 2005;53(1):37–40. doi: 10.4103/0028-3886.15050. discussion 40. [DOI] [PubMed] [Google Scholar]
- 32.Thomas CE, Mayer SA, Gungor Y, et al. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48(5):1253–1260. doi: 10.1212/wnl.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 33.Lawn ND, Wijdicks EFM. Post-intubation pulmonary function test in Guillain-Barré syndrome. Muscle Nerve. 2000;23(4):613–616. doi: 10.1002/(sici)1097-4598(200004)23:4<613::aid-mus21>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Ali MI, Fernández-Pérez ER, Pendem S, Brown DR, Wijdicks EF, Gajic O. Mechanical ventilation in patients with Guillain-Barré syndrome. Respir Care. 2006;51(12):1403–1407. [PubMed] [Google Scholar]
- 35.Rabinstein AA, Mueller-Kronast N. Risk of extubation failure in patients with myasthenic crisis. Neurocrit Care. 2005;3(3):213–215. doi: 10.1385/NCC:3:3:213. [DOI] [PubMed] [Google Scholar]
- 36.Gajdos P, Chevret S, Toyka K. Plasma exchange for myasthenia gravis. Cochrane Database Syst Rev. 2002;(4):CD002275. doi: 10.1002/14651858.CD002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raphaël J-C, Chevret S, Hughes RAC, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2002;(2):CD001798. doi: 10.1002/14651858.CD001798. [DOI] [PubMed] [Google Scholar]
- 38.Hughes RAC, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010;(6):CD002063. doi: 10.1002/14651858.CD002063.pub. [DOI] [PubMed] [Google Scholar]
- 39.Gajdos P, Chevret S, Toyka KV. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev. 2008;(1):CD002277. doi: 10.1002/14651858.CD002277.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Hughes RAC, Swan AV, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010;(2):CD001446. doi: 10.1002/14651858.CD001446.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Schneider-Gold C, Gajdos P, Toyka KV, Hohlfeld RR. Corticosteroids for myasthenia gravis. Cochrane Database Syst Rev. 2005;(2):CD002828. doi: 10.1002/14651858.CD002828.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angelini C. Diagnosis and management of autoimmune myasthenia gravis. Clin Drug Investig. 2011;31(1):1–14. doi: 10.2165/11584740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Hadden RDM, Karch H, Hartung H-P, et al. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology. 2001;56(6):758–765. doi: 10.1212/wnl.56.6.758. [DOI] [PubMed] [Google Scholar]
- 44.Varelas PN, Chua HC, Natterman J, et al. Ventilatory care in myasthenia gravis crisis: assessing the baseline adverse event rate. Crit Care Med. 2002;30(12):2663–2668. doi: 10.1097/00003246-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Seneviratne J, Mandrekar J, Wijdicks EFM, Rabinstein AA. Predictors of extubation failure in myasthenic crisis. Arch Neurol. 2008;65(7):929–933. doi: 10.1001/archneur.65.7.929. [DOI] [PubMed] [Google Scholar]
- 46.Mayer SA. Intensive care of the myasthenic patient. Neurology. 1997;48:S70–S75. [Google Scholar]
- 47.Mannam P, Siegel MD. Analytic review: management of life-threatening asthma in adults. J Intensive Care Med. 2010;25(1):3–15. doi: 10.1177/0885066609350866. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Roisin R. Acute severe asthma: pathophysiology and pathobiology of gas exchange abnormalities. Eur Respir J. 1997;10(6):1359–1371. doi: 10.1183/09031936.97.10061359. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a eview. Chest. 2004;125(3):1081–1102. doi: 10.1378/chest.125.3.1081. [DOI] [PubMed] [Google Scholar]
- 50.Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical eview: severe asthma. Crit Care. 2002;6(1):30–44. doi: 10.1186/cc1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Heart, Lung, and Blood Institute. [Accessed September 3, 2011];Morbidity and ortality: 2010 chart book on cardiovascular, lung and blood diseases. http://www.nhlbi.nih.gov/about/factbook/hapter4.htm.
- 52.Salmeron S, Liard R, Elkharrat D, Muir J, Neukirch F, llrodt A. Asthma severity and adequacy of management in accident and emergency departments in France: a prospective tudy. Lancet. 2001;358(9282):629–635. doi: 10.1016/s0140-6736(01)05779-8. [DOI] [PubMed] [Google Scholar]
- 53.Pendergraft TB, Stanford RH, Beasley R, Stempel DA, oberts C, McLaughlin T. Rates and characteristics of intensive care unit admissions and intubations among sthma-related hospitalizations. Ann Allergy Asthma Immunol. 2004;93(1):29–35. doi: 10.1016/S1081-1206(10)61444-5. [DOI] [PubMed] [Google Scholar]
- 54.British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. horax. 2008;63(Suppl 4):iv1–121. doi: 10.1136/thx.2008.097741. No abstract available. [DOI] [PubMed] [Google Scholar]
- 55.Holley AD, Boots RJ. Review article: management of acute evere and near-fatal asthma. Emerg Med Australas. 2009;1(4):259–268. doi: 10.1111/j.1742-6723.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- 56.Travers A, Jones AP, Kelly K, Barker SJ, Camargo CA, Rowe BH. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database Syst Rev. 2001;(2):CD002988. doi: 10.1002/14651858.CD002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abroug F, Nouira S, Bchir A, Boujdaria R, Elatrous S, Bouchoucha S. A controlled trial of nebulized salbutamol and adrenaline in acute severe asthma. Intensive Care Med. 1995;21(1):18–23. doi: 10.1007/BF02425149. [DOI] [PubMed] [Google Scholar]
- 58.Stoodley RG, Aaron SD, Dales RE. The role of ipratropium bromide in the emergency management of acute asthma exacerbation: a metaanalysis of randomized clinical trials. Ann Emerg Med. 1999;34(1):8–18. doi: 10.1016/s0196-0644(99)70266-0. [DOI] [PubMed] [Google Scholar]
- 59.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178. doi: 10.1002/14651858.CD002178. [DOI] [PubMed] [Google Scholar]
- 60.Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA., Jr Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;(2):CD001490. doi: 10.1002/14651858. CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parameswaran K, Belda J, Rowe BH. Addition of intravenous aminophylline to beta2-agonists in adults with acute asthma. Cochrane Database Syst Rev. 2000;(4):CD002742. doi: 10.1002/14651858.CD002742. [DOI] [PubMed] [Google Scholar]
- 62.Camargo CA, Jr, Gurner DM, Smithline HA, et al. A randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthma. J Allergy Clin Immunol. 2010;125(2):374–380. doi: 10.1016/j.jaci.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Silverman RA, Nowak RM, Korenblat PE, et al. Zafirlukast treatment for acute asthma: evaluation in a randomized, double-blind, multicenter trial. Chest. 2004;126(5):1480–1489. doi: 10.1378/chest.126.5.1480. [DOI] [PubMed] [Google Scholar]
- 64.Hess DR, Fink JB, Venkataraman ST, Kim IK, Myers TR, Tano BD. The history and physics of heliox. Respir Care. 2006;51(6):608–612. [PubMed] [Google Scholar]
- 65.Rodrigo G, Pollack C, Rodrigo C, Rowe BH. Heliox for nonintubated acute asthma patients. Cochrane Database Syst Rev. 2006;(4):CD002884. doi: 10.1002/14651858.CD002884.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wigmore T, Stachowski E. A review of the use of heliox in the critically ill. Crit Care Resusc. 2006;8(1):64–72. [PubMed] [Google Scholar]
- 67.Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest. 2003;123(4):1018–1025. doi: 10.1378/chest.123.4.1018. [DOI] [PubMed] [Google Scholar]
- 68.Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(1):CD004360. doi: 10.1002/14651858.CD004360.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Brenner B, Corbridge T, Kazzi A. Intubation and mechanical ventilation of the asthmatic patient in respiratory failure. Proc Am Thorac Soc. 2009;6(4):371–379. doi: 10.1513/pats.P09ST4. [DOI] [PubMed] [Google Scholar]
- 70.McCourt KC, Salmela L, Mirakhur RK, et al. Comparison of rocuronium and suxamethonium for use during rapid sequence induction of anaesthesia. Anaesthesia. 1998;53(9):867–871. doi: 10.1046/j.1365-2044.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 71.Hogg RMG, Lappin E, Shields MO, et al. Sugammadex allows the use of rocuronium in place of succinylcholine during rapid sequence induction of anaesthesia. Br J Anaesth. 2010;105:726P–727P. [Google Scholar]
- 72.Phipps P, Garrard CS. The pulmonary physician in critical care. 12: acute severe asthma in the intensive care unit. Thorax. 2003;58(1):81–88. doi: 10.1136/thorax.58.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stather DR, Stewart TE. Clinical review: mechanical ventilation in severe asthma. Crit Care. 2005;9(6):581–587. doi: 10.1186/cc3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemmingsen C, Nielsen PK, Odorico J. Ketamine in the treatment of bronchospasm during mechanical ventilation. Am J Emerg Med. 1994;12(4):417–420. doi: 10.1016/0735-6757(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 75.Sarma VJ. Use of ketamine in acute severe asthma. Acta Anaesthesiol Scand. 1992;36(1):106–107. doi: 10.1111/j.1399-6576.1992.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 76.Saulnier FF, Durocher AV, Deturck RA, Lefèbvre MC, Wattel FE. Respiratory and hemodynamic effects of halothane in status asthmaticus. Intensive Care Med. 1990;16(2):104–107. doi: 10.1007/BF02575303. [DOI] [PubMed] [Google Scholar]
- 77.Maltais F, Sovilj M, Goldberg P, Gottfried SB. Respiratory mechanics in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401–1406. doi: 10.1378/chest.106.5.1401. [DOI] [PubMed] [Google Scholar]
- 78.Elliot SC, Paramasivam K, Oram J, Bodenham AR, Howell SJ, Mallick A. Pumpless extracorporeal carbon dioxide removal for life-threatening asthma. Crit Care Med. 2007;35(3):945–948. doi: 10.1097/01.CCM.0000257462.04514.15. [DOI] [PubMed] [Google Scholar]
- 79.Marquette CH, Saulnier F, Leroy O, et al. Long-term prognosis of near-fatal asthma. A 6-year follow-up study of 145 asthmatic patients who underwent mechanical ventilation for a near-fatal attack of asthma. Am Rev Respir Dis. 1992;146(1):76–81. doi: 10.1164/ajrccm/146.1.76. [DOI] [PubMed] [Google Scholar]
- 80.Barnes PJ. Mechanisms in COPD: differences from asthma. Chest. 2000;117(2, Suppl):10S–14S. doi: 10.1378/chest.117.2_suppl.10s. [DOI] [PubMed] [Google Scholar]
- 81.Bleecker ER. Similarities and differences in asthma and COPD. The Dutch hypothesis. Chest. 2004;126(2, Suppl):93S–95S. doi: 10.1378/chest.126.2_suppl_1.93S. discussion 159S–161S. [DOI] [PubMed] [Google Scholar]
- 82.Ali NK. Evidence-based approach to acute exacerbations of chronic obstructive pulmonary disease. Hosp Physician. 2009;45:9–17. Available at: http://www.turner-white.com/cgi-bin/hmain2.cgi. The article can be accessed at http://www.turner-white.com/memberfile.php?PubCode=hp_feb09_pulmonary.pdf. [Google Scholar]
- 83.Turato G, Zuin R, Saetta M. Pathogenesis and pathology of COPD. Respiration. 2001;68(2):117–128. doi: 10.1159/000050478. [DOI] [PubMed] [Google Scholar]
- 84.Davidson AC. The pulmonary physician in critical care. 11: critical care management of respiratory failure resulting from COPD. Thorax. 2002;57(12):1079–1084. doi: 10.1136/thorax.57.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 86.Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274(23):1852–1857. [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention. Deaths from chronic obstructive pulmonary disease—United States, 2000–2005. MMWR Morb Mortal Wkly Rep. 2008;57(45):1229–1232. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a4.htm. [PubMed] [Google Scholar]
- 88.MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):530–535. doi: 10.1513/pats.200707-088ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Driscoll BR, Howard LS, Davison AG British Thoracic Society. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63(Suppl 6):vi1–vi68. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 90.Hanson CWI, III, Marshall BE, Frasch HF, Marshall C. Causes of hypercarbia with oxygen therapy in patients with chronic obstructive pulmonary disease. Crit Care Med. 1996;24(1):23–28. doi: 10.1097/00003246-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Karpel JP. Bronchodilator responses to anticholinergic and beta-adrenergic agents in acute and stable COPD. Chest. 1991;99(4):871–876. doi: 10.1378/chest.99.4.871. [DOI] [PubMed] [Google Scholar]
- 92.Bach PB, Brown C, Gelfand SE, McCrory DC American College of Physicians-American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001;134(7):600–620. doi: 10.7326/0003-4819-134-7-200104030-00016. [DOI] [PubMed] [Google Scholar]
- 93.Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol. 2006;533(1–3):36–39. doi: 10.1016/j.ejphar.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 94.Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;(1):CD001288. doi: 10.1002/14651858.CD001288.pub2. [DOI] [PubMed] [Google Scholar]
- 95.Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trial. Lancet. 2001;358(9298):2020–2025. doi: 10.1016/S0140-6736(01)07097-0. [DOI] [PubMed] [Google Scholar]
- 96.National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre; 2010. http://guidance.nice.org.uk/CG101. [Google Scholar]
- 97.Barr RG, Rowe BH, Camargo CA., Jr Methylxanthines for exacerbations of chronic obstructive pulmonary disease: metaanalysis of randomised trials. BMJ. 2003;327(7416):643. doi: 10.1136/bmj.327.7416.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104. doi: 10.1002/14651858.CD004104.pub3. [DOI] [PubMed] [Google Scholar]
- 99.Moretti M, Cilione C, Tampieri A, Fracchia C, Marchioni A, Nava S. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000;55(10):819–825. doi: 10.1136/thorax.55.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med. 1999;160(1):86–92. doi: 10.1164/ajrccm.160.1.9802120. [DOI] [PubMed] [Google Scholar]
- 101.Esteban A, Anzueto A, Frutos F, et al. Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 102.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–2646. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 103.Salpeter SR, Buckley NS, Salpeter EE. Meta-analysis: anticholinergics, but not b-agonists, reduce severe exacerbations and respiratory mortality in COPD. J Gen Intern Med. 2006;21(10):1011–1019. doi: 10.1111/j.1525-1497.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting b-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144(12):904–912. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 105.Noveanu M, Breidthardt T, Reichlin T, et al. Effect of oral beta-blocker on short and long-term mortality in patients with acute respiratory failure: results from the BASEL-IIICU study. Crit Care. 2010;14(6):R198. doi: 10.1186/cc9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 107.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 108.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 109.Papazian L, Forel J-M, Gacouin A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 110.Zambon M, Vincent J-L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 111.Wheeler AP, Bernard GR, Thompson BT, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonaryartery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 112.Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158(1):3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 113.Atabai K, Matthay MA. The pulmonary physician in critical care, V: Acute lung injury and the acute respiratory distress syndrome: definitions and epidemiology. Thorax. 2002;57(5):452–458. doi: 10.1136/thorax.57.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flores C, Pino-Yanes MM, Casula M, Villar J. Genetics of acute lung injury: past, present and future. Minerva Anestesiol. 2010;76(10):860–864. [PubMed] [Google Scholar]
- 115.Bellingan GJ. The pulmonary physician in critical care * 6: the pathogenesis of ALI/ARDS. Thorax. 2002;57(6):540– 546. doi: 10.1136/thorax.57.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bernard GR, Artigas A, Brigham KL, et al. The American- European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818– 824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 117.Abraham E, Matthay MA, Dinarello CA, et al. Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Crit Care Med. 2000;28(1):232–235. doi: 10.1097/00003246-200001000-00039. [DOI] [PubMed] [Google Scholar]
- 118.Villar J, Pérez-Méndez L, Kacmarek RM. Current definitions of acute lung injury and the acute respiratory distress syndrome do not reflect their true severity and outcome. Intensive Care Med. 1999;25(9):930–935. doi: 10.1007/s001340050984. [DOI] [PubMed] [Google Scholar]
- 119.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116(5):1347–1353. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 120.Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002;28(8):1073–1077. doi: 10.1007/s00134-002-1354-y. [DOI] [PubMed] [Google Scholar]
- 121.Iberti TJ, Fischer EP, Leibowitz AB, Panacek EA, Silverstein JH, Albertson TE Pulmonary Artery Catheter Study Group. A multicenter study of physicians’ knowledge of the pulmonary artery catheter. JAMA. 1990;264(22):2928–2932. [PubMed] [Google Scholar]
- 122.Iberti TJ, Daily EK, Leibowitz AB, Schecter CB, Fischer EP, Silverstein JH The Pulmonary Artery Catheter Study Group. Assessment of critical care nurses’ knowledge of the pulmonary artery catheter. Crit Care Med. 1994;22(10):1674–1678. [PubMed] [Google Scholar]
- 123.Esteban A, Fernández-Segoviano P, Frutos-Vivar F, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med. 2004;141(6):440–445. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 124.Tzouvelekis A, Pneumatikos I, Bouros D. Serum biomarkers in acute respiratory distress syndrome an ailing prognosticator. Respir Res. 2005;6:62. doi: 10.1186/1465-9921-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary deadspace fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 126.Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care. 2010;55(12):1653–1660. [PubMed] [Google Scholar]
- 127.Ortiz G, Frutos-Vivar F, Ferguson ND, et al. Ventila Group. Outcomes of patients ventilated with synchronized intermittent mandatory ventilation with pressure support: a comparative propensity score study. Chest. 2010;137(6):1265–1277. doi: 10.1378/chest.09-2131. [DOI] [PubMed] [Google Scholar]
- 128.Rodriguez P, Michel D, Brochard L. Mechanical ventilation: changing concepts. Indian J Crit Care Med. 2005;9:235–243. [Google Scholar]
- 129.Davis K, Jr, Branson RD, Campbell RS, Porembka DT. Comparison of volume control and pressure control ventilation: is flow waveform the difference? J Trauma. 1996;41(5):808–814. doi: 10.1097/00005373-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 130.Piotrowski A, Sobala W, Kawczy ski P. Patient-initiated, pressure-regulated, volume-controlled ventilation compared with intermittent mandatory ventilation in neonates: a prospective, randomised study. Intensive Care Med. 1997;23(9):975–981. doi: 10.1007/s001340050441. [DOI] [PubMed] [Google Scholar]
- 131.Amato MB, Barbas CS, Bonassa J, Saldiva PH, Zin WA, de Carvalho CR. Volume-assured pressure support ventilation (VAPSV): a new approach for reducing muscle workload during acute respiratory failure. Chest. 1992;102(4):1225– 1234. doi: 10.1378/chest.102.4.1225. [DOI] [PubMed] [Google Scholar]
- 132.Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med. 1987;15(5):462–466. doi: 10.1097/00003246-198705000-00002. [DOI] [PubMed] [Google Scholar]
- 133.Sinderby C, Navalesi P, Beck J, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5(12):1433–1436. doi: 10.1038/71012. [DOI] [PubMed] [Google Scholar]
- 134.Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation: results of an initial clinical trial. Am Rev Respir Dis. 1992;145(1):121–129. doi: 10.1164/ajrccm/145.1.121. [DOI] [PubMed] [Google Scholar]
- 135.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110(5):556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 136.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137(5):1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 138.Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116(1, Suppl):9S–15S. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 139.Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians. 1998;110(6):482–488. [PubMed] [Google Scholar]
- 140.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 141.Register SD, Downs JB, Stock MC, Kirby RR. Is 50% oxygen harmful? Crit Care Med. 1987;15(6):598–601. doi: 10.1097/00003246-198706000-00012. [DOI] [PubMed] [Google Scholar]
- 142.Hedenstierna G, Rothen HU. Atelectasis formation during anesthesia: causes and measures to prevent it. J Clin Monit Comput. 2000;16(5–6):329–335. doi: 10.1023/a:1011491231934. [DOI] [PubMed] [Google Scholar]
- 143.Gattinoni L, Pesenti A. ARDS: the nonhomogeneous lung; facts and hypothesis. Intensive Crit Care Dig. 1987;6:1–4. [Google Scholar]
- 144.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 145.Hager DN, Krishnan JA, Hayden DL, Brower RG ARDS Clinical Trials Network. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172(10):1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rimensberger PC, Cox PN, Frndova H, Bryan AC. The open lung during small tidal volume ventilation: concepts of recruitment and ‘optimal’ positive end-expiratory pressure. Crit Care Med. 1999;27(9):1946–1952. doi: 10.1097/00003246-199909000-00038. [DOI] [PubMed] [Google Scholar]
- 147.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151(8):566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 148.Steinberg KP, Kacmarek RM. Respiratory controversies in the critical care setting. Should tidal volume be 6 mL/kg predicted body weight in virtually all patients with acute respiratory failure? Respir Care. 2007;52(5):556–564. discussion 565–567. [PubMed] [Google Scholar]
- 149.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogué S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851–1858. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 151.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 152.Esteban A, Frutos F, Tobin MJ, et al. Spanish Lung Failure Collaborative Group. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332(6):345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 153.Hall RI, Sandham D, Cardinal P, et al. Study Investigators. Propofol vs midazolam for ICU sedation: a Canadian multicenter randomized trial. Chest. 2001;119(4):1151–1159. doi: 10.1378/chest.119.4.1151. [DOI] [PubMed] [Google Scholar]
- 154.Meade M, Guyatt G, Cook D, et al. Predicting success in weaning from mechanical ventilation. Chest. 2001;120(6, Suppl):400S–424S. doi: 10.1378/chest.120.6_suppl.400s. [DOI] [PubMed] [Google Scholar]
- 155.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324(21):1445–1450. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 156.O’Croinin D, Ni Chonghaile M, Higgins B, Laffey JG. Bench-to-bedside review: permissive hypercapnia. Crit Care. 2005;9(1):51–59. doi: 10.1186/cc2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Armstrong BWJ, Jr, MacIntyre NR. Pressure-controlled, inverse ratio ventilation that avoids air trapping in the adult respiratory distress syndrome. Crit Care Med. 1995;23(2):279–285. doi: 10.1097/00003246-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 158.Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20(4):1017–1028. doi: 10.1183/09031936.02.00401702. [DOI] [PubMed] [Google Scholar]
- 159.Fan E, Wilcox ME, Brower RG, et al. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med. 2008;178(11):1156–1163. doi: 10.1164/rccm.200802-335OC. [DOI] [PubMed] [Google Scholar]
- 160.Sud S, Sud M, Friedrich JO, Adhikari NKJ. Effect of mechanical ventilation in the prone position on clinical outcomes in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. CMAJ. 2008;178(9):1153–1161. doi: 10.1503/cmaj.071802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gattinoni L, Carlesso E, Taccone P, Polli F, Guérin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010;76(6):448–454. [PubMed] [Google Scholar]
- 162.Hess DR, Gillette MA. Tracheal gas insufflation and related techniques to introduce gas flow into the trachea. Respir Care. 2001;46(2):119–129. [PubMed] [Google Scholar]
- 163.Goldhill DR, Imhoff M, McLean B, Waldmann C. Rotational bed therapy to prevent and treat respiratory complications: a review and meta-analysis. Am J Crit Care. 2007;16(1):50–61. quiz 62. [PubMed] [Google Scholar]
- 164.Wiedemann HP, Wheeler AP, Bernard GR, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 165.Humphrey H, Hall J, Sznajder I, Silverstein M, Wood L. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97(5):1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 166.Sakr Y, Vincent J-L, Reinhart K, et al. Sepsis Occurence in Acutely Ill Patients Investigators. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128(5):3098–3108. doi: 10.1378/chest.128.5.3098. [DOI] [PubMed] [Google Scholar]
- 167.Afshari A, Brok J, Møller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with metaanalysis and trial sequential analysis. Anesth Analg. 2011;112(6):1411–1421. doi: 10.1213/ANE.0b013e31820bd185. [DOI] [PubMed] [Google Scholar]
- 168.Abraham E, Park YC, Covington P, Conrad SA, Schwartz M. Liposomal prostaglandin E1 in acute respiratory distress syndrome: a placebo-controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med. 1996;24(1):10–15. doi: 10.1097/00003246-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 169.Reyes A, Roca J, Rodriguez-Roisin R, Torres A, Ussetti P, Wagner PD. Effect of almitrine on ventilation-perfusion distribution in adult respiratory distress syndrome. Am Rev Respir Dis. 1988;137(5):1062–1067. doi: 10.1164/ajrccm/137.5.1062. [DOI] [PubMed] [Google Scholar]
- 170.Gallart L, Lu QIN, Puybasset L, Umamaheswara Rao GS, Coriat P, Rouby JJ The NO Almitrine Study Group. Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158(6):1770–1777. doi: 10.1164/ajrccm.158.6.9804066. [DOI] [PubMed] [Google Scholar]
- 171.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178(6):618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Adhikari NKJ, Scales DC. Corticosteroids for acute respiratory distress syndrome. BMJ. 2008;336(7651):969–970. doi: 10.1136/bmj.39553.408924.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.The ARDS Network. Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2000;283(15):1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 174.Montravers P, Fagon JY, Gilbert C, Blanchet F, Novara A, Chastre J. Pilot study of cardiopulmonary risk from pentoxifylline in adult respiratory distress syndrome. Chest. 1993;103(4):1017–1022. doi: 10.1378/chest.103.4.1017. [DOI] [PubMed] [Google Scholar]
- 175.The Acute Respiratory Disease Syndrome Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30(1):1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 176.Raoof S, Goulet K, Esan A, Hess DR, Sessler CN. Severe hypoxemic respiratory failure, II: Nonventilatory strategies. Chest. 2010;137(6):1437–1448. doi: 10.1378/chest.09-2416. [DOI] [PubMed] [Google Scholar]
- 177.Spragg RG, Lewis JF, Wurst W, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167(11):1562–1566. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 178.Matthay MA, Brower R, Thompson BT, et al. Randomized, placebo controlled trial of an aerosolized beta-2 adrenergic agonist (albuterol) for the treatment of acute lung injury. Am J Respir Crit Care Med. 2009;179:A2166. [Google Scholar]
- 179.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 180.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336(7651):1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Matthay MA, Thompson BT, Read EJ, et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest. 2010;138(4):965–972. doi: 10.1378/chest.10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L924–L940. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 183.Downar J, Mehta S. Bench-to-bedside review: highfrequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care. 2006;10(6):240. doi: 10.1186/cc5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chan KPW, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest. 2007;131(6):1907–1916. doi: 10.1378/chest.06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sud S, Sud M, Friedrich JO, et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ. 2010;340:c2327. doi: 10.1136/bmj.c2327. [DOI] [PubMed] [Google Scholar]
- 186.Kaisers U, Kelly KP, Busch T. Liquid ventilation. Br J Anaesth. 2003;91(1):143–151. doi: 10.1093/bja/aeg147. [DOI] [PubMed] [Google Scholar]
- 187.Kacmarek RM, Wiedemann HP, Lavin PT, Wedel MK, Tütüncü AS, Slutsky AS. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173(8):882–889. doi: 10.1164/rccm.200508-1196OC. [DOI] [PubMed] [Google Scholar]
- 188.Davies A, Jones D, Bailey M, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 189.Matheis G. New technologies for respiratory assist. Perfusion. 2003;18(4):245–251. doi: 10.1191/0267659103pf684oa. [DOI] [PubMed] [Google Scholar]
- 190.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242(20):2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 191.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(2 Pt 1):295–305. doi: 10.1164/ajrccm.149.2.8306022. Erratum appears in Am J Respir Crit Care Med 1994;149(3 Pt 1):838. [DOI] [PubMed] [Google Scholar]
- 192.Zwischenberger JB, Lynch JE. Will CESAR answer the adult ECMO debate? Lancet. 2009;374(9698):1307–1308. doi: 10.1016/S0140-6736(09)61630-5. [DOI] [PubMed] [Google Scholar]
- 193.Peek GJ, Mugford M, Tiruvoipati R, et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 194.Extracorporeal Life Support Organization. [Accessed March 20, 2011];ELSO Registry Information. http://www.elso.med.umich.edu/Registry.html.
- 195.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 196.Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1985;132(3):485– 489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- 197.Vincent JL, Sakr Y, Ranieri VM. Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31(4, Suppl):S296–S299. doi: 10.1097/01.CCM.0000057906.89552.8F. [DOI] [PubMed] [Google Scholar]
- 198.Cottrell TS, Levine OR, Senior RM, Wiener J, Spiro D, Fishman AP. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res. 1967;21(6):783–797. doi: 10.1161/01.res.21.6.783. [DOI] [PubMed] [Google Scholar]
- 199.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 200.Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest. 2004;125(2):669–682. doi: 10.1378/chest.125.2.669. [DOI] [PubMed] [Google Scholar]
- 201.Cleland JG, Yassin AS, Khadjooi K. Acute heart failure: focusing on acute cardiogenic pulmonary oedema. Clin Med. 2010;10(1):59–64. doi: 10.7861/clinmedicine.10-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 203.Schappert S, Rechtsteiner E. Ambulatory Medical Care Utilization Estimates for 2006. [Accessed March 15, 2011];National Health Statistics Reports. 2008 http://198.246.98.21/nchs/data/nhsr/nhsr.pdf. [PubMed]
- 204.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359(2):142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 205.Edoute Y, Roguin A, Behar D, Reisner SA. Prospective evaluation of pulmonary edema. Crit Care Med. 2000;28(2):330–335. doi: 10.1097/00003246-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 206.Roguin A, Behar DM, Ben Ami H, et al. Long-term prognosis of acute pulmonary oedema—an ominous outcome. Eur J Heart Fail. 2000;2(2):137–144. doi: 10.1016/s1388-9842(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 207.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345(8):574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 208.Gluecker T, Capasso P, Schnyder P, et al. Clinical and radiologic features of pulmonary edema. Radiographics. 1999;19(6):1507–1531. doi: 10.1148/radiographics.19.6.g99no211507. discussion 1532–1533. [DOI] [PubMed] [Google Scholar]
- 209.Aberle DR, Wiener-Kronish JP, Webb WR, Matthay MA. Hydrostatic versus increased permeability pulmonary edema: diagnosis based on radiographic criteria in critically ill patients. Radiology. 1988;168(1):73– 79. doi: 10.1148/radiology.168.1.3380985. [DOI] [PubMed] [Google Scholar]
- 210.Collins SP, Lindsell CJ, Storrow AB, Abraham WT ADHERE Scientific Advisory Committee, Investigators and Study Group. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18. doi: 10.1016/j.annemergmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 211.Pistolesi M, Giuntini C. Assessment of extravascular lung water. Radiol Clin North Am. 1978;16(3):551–574. [PubMed] [Google Scholar]
- 212.Worster A, Balion CM, Hill SA, et al. Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem. 2008;41(4–5):250–259. doi: 10.1016/j.clinbiochem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 213.Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944– 1956. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 214.Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348(10):933–940. doi: 10.1056/NEJMra022700. [DOI] [PubMed] [Google Scholar]
- 215.Price S, Nicol E, Gibson DG, Evans TW. Echocardiography in the critically ill: current and potential roles. Intensive Care Med. 2006;32(1):48–59. doi: 10.1007/s00134-005-2834-7. [DOI] [PubMed] [Google Scholar]
- 216.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Harvey S, Young D, Brampton W, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2006;3(3):CD003408. doi: 10.1002/14651858.CD003408.pub2. [DOI] [PubMed] [Google Scholar]
- 218.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2010;35(2):331–337. doi: 10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Haque WA, Boehmer J, Clemson BS, Leuenberger UA, Silber DH, Sinoway LI. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol. 1996;27(2):353–357. doi: 10.1016/0735-1097(95)00474-2. [DOI] [PubMed] [Google Scholar]
- 220.Prinianakis G, Klimathianaki M, Georgopoulos D. Physiological rationale of noninvasive mechanical ventilation use in acute respiratory failure. Eur Respir Monogr. 2008;41:3–23. [Google Scholar]
- 221.Weng C-L, Zhao Y-T, Liu Q-H, et al. Meta-analysis: noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590–600. doi: 10.7326/0003-4819-152-9-201005040-00009. [DOI] [PubMed] [Google Scholar]
- 222.Sosnowski MA. Review article: lack of effect of opiates in the treatment of acute cardiogenic pulmonary oedema. Emerg Med Australas. 2008;20(5):384–390. doi: 10.1111/j.1742-6723.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- 223.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Committee for Practice Guidelines (CPG) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive CareMedicine (ESICM) Eur J Heart Fail. 2008;10(10):933–989. doi: 10.1016/j.ejheart.2008.08.005. Corrected in Eur J Heart Fail 2010;12(4):416 (note: dosage error in article text) [DOI] [PubMed] [Google Scholar]
- 224.Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351(9100):389–393. doi: 10.1016/S0140-6736(97)08417-1. [DOI] [PubMed] [Google Scholar]
- 225.Jhund PS, McMurray JJV, Davie AP. The acute vascular effects of frusemide in heart failure. Br J Clin Pharmacol. 2000;50(1):9–13. doi: 10.1046/j.1365-2125.2000.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Singer M, Glynne P. Treating critical illness: the importance of first doing no harm. PLoS Med. 2005;2(6):e167. doi: 10.1371/journal.pmed.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293(15):1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 228.Hamilton RJ, Carter WA, Gallagher EJ. Rapid improvement of acute pulmonary edema with sublingual captopril. Acad Emerg Med. 1996;3(3):205–212. doi: 10.1111/j.1553-2712.1996.tb03422.x. [DOI] [PubMed] [Google Scholar]
- 229.Annane D, Bellissant E, Pussard E, et al. Placebocontrolled, randomized, double-blind study of intravenous enalaprilat efficacy and safety in acute cardiogenic pulmonary edema. Circulation. 1996;94(6):1316–1324. doi: 10.1161/01.cir.94.6.1316. [DOI] [PubMed] [Google Scholar]
- 230.Petersen JW, Felker GM. Inotropes in the management of acute heart failure. Crit Care Med. 2008;36(1, Suppl):S106– S111. doi: 10.1097/01.CCM.0000296273.72952.39. [DOI] [PubMed] [Google Scholar]
- 231.Scheidt S, Wilner G, Mueller H, et al. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a cooperative clinical trial. N Engl J Med. 1973;288(19):979–984. doi: 10.1056/NEJM197305102881901. [DOI] [PubMed] [Google Scholar]
- 232.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for endstage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 233.Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators. Advanced heart failure treated with continuous- flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 234.Combes A, Leprince P, Luyt C-E, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 235.Goldhaber SZ, Elliott CG. Acute pulmonary embolism: part I: epidemiology, pathophysiology, and diagnosis. Circulation. 2003;108(22):2726–2729. doi: 10.1161/01.CIR.0000097829.89204.0C. [DOI] [PubMed] [Google Scholar]
- 236.McIntyre KM, Sasahara AA. The hemodynamic response to pulmonary embolism in patients without prior cardiopulmonary disease. Am J Cardiol. 1971;28(3):288–294. doi: 10.1016/0002-9149(71)90116-0. [DOI] [PubMed] [Google Scholar]
- 237.Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48(1):23–33. doi: 10.1016/s0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 238.Piazza G, Goldhaber SZ. Management of submassive pulmonary embolism. Circulation. 2010;122(11):1124–1129. doi: 10.1161/CIRCULATIONAHA.110.961136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kasper W, Geibel A, Tiede N, Just H. Patent foramen ovale in patients with haemodynamically significant pulmonary embolism. Lancet. 1992;340(8819):561–564. doi: 10.1016/0140-6736(92)92102-l. [DOI] [PubMed] [Google Scholar]
- 240.Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17(4):259–270. doi: 10.1016/s0033-0620(75)80017-x. [DOI] [PubMed] [Google Scholar]
- 241.Torbicki A, Perrier A, Konstantinides S, et al. ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29(18):2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 242.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 243.Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30(5):1165–1171. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- 244.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23, Suppl 1):I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 245.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23, Suppl 1):I22–I30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 246.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416– 420. [PubMed] [Google Scholar]
- 247.Wicki J, Perneger TV, Junod AF, Bounameaux H, Perrier A. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med. 2001;161(1):92–97. doi: 10.1001/archinte.161.1.92. [DOI] [PubMed] [Google Scholar]
- 248.Le Gal G, Righini M, Roy P-M, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144(3):165–171. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 249.Chunilal SD, Eikelboom JW, Attia J, et al. Does this patient have pulmonary embolism? JAMA. 2003;290(21):2849–2858. doi: 10.1001/jama.290.21.2849. [DOI] [PubMed] [Google Scholar]
- 250.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363(3):266–274. doi: 10.1056/NEJMra0907731. [DOI] [PubMed] [Google Scholar]
- 251.Carlbom DJ, Davidson BL. Pulmonary embolism in the critically ill. Chest. 2007;132(1):313–324. doi: 10.1378/chest.06-1854. [DOI] [PubMed] [Google Scholar]
- 252.Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med. 2002;136(9):691– 700. doi: 10.7326/0003-4819-136-9-200205070-00012. [DOI] [PubMed] [Google Scholar]
- 253.Chartier L, Béra J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation. 1999;99(21):2779–2783. doi: 10.1161/01.cir.99.21.2779. [DOI] [PubMed] [Google Scholar]
- 254.Ghaye B, Szapiro D, Mastora I, et al. Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology. 2001;219(3):629–636. doi: 10.1148/radiology.219.3.r01jn32629. [DOI] [PubMed] [Google Scholar]
- 255.Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN. Pulmonary embolism: prognostic CT findings. Radiology. 2007;242(3):889–897. doi: 10.1148/radiol.2423051441. [DOI] [PubMed] [Google Scholar]
- 256.van der Meer RW, Pattynama PMT, van Strijen MJL, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3- month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235(3):798–803. doi: 10.1148/radiol.2353040593. [DOI] [PubMed] [Google Scholar]
- 257.Mercat A, Diehl J-L, Meyer G, Teboul J-L, Sors H. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med. 1999;27(3):540–544. doi: 10.1097/00003246-199903000-00032. [DOI] [PubMed] [Google Scholar]
- 258.Layish DT, Tapson VF. Pharmacologic hemodynamic support in massive pulmonary embolism. Chest. 1997;111(1):218–224. doi: 10.1378/chest.111.1.218. [DOI] [PubMed] [Google Scholar]
- 259.Bursztein S, Taitelman U, De Myttenaere S, et al. Reduced oxygen consumption in catabolic states with mechanical ventilation. Crit Care Med. 1978;6(3):162–164. doi: 10.1097/00003246-197805000-00008. [DOI] [PubMed] [Google Scholar]
- 260.Kress JP, O’Connor MF, Pohlman AS, et al. Sedation of critically ill patients during mechanical ventilation: a comparison of propofol and midazolam. Am J Respir Crit Care Med. 1996;153(3):1012–1018. doi: 10.1164/ajrccm.153.3.8630539. [DOI] [PubMed] [Google Scholar]
- 261.Spence TH, Newton WD. Pulmonary embolism: improvement in hemodynamic function with amrinone therapy. South Med J. 1989;82(10):1267–1268. [PubMed] [Google Scholar]
- 262.Boulain T, Lanotte R, Legras A, Perrotin D. Efficacy of epinephrine therapy in shock complicating pulmonary embolism. Chest. 1993;104(1):300–302. doi: 10.1378/chest.104.1.300. [DOI] [PubMed] [Google Scholar]
- 263.McDonald IG, Hirsh J, Hale GS, Cade JF, McCarthy RA. Isoproterenol in massive pulmonary embolism: haemodynamic and clinical effects. Med J Aust. 1968;2(5):201–205. doi: 10.5694/j.1326-5377.1968.tb82731.x. [DOI] [PubMed] [Google Scholar]
- 264.Jardin F, Genevray B, Brun-Ney D, Margairaz A. Dobutamine: a hemodynamic evaluation in pulmonary embolism shock. Crit Care Med. 1985;13(12):1009–1012. doi: 10.1097/00003246-198512000-00004. [DOI] [PubMed] [Google Scholar]
- 265.Manier G, Castaing Y. Influence of cardiac output on oxygen exchange in acute pulmonary embolism. Am Rev Respir Dis. 1992;145(1):130–136. doi: 10.1164/ajrccm/145.1.130. [DOI] [PubMed] [Google Scholar]
- 266.Webb SA, Stott S, van Heerden PV. The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intensive Care Med. 1996;22(4):353–355. doi: 10.1007/BF01700458. [DOI] [PubMed] [Google Scholar]
- 267.Ganière V, Feihl F, Tagan D. Dramatic beneficial effects of sildenafil in recurrent massive pulmonary embolism. Intensive Care Med. 2006;32(3):452–454. doi: 10.1007/s00134-005-0058-5. [DOI] [PubMed] [Google Scholar]
- 268.Capellier G, Jacques T, Balvay P, Blasco G, Belle E, Barale F. Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med. 1997;23(10):1089–1092. doi: 10.1007/s001340050461. [DOI] [PubMed] [Google Scholar]
- 269.Szold O, Khoury W, Biderman P, Klausner JM, Halpern P, Weinbroum AA. Inhaled nitric oxide improves pulmonary functions following massive pulmonary embolism: a report of four patients and review of the literature. Lung. 2006;184(1):1–5. doi: 10.1007/s00408-005-2550-7. [DOI] [PubMed] [Google Scholar]
- 270.Huet Y, Brun-Buisson C, Lemaire F, Teisseire B, Lhoste F, Rapin M. Cardiopulmonary effects of ketanserin infusion in human pulmonary embolism. Am Rev Respir Dis. 1987;135(1):114–117. doi: 10.1164/arrd.1987.135.1.114. [DOI] [PubMed] [Google Scholar]
- 271.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133(6, Suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 272.Verstraete M, Miller GA, Bounameaux H, et al. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation. 1988;77(2):353–360. doi: 10.1161/01.cir.77.2.353. [DOI] [PubMed] [Google Scholar]
- 273.Meneveau N, Séronde M-F, Blonde M-C, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006;129(4):1043–1050. doi: 10.1378/chest.129.4.1043. [DOI] [PubMed] [Google Scholar]
- 274.Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358(10):1037–1052. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 275.Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744–749. doi: 10.1161/01.CIR.0000137826.09715.9C. [DOI] [PubMed] [Google Scholar]
- 276.Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657–663. doi: 10.1378/chest.07-0665. [DOI] [PubMed] [Google Scholar]
- 277.Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005;129(5):1018–1023. doi: 10.1016/j.jtcvs.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 278.Eid-Lidt G, Gaspar J, Sandoval J, et al. Combined clot fragmentation and aspiration in patients with acute pulmonary embolism. Chest. 2008;134(1):54–60. doi: 10.1378/chest.07-2656. [DOI] [PubMed] [Google Scholar]
- 279.Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62(3):570–576. doi: 10.1097/TA.0b013e318031cd0c. [DOI] [PubMed] [Google Scholar]
- 280.Pengo V, Lensing AWA, Prins MH, et al. Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]