Abstract
The blockade of the PD-1 pathway can increase the production of interferon γ by tumor-specific T cells located within or in the proximity of the malignant lesion, thereby increasing the chemokine-dependent trafficking of immune effector cells. This can boost the efficacy of adoptive T-cell therapy to achieve superior antitumor responses.
Keywords: adoptive cell therapy, cancer immunotherapy, checkpoint blockade, melanoma, PD-1, PD-L1
An increasing knowledge of the molecular basis of oncogenesis and tumor progression has recently led to the emergence of several new FDA-approved antitumor drugs. These agents have revolutionized the treatment of melanoma patients, with the BRAF-specific inhibitor vemurafenib promoting clinical responses in more than 50% of patients bearing the BRAFV600 mutation.1 Oncogene-targeted therapies for patients with advanced solid tumors have been a very exciting development that can potentially apply to many other cancer types. While the development of drug resistance remains to be addressed, these findings have raised the bar for the next generation of anticancer therapies, from simply extending the survival time of patients to achieving prolonged, complete antitumor responses.
Employing rational combinations of agents appears to be a promising approach for improving long-term survival among melanoma patients. In line with this philosophy, we have focused our attention on combining—in a pre-clinical model—two among the most promising immunotherapies available to date, the blockade of the T-cell inhibitory PD-1 pathway and adoptive cell therapy (ACT). Among several immunotherapeutic strategies evaluated in the clinic over the past 30 years, PD-1 checkpoint blockade and ACT have induced the highest rates of objective responses in melanoma patients (~25% and ~50%, respectively).2-4 The rationale for this combination stems from the fact that the PD-1 pathway greatly contributes to immunosuppression in the tumor microenvironment, and hence presumably plays a role in the lack of clinical responses observed in some patients treated with ACT. Moreover, tumor-reactive T-cells used for adoptive transfer frequently express the inhibitory PD-1 receptor.
Similar to what is observed in cancer patients, in our pre-clinical ACT model we observed the upregulation of PD-1 by adoptively transferred tumor-specific T cells at the tumor site, but not in peripheral blood or lymphoid organs. Given that interferon γ (IFNγ) has been reported to upregulate the expression of PD-L1 by tumor cells in vitro, it is reasonable to expect that IFNγ produced by T cells would increase PD-L1 expression by malignant cells in vivo. These observations suggest that the activation of the PD-1 pathway serves as an important checkpoint influencing the effectiveness of ACT.5,6 Therefore, we hypothesized that combining PD-1 checkpoint blockade and ACT may lead to therapeutic synergy and hence to higher rates of complete and durable responses.
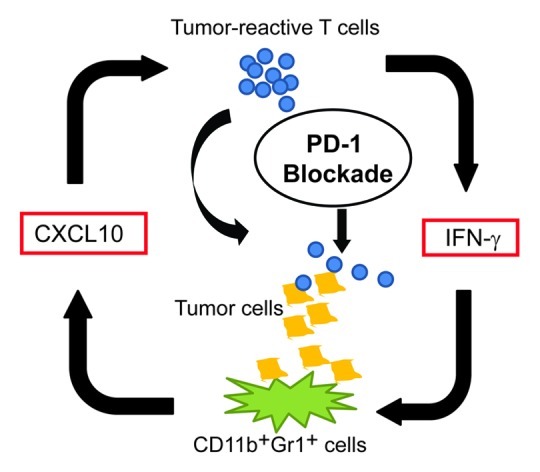
To test this hypothesis, we treated gp100-expressing tumor-bearing mice with adoptively transferred pmel-1 T cells that express a TCR specifically recognizing a gp100-derived peptide in an H-2Db-restricted manner.7 In two gp100-expressing tumor models, we found that mice treated with a combination of anti-PD-1 antibodies and ACT consistently showed increased numbers of transferred T cells at the tumor site and enhanced tumor regression, compared with mice treated with either agent alone. Although anti-PD-1 antibodies did not alter the number of immunosuppressive regulatory T cells (Tregs) or myeloid-derived suppressor cells (MDSCs), blockade of the PD-1 pathway resulted in increased production of IFNγ as well as of the IFNγ-inducible chemokine CXCL10. Our findings concur with recent data showing that anti-PD-1 antibodies can enhance IFNγ production at the tumor sites of mice receiving an anticancer vaccine.8 More interesting, our bone marrow chimera experiments with Ifngr1−/− mice and CXCL10-deficient mice implicated IFNγ-CXCL10 as a major nexus point in the control of PD-1 blockade-induced tumor infiltration by T cells. Although CXCL10 is secreted by various cell types, including neutrophils, monocytes and dendritic cells, we found that CD11b+ myeloid cells that share some surface markers with granulocytic MDSCs are the major source of CXCL10 in the tumor microenvironment of these models. Collectively, these data suggest that anti-PD-1 therapies may re-educate MDSCs, an important immunosuppressive cell population of the tumor microenvironment, to favor the recruitment of adoptively transferred, tumor-specific T cells, thus leading to an improved antitumor response (Fig. 1).
Figure 1.
Our preclinical results imply that immunotherapeutic regimens combining anti-PD-1 antibodies with ACT may achieve superior clinical responses in cancer patients. It will be interesting to test this approach in the context of oncogene-targeted (e.g., vemurafenib-based) therapies, as it is possible that—in response to immunostimulatory signals—tumor-reactive T cells might be able to control the growth of drug-resistant tumor cells.
Mechanism whereby PD-1 blockade may improve the efficacy of adoptive cell transfer. Blockade of the PD-1 pathway appears to trigger a positive feedback loop, increasing T-cell proliferation and interferon γ (IFNγ) levels. IFNγ in turn promotes the production of CXCL10 by bone marrow derived myeloid (CD11b+Gr1+) cells to enhance the intratumoral accumulation of effector T cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22691
References
- 1.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, et al. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217:385–93. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37. [DOI] [PMC free article] [PubMed]
- 7.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 Blockade Enhances T-cell Migration to Tumors by Elevating IFN-γ Inducible Chemokines. Cancer Res. 2012;72:5209–18. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omori R, Eguchi J, Hiroishi K, Ishii S, Hiraide A, Sakaki M, et al. Effects of interferon-α-transduced tumor cell vaccines and blockade of programmed cell death-1 on the growth of established tumors. Cancer Gene Ther. 2012;19:637–43. doi: 10.1038/cgt.2012.42. [DOI] [PubMed] [Google Scholar]


