Abstract
Malnutrition may manifest as either obesity or undernutrition. Accumulating evidence suggests that the gut microbiota plays an important role in the harvest, storage, and expenditure of energy obtained from the diet. The composition of the gut microbiota has been shown to differ between lean and obese humans and mice; however, the specific roles that individual gut microbes play in energy harvest remain uncertain. The gut microbiota may also influence the development of conditions characterized by chronic low-level inflammation, such as obesity, through systemic exposure to bacterial lipopolysaccharide derived from the gut microbiota. In this review, the role of the gut microbiota in energy harvest and fat storage is explored, as well as differences in the microbiota in obesity and undernutrition.
Keywords: microbiome, metagenome, obesity, malnutrition, absorption, energy metabolism
Malnutrition can be defined as either the inadequate or excessive consumption of dietary substances ultimately leading to the development of undernutrition or obesity, respectively, and their corresponding health sequelae. Selective pressures throughout evolution appear to have programmed animals to protect energy stores. In part, the obesity epidemic may be related to these physiologic biases such that, although the availability and stability of the food supply have improved over the past several centuries, humans remain physiologically predisposed to protect energy stores through the accumulation of adipose tissue. Therefore, as diets have changed and energy-dense foods have become readily available, obesity rather than undernutrition has become the primary concern in developed nations.
The microbes present within the gastrointestinal tract (ie, gut microbiota) have coevolved with the human host to perform a number of functions the host would otherwise be unable to accomplish on its own. Although incompletely understood, the gut microbiota is implicated in a variety of host functions involving intestinal development and function, micronutrient synthesis, and drug metabolism. Accumulating evidence suggests that the gut microbiota also plays an important role in the harvest, storage, and expenditure of energy obtained from the diet. The preponderance of the evidence demonstrates that germ-free mice are protected against obesity and that the transfer of gut microbes from conventionally raised animals to germ-free animals results in dramatic increases in body fat content and insulin resistance. Moreover, the composition of the gut microbiota has been shown to differ between lean and obese humans and mice and to change rapidly in response to dietary factors. The gut microbiota may also influence the development of conditions characterized by chronic low-level inflammation, such as obesity and type 2 diabetes, through systemic exposure to bacterial lipopolysaccharide derived from the intestinal microbiota.
To better define our nutrition status, an understanding of our microbial differences and their origins is necessary. In this review, the role of the gut microbiota in energy harvest and fat storage will be explored as will differences in the microbiota in obesity and undernutrition. Finally, potential mechanisms for modifying the gut microbiota as a therapeutic strategy for undernutrition, obesity, and other metabolic disorders will be examined.
Development and Plasticity of the Commensal Gut Microbiota
The gut microbiota can be classified into 3 domains based on molecular phylogeny (ie, 16S ribosomal ribonucleic acid [rRNA] sequence similarities and differences): Eukarya, Bacteria, and Archaea. Recall that Eukarya consists of organisms with cells that contain complex structures enclosed within membranes, most notably the nucleus. In contrast, Bacteria are the predominant members of the gut microbiota. The sequencing of 16S rRNA genes from amplified bacterial nucleic acids extracted from fecal material or mucosal samples using high-throughput techniques has greatly improved the ability to identify and classify bacteria.1 Using these techniques, the gastrointestinal tract in an individual adult human has been estimated to contain approximately 500 to 1000 distinct bacterial species.2,3 Two divisions of Bacteria, Bacteroidetes and Firmicutes, dominate, accounting for >90% of all phylotypes, whereas a single hydrogen-consuming methanogen, Methanobrevibacter smithii, dominates the Archaea domain.3
Infancy is characterized by microbial plasticity, whereby the rapid colonization by groups of microbes can change in response to events such as illness, medications, or changes in diet. The age-related mechanisms involved in the development of the adult human microbiota are poorly understood. Current evidence suggests that the gastrointestinal microbiota is established within the first few years of life in a process that is influenced by a variety of host (genetics) and external factors (diet, environmental exposures).4,5
In the newborn, most bacterial species are acquired during the birthing process. Birth by vaginal delivery or caesarean section (C-section) affects the newborn’s initial microbial profile. The vaginal microbial communities appear to change during pregnancy to provide newborns with beneficial microbes; at the time of delivery, the vagina is dominated by Lactobacillus and Prevotella spp.6 Vaginally delivered newborns are initially colonized with the same microbiota as their mothers and only later develop the distinct microbial communities found as adults. In contrast, infants born by C-section harbor microbial communities that initially resemble those of the skin, comprising Staphylococcus, Corynebacterium, and Propionibacterium spp,5,6 and maintain differences in their gut microbiota for several months after birth. These differences in the microbial communities resulting from the method of delivery may have important implications for infant development and health, including the observation of an increased susceptibility to certain pathogens and atopic diseases in babies delivered by C-section.7,8
Most important in the transformation of the microbiota from the infant to the adult type are effects of the microbiota itself, developmental changes in the gut environment, and the transition to an adult diet.9 The specific concentration and type of bacteria within the gastrointestinal tract are influenced by microhabitat variations throughout the gut involving such factors as pH, oxygen content, and exposure to bile, pancreatic secretions, and nutrients. The microbial habitats of the host select for a group of microbial communities from the microbiota available for colonization, and host genetics influence the composition of the microbiota by influencing the environmental conditions of the habitats.10 Bacterial counts rise from the proximal to the distal end of the gastrointestinal tract, and a gradual transition from aerobic to anaerobic organisms occurs in more distal segments of the gut. Once across the ileocecal valve, bacterial counts rise from 107–109 organisms/mL in the terminal ileum to approximately 1010–1012 organisms/mL in the colon.11
Diet is one of the most important determinants of microbial diversity within the gut.12 A recent study comparing the gut microbiome of rural children in Burkina Faso with children in Italy showed that Bacteroidetes were far more abundant in the microbiomes of African children and that the specific types that were increased were well suited to harvest energy from their plant-rich diet.13 These findings raise the possibility that microbiomes vary geographically with their hosts, in part because they are adapted to local diets. Other dietary factors that have occurred over the past few decades that have likely affected the human gut microbiome include differences in overall energy intake and the relative proportions of the different macronutrients and micronutrients in the diet, as well as differences in food modification and preparation.
Although the gut microbiota is thought to remain fairly stable throughout the lifetime of an individual,14 transient changes may occur, and some factors can lead to long-term changes in the human gut microbiota. In particular, antibiotics have a dramatic effect on the gut microbiota, and restoration to its prior diversity varies among individuals.15 Changes in diet may also cause significant, and potentially long-term, alterations in the gut microbiota. Jumpertz et al16 recently showed that in as short as 3 days, the gut microbiota structure in humans can be drastically altered by variations in calorie intake (from 2400–3400 kcal/d). A study of mice recipients of microbiome transplantation also showed that switching from a low-fat, plant polysaccharide diet to a high-fat, high-sugar Western diet altered gut community structure and metabolic function within 1 day.17 Aging itself also has an effect on the stability of gut microbial communities. Aging is associated with reduced immune function, increased disease and use of medications, and changes in nutrition—all of which may modify the gut microbiota.18 Nevertheless, the general intraindividual stability of the gut microbiota is largely based on recognition and tolerance of the infant-acquired microbiota by the gut immune system, which, by being exposed to and sampling microbial antigens, identifies these as normal.19 In contrast to the intraindividual stability described, interindividual differences in intestinal microbiota show more diversity, as do differences between luminal (ie, stool) and mucosal (ie, epithelial) compositions.3 Importantly, comparative studies of the gut microbiota of monozygotic and dizygotic twins have emphasized the influence of host genotype over diet, age, and lifestyle,20 although conflicting information exists.21
Despite these interindividual differences in gut microbiota, findings from a recent study provide further insights between human fecal microbiomes across different geographic populations by identifying 3 robust clusters referred to as “enterotypes.”22 The enterotypes were found to be mostly driven by species composition; however, functional differences were also important, reflecting a possible impact on symbiotic interrelations with the human host. Interestingly, host properties such as body mass index, age, and gender did not explain the observed enterotype clusters. The presence of these 3 enterotypes was recently confirmed23 and was shown to be linked to long-term (but not short-term) dietary patterns, particularly protein and animal fat vs carbohydrates.
Role of Microorganisms in Energy Regulation
Production of Short-Chain Fatty Acids and Hydrogen Metabolism
The stomach and proximal small intestine are responsible for most nutrient digestion and absorption in humans. In an otherwise healthy individual without prior surgical resection of the small bowel, about 85% of carbohydrates, 66%–95% of proteins, and all fats are absorbed before entering the large intestine.24,25 The indigestible carbohydrates and proteins that the colon receives represent from 10%–30% of the total ingested energy26,27 and, without the activity of the colonic microbiota, would generally be eliminated via the stool without further absorption because the human large intestine has limited digestive capability.
In the colon, microorganisms ferment starch (including resistant starch), unabsorbed sugars, cellulosic and noncellulosic polysaccharides,28 and mucins29 into short-chain fatty acids (SCFAs)30 and gases such as CO2, CH4, and H2.31,32 The type and quantity of SCFA and gases produced in the gut depend on multiple factors,32 including age, diet,33 especially the availability of nondigested carbohydrates,34 the gut microbial community composition,35 gut transit time,36 pH of the colon,37 and the segment of the colon.38 The major SCFAs produced as a result of carbohydrate and protein fermentation are acetate, propionate, and butyrate.28 In addition to the major SCFAs, formate, valerate, caproate, isobutyrate, 2-methyl-butyrate, and isovalerate can be produced during the breakdown of branched-chain amino acids.28
Microorganisms in the gut work in symbiosis; the manner in which they interact with each other and with their environment will determine the final metabolic and environmental outcome. Because much of the gut is anaerobic, disposal of H2 strongly influences microbial interactions. As illustrated in Figure 1, fermentation breaks down complex organic compounds producing SCFA and H2. H2 is then excreted in the breath39 and/or consumed (oxidized) by 3 groups of microorganisms: methanogens, acetogens (homo-acetogens), and/or sulfate reducers, all of which coexist in the colon in differing proportions.40 These H2-based interactions are critical for fermentation to proceed because accumulation of H2 in the colon inhibits further fermentation.30 Our group and others have verified the enrichment of H2-oxidizing methanogens in obese compared with normal-weight individuals.41–43 Of the homo-acetogens detected in the human gut,44–46 many belong to the Firmicutes phylum, and this may partly explain why an increase in Firmicutes has been demonstrated in obesity.47 Sulfate-reducing bacteria (SRB) may be present within the gut even when sulfate is absent as an electron acceptor. In this case, they act as fermenters and, in some instances, may function as H2-producing acetogens, 48 which convert acetate to CO2 and H2.
Figure 1.
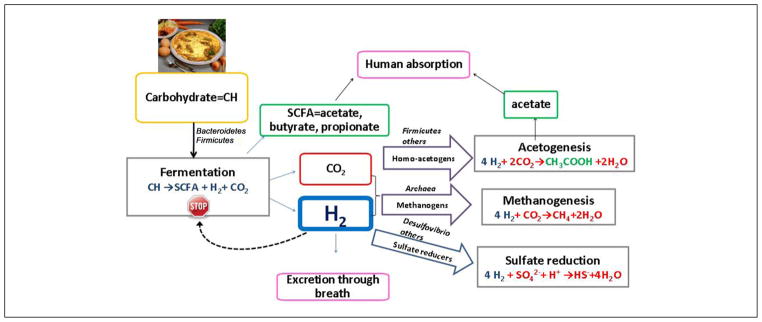
Microbial H2-producing and consuming reactions in the human intestine. SCFA, short-chain fatty acid.
With reference to the 3 enterotypes described previously,22 each enterotype was defined by the variation of 3 genera: Bacteroides (enterotype 1), Prevotella (enterotype 2), and Ruminococcus (enterotype 3).22 The key microorganisms within each enterotype are involved in degradation of polymers such as plant carbohydrates. The principal microorganisms in enterotypes 1 and 2 interact with other community members to achieve sugar or mucin degradation and are intimately involved in H2 transfer and disposal. Interestingly, the enriched genera within each enterotype were shown to use different routes to generate energy from fermentable colonic substrates, suggesting that they could be triggered by the 3 distinct pathways for hydrogen disposal described above. Indeed, despite their low abundance, the sulfate reducer, Desulfovibrio, and methanogen, Methanobrevibacter, were found to be enriched in enterotypes 1 and 3, respectively.
Absorption of Monosaccharides and SCFA by the Host Epithelium
Monosaccharides are directly absorbed by the intestinal epithelium via monosaccharide transporters.49,50 In a healthy individual consuming a typical Western diet, about 100–200 mM SCFAs are produced per day in the large intestine,51,52 of which about 90%–95% are absorbed in the colon.25 The molar ratio of acetate to propionate and to butyrate varies around 40:40:20 to 75:15:10 depending on the diet consumed.26,28,53 The absorption of SCFA is an efficient process involving passive diffusion or ion exchange.51,54
Absorbed SCFAs are used as energy for the colonocytes or transported to various peripheral tissues for further metabolism.32 Butyrate is the colonic epithelial cells’ preferred nutrient for their metabolism and development.51,52 Substantial amounts of propionate traverse the colonocyte and are transported to the liver,55 where it serves as a substrate for gluconeogenesis or regulates cholesterol synthesis.55,56 Acetate is the principal SCFA in the blood and is an important energy source to peripheral tissues, including the liver, where acetate is used for lipogenesis and cholesterol synthesis.26,51 SCFAs absorbed in the colon contribute 6%–10% of the entire energy requirements in humans, and their contribution likely increases in humans who ingest more dietary fiber.26,57 Although SCFAs account for a relatively small portion of energy acquisition, their impact on energy balance in humans is significant because of other roles they play in energy regulation as discussed in the following sections.56
SCFAs and Obesity
The abundance of SCFAs and their concentration in the colon has recently been associated with the health of humans31,32,58 and, pertinent to this review, linked to obesity.59,60 Transplantation of the gut microbiome from obese and lean mice to germ-free mice resulted in higher acetate and butyrate production in obese microbiome recipients.61 In humans, Schwiertz et al35 reported a greater concentration of total SCFAs, particularly propionate, in fecal samples of obese adults compared with their lean counterparts. These observations are supported by a study conducted in obese and normal-weight children.60 Although fecal acetate, glucose, and lactate concentrations were relatively similar in both groups, butyrate, propionate, and intermediate fermentation compounds such as formate and isobutyrate were significantly higher in the obese children.
Host-Microbial Mutualism in Energy Harvest
Microbes in the large intestine allow the host to salvage energy from otherwise indigestible carbohydrates and proteins by providing a variety of enzymes required for their metabolism.62,63 For example, Bacteroides thetaiotaomicron (B theta), a prominent commensal gut microbe, produces 226 predicted glycoside hydrolases and 15 polysaccharide lyases, whereas the human genome only contains 98 potential glycoside hydrolases.63,64 Therefore, the gut microbiota provides the human host an ability to degrade plant polysaccharides, enhancing the host’s energy balance. The genes involved in the metabolism of starch, sucrose, glucose, galactose, fructose, arabinose, mannose, and xylose, as well as fucose from host mucus, are enriched in the distal colon microbiome.2 Cluster of Orthologous Groups (COG) analysis has shown that the gut microbiome is dominated by genes that drive production of acetate, butyrate, lactate, and succinate.2 The gut microbiome is also enriched in genes that code for amino acid and vitamin synthesis and enzymes that detoxify xenobiotics such as β-glucosidase.2
Enrichment of some bacterial genes in the gut microbiome has recently been linked to obesity. In an obese microbiome, Eubacterium rectale (a Firmicutes) genes that encode for primary fermentation enzymes that digest dietary polysaccharides, α- and β-galactosidases that generate acetate and butyrate, and ABC transporters were all found to be enriched.61 Another study involving monozygotic and dizygotic twins showed a higher abundance of Bacteroidetes and an enrichment of genes related to carbohydrate metabolism in lean individual microbiomes, whereas Firmicutes dominated the obese microbiome, which was enriched with genes related to nutrient transporters.21 In addition to a transporter-related mechanism, members of Firmicutes such as the Ruminococcus genus can also degrade cellulose and produce acetate, succinate, and ethanol.65
Fat Storage: Lipoprotein Lipase Activity and Gut Microorganisms
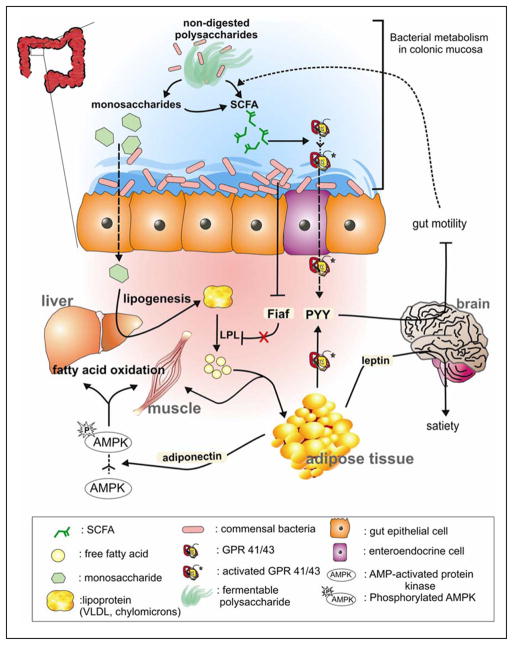
Intestinal microbes affect energy balance through metabolites they produce by regulating gene expression via complex mechanisms initiated by monosaccharides and SCFAs (Figure 2).64 The gut microbiota induces monosaccharide cellular uptake66 and stimulates hepatic triglyceride production (lipogenesis) by activating the transcription factors, carbohydrate response element binding protein (ChREBP), and sterol response element binding protein (SREBP).50,67 The product of hepatic lipogenesis, triacylglycerols, are secreted from the liver to the bloodstream in the form of very low-density lipoprotein (VLDL) and chylomicrons and influence host nutrient balance and insulin resistance.
Figure 2.
The gut microbiome has a regulatory function on host energy metabolism. By breaking down nondigestible polysaccharides, gut microorganisms produce monosaccharides and short-chain fatty acids (SCFAs). SCFAs bind to GPR 41/43 receptors and stimulate peptide YY (PYY) production, which inhibits gut motility and allows gut microbes to digest more polysaccharides. Gut microbes also regulate energy metabolism by reducing the expression of fasting-induced adipocyte factor (Fiaf) from gut epithelial cells. Suppressed Fiaf release results in the degradation of lipoproteins and deposition of free fatty acids in adipose tissues. The adiposity in liver and skeletal muscles is also regulated by microorganisms through the changes of phosphorylated adenosine monophosphate-activated protein kinase (AMPK) levels. LPL, lipoprotein lipase; VLDL, very low-density lipoprotein.
SCFAs also act as signaling molecules that interact with the G-protein-coupled receptors, Gpr41 and Gpr43, expressed on adipocytes and intestinal epithelium.68–70 Propionate and butyrate have higher activity than acetate toward the Gpr41 receptor; however, all 3 SCFAs share equal potency toward GPR43.68,71 Following Gpr41 activation, SCFAs stimulate leptin expression, which suppresses appetite.70 Samuel et al72 emphasized the role of Gpr41 as a key regulator between microbial-host communications. In their investigation, mice deficient in Gpr41 (Gpr41−/−) weighed significantly less than germ-free and wild-type mice when both types of mice were colonized by gut microbes.72 It was speculated that the weight gain in the wild-type mice was due to an increased expression of the gut-derived hormone, peptide YY (PYY), which inhibits gut motility, thereby allowing more intestinal epithelial contact time in which to extract and absorb energy.72,73 Although Gpr43 binding by SCFAs is known to inhibit inflammatory responses,74 it also participates in energy regulation. Xiong et al70 observed an increase in both leptin expression and adipogenesis via interactions with Gpr43 in the adipose tissue of mice.75
Energy balance is also associated with another key modulator, fasting-induced adipocyte factor (Fiaf). Reduced expression of Fiaf induces the activity of circulating lipoprotein lipase (LPL),50 which hydrolyzes circulating triacylglycerols to free fatty acid (FFA) at various peripheral tissues.76 The distribution of FFA deposit is not well known, but a high ratio of adipose tissue to muscle-LPL activity directs more FFA to adipose tissues, while a low ratio induces more FFA deposit to muscles.77,78 The expansion of adipose tissue, in turn, results in an inability to store surplus FFA, which raises blood FFA levels and contributes to insulin resistance.79 Backhed et al80 showed that B theta monocolonized mice increased their body fat because of bacterial suppression of Fiaf.22 Germ-free (GF) knockout mice lacking Fiaf (Fiaf−/−) have also been shown to be susceptible to diet-induced obesity because of a lower expression of the peroxisomal proliferator-activated receptor coactivator (Pgc-1α) that increases expression of genes regulating fatty acid oxidation.
Adipocytes have an important role in obesity as a source of hormones, such as leptin and adiponectin.81 Adipocytes sense the increase of SCFA levels produced by intestinal microbe fermentation and respond by inducing leptin production. Leptin signals the brain to regulate the appetite and energy expenditure, thereby tightly connecting host energy balance.82 Adiponectin is associated with adenosine monophosphate-activated protein kinase (AMPK), an enzyme that monitors cellular energy status and stimulates fatty acid oxidation in peripheral tissues. Increased AMPK activity was shown to prevent GF mice from diet-induced obesity.80 In obesity, the level of adiponectin decreases,83 causing a deactivation of AMPK and leading to a reduction in fatty acid oxidation and an increased influx of free fatty acids into the liver.84
Gut Microbes and Undernutrition
Worldwide, nearly a billion people suffer from undernutrition of varying degrees.85 Undernutrition may present with a spectrum of clinical manifestations ranging from asymptomatic micronutrient deficiencies to severely symptomatic protein-energy malnutrition in the forms of kwashiorkor and marasmus. Importantly, undernutrition contributes in some manner to over half the deaths occurring annually in the nearly 10 million children younger than age 5 years.86 Even the children who survive periods of undernutrition can suffer long-term sequelae, including growth retardation and neurodevelopmental deficits.87 Furthermore, the exposure to undernutrition in utero may lead to large and long-term negative mental and physical sequelae.88 Although food availability, socioeconomic factors, and genetics influence the development of undernutrition, the underlying mechanisms remain poorly defined. The gut microbiota may contribute to the risk and pathogenesis of undernutrition through effects on nutrient metabolism and immune function. In addition, under-nutrition in childhood could affect the development of the gut microbiome and its metabolic capabilities, leading to metabolic dysfunction and contributing to the sequelae of undernutrition. Very little is known about the microbial diversity in undernutrition. Further study in this area is critical because identifying how undernutrition affects the gut microbiota will likely have important implications for improving its clinical consequences. Furthermore, modifying the gut microbiota may be used as a strategy to counter undernutrition.
Gut Microbes and Obesity
Abundance of Microbial Divisions and Important Functions in the Host
The development of metagenomics and high-throughput sequencing has expanded our knowledge of the microbial composition and gene content of the gut microbiome and has led to insights into the role of the gut microbiota in health and disease.36 Major differences in the abundance of major phyla, reduction in bacterial diversity, and changes in bacterial gene expression and, therefore, the metabolic function of the distal gut have been detected in association with numerous disease states, including obesity.17,89 Based on a recent metagenomics study, 75% of microbial genes associated with obesity belonged to Actinobacteria and 25% to Firmicutes, whereas 42% of genes associated with leanness belonged to Bacteroidetes.17
Comparisons of the dominant phyla from obese and lean gut microbiomes have produced conflicting results. Although some studies involving mouse models61,89 and humans21,90 have demonstrated a significant increase in the ratio of Firmicutes to Bacteroidetes in the obese microbiome, others16,43,91,92 have not observed a change in this ratio or the abundance of any specific phyla. For example, 16S rDNA sequencing performed on genetically obese (ob/ob) and lean mice revealed major differences in the microbial community structure between obese and lean mice at the phyla level.89 Specifically, genetically obese (ob/ob) mice had at least a 50% reduction of Bacteroidetes and a significant increase of Firmicutes.89 In another study, obese mice exhibited more Firmicutes (71%) and less Bacteroidetes (26%) than ob/+ and +/+ mice despite similar chow consumption and energy expenditure.90 Phylum-level alterations between obese and lean microbiomes have also been observed with human studies. Reduction of Bacteroidetes41,93 and increase in Actinobacteria21 and Prevotella93 were observed with obesity. In contrast, when a group of anorexic individuals was compared with lean and obese individuals, the abundance of Firmicutes did not correlate with obesity.41 These phylogenetic differences may arise from many variables, including diet, sample handling, sequencing techniques, and data analysis tools.
Interestingly, the shifts in gut microbiota based on body mass index (BMI) at the division level do not always correlate with the shifts at lower (more defined) taxonomic levels such as the genus level. For example, a reduction in Bacteroidetes does not mean there cannot be an increase in the Bacteroides genus. Indeed, Bacteroides strains known to degrade plant polysaccharides were found to be more abundant in obese adults35 but were not significantly different between obese and nonobese Indian children.94 Another plant polysaccharide (cellulose) degrader, Ruminococcus spp,65 which belongs to Firmicutes, and Bifidobacterium were present in lower abundance in obese individuals.35,95 Higher counts of the Lactobacillus genus were observed in some obese individuals41,95 in contrast to no difference between obese and non-obese Indian children.94 Abundance of the Prevotella genus, an H2-producing genus, did not correlate with obesity among children; however, it was more abundant in obese adults.43 Finally, fecal counts of Faecalibacterium prausnitzii were higher in obese than in nonobese children.94
M smithii, the dominant archaeon in the human gut, is associated with increased energy harvest and adiposity.96 Two scenarios may help to explain the differences in the abundance of M smithii noted in obese and lean microbiomes. In the first scenario in which M smithii is more abundant in the obese microbiome than in the lean microbiome,35,42,43 it may be hypothesized that obese individuals produce more SCFAs due to an increased efficiency of the fermentation process caused by the increased concentration of M smithii. In the second scenario in which underweight individuals harbor more M smithii than obese individuals,41,95 it may be hypothesized that a lower calorie intake stimulates enhanced energy extraction through more efficient fermentation and SCFA production via M smithii.
Diet and Its Effect on Gut Microorganisms
The effect of diet on the pathogenesis of obesity is a key contributing factor; however, the impact of diet on the gut microbiome structure remains poorly understood. Studies using high-throughput sequencing to compare variations in microbial community composition in animals and humans following different diets13,97 suggest that differences in the diet modify the relative abundance of gut microorganisms. High-fat, high-sugar (ie, Western-type diet), or high-plant polysaccharide-containing diets have been shown to significantly alter the microbiome composition at different phylogenetic levels.
Genetically modified mouse models that display obesity-resistant or obesity-prone phenotypes when fed a high-fat diet have been employed to better identify the effect of diet on gut microbial composition independent of the obese state. When fed a high-fat diet, the microbiomes of both Sprague-Dawley rats98 and RELMβ knockout (KO) mice,99 which do not become obese despite ingesting a high-fat diet, were found to be enriched in Clostridiales in the Firmicutes phylum. The relative abundance of Bacteroidales in the Bacteroidetes phylum was also higher in rodents fed with a high-fat diet, independent of the phenotype.98 From the Proteobacteria phyla, Enterobacteriales were significantly more abundant in obesity-prone rats than obesity-resistant and low-fat-fed animals; however, the total number of bacteria based on 16S rDNA copy numbers was lower in rats that were fed a high-fat diet.98 A high-fat diet significantly reduced phylogenetic orders, including Bacteroidaceae, Prevotellaceae, and Rickenellaceae,99 while transiently increasing Bifidobacterium and Actinobacteria orders (see Table 1). These studies confirm the strong correlation between high-fat diet and the variations in gut microbial community composition.
Table 1.
Impact of the Diet on the Abundance of the Gut Microbiota
| Donor | Sample | Phenotypic Changes | Diet | Important Changes in Microbiota |
|---|---|---|---|---|
| Sprague-Dawley rats98 | Cecal | Increase in body fat on obesity prone rats | High fat |
Bacteroidales ↑ Clostridiales ↑ |
| Mice97 | Fecal | Increase in body fat | Western | Eubacterium dolichum ↑ |
| RELMβ KO and wild-type mice99 | Fecal | Tendency to obesity in wild-type mice RELMβ KO mice not affected |
Chow to high fat |
Clostridiales ↑ Mollicutes ↑a Desulfovibrionaceae ↑ Bacteroidaceae ↓ Prevotella ↓ Rickenellaceae ↓ |
| ob/ob mice33 | Fecal and cecal | Increase in body weight | High fat |
Bifidobacteria ↑ Actinobacteria ↑ Proteobacteria ↓ |
| Gnotobiotic mice (obese human microbiome)17 | Fecal | Increase in adiposity | Low fat to Westernb |
Bacilli (Enterococcus) ↑ Erysipelotrichi ↑ |
| Gnotobiotic mice (co-colonization)157 | Cecal | NA | Western to low- fat, high-plant polysaccharide |
Eubacterium rectale ↓ Bacteroides thetaiotaomicron = |
| Normal-weight individuals100 | Fecal | Stable weight | Low carbohydrate High carbohydrate |
Bifidobacterium ↓ Fecal Lactobacillus = Fecal Lactobacillus = |
| Normal-weight individuals58 | Fecal | Stable weight | Low carbohydrate, high protein | Roseburia/E rectale ↓ |
| Obese men158 | Fecal | NA | Resistant starch in comparison with NSP Weight loss diet |
E rectale ↑ Ruminococcus bromii ↑ Oscillibacter valericigenes ↑ Bacteroides =Faecalibacterium prausnitzii = Collinsella aerofaciens ↓ |
| Obese men101 | Fecal | NA | High protein, low carbohydrate to medium carbohydrate |
Roseburia/Eubacterium ↑ Bifidobacterium ↑ |
| Children13 | Fecal | NA | High fiber, polysaccharide |
Prevotella ↑ Xylanibacter ↑ |
KO, knockout; NA, not available; NSP, nonsugar polysaccharide; ↑, increase; ↓, decrease; =, no change observed.
Significantly less important.
Western diet refers to high-sugar and high-fat diet.
Carbohydrate intake also alters the gut microbiome composition. Bifidobacterium levels decreased in normal-weight mice that were switched from a high- to a low-carbohydrate diet, and the fecal Lactobacilli, often associated with the obese phenotype, did not change as a result of either diet.100 Low carbohydrate accompanied by high protein in the diet led to significant reduction in the Roseburia/E rectale group, which might negatively affect host health by leading to a reduction in butyrate production.58
As would be expected, the introduction of a diet high in both fat and sugar (ie, Western diet) also results in changes in the gut microbiome. Mice that underwent transplantation with human gut microbiota and were switched from a low-fat plant polysaccharide diet to a Western diet exhibited a higher abundance of Bacilli (mainly Enterococcus) and Erysipelotrichi from Firmicutes and less Bacteroidetes.17 In a study in which mice became obese following consumption of a Western diet, pyrosequencing also demonstrated an increase in Firmicutes owing mostly to a bloom in the Mollicutes class.97 The impact of Western and plant polysaccharide-rich diets on the gut microbiota was investigated in a study of children in the European Union and Burkina Faso, Africa. African children consuming a high-fiber, polysaccharide diet had a higher Bacteroidetes/Firmicutes ratio in their gut microbiota than did European Union children consuming a high-fat, high-protein diet. Although Actinobacteria were found to be more abundant in African children, Proteobacteria were more abundant in European children. African children also had an enriched reservoir of the genera Prevotella and Xylanibacter accompanied by high SCFA production. The nutrient-rich, fiber-poor Western diet reduced the diversity of the gut microbiota, whereas the fiber-rich diet was associated with an increased diversity of the gut microbiota, resulting in a concomitant increase in the diversity of enzymes that can produce a variety of SCFAs.13
Shifts in gut microbial composition due to dietary variations have been observed at different phylogenetic levels. At the phylum level, the abundance of 2 major phyla, Bacteroidetes and Firmicutes, in response to changes in the diet has not been clearly established. Many studies with different diet regimens in both mice and humans have shown mixed findings. For example, in a weight loss intervention study with obese individuals, the Bacteroidetes/Firmicutes ratio did not change following 4 weeks of low-carbohydrate followed by another 4 weeks of medium-carbohydrate weight loss diets.91 In contrast, Turnbaugh et al97 showed a reduced abundance of Bacteroidetes following a low-carbohydrate weight loss diet, and Duncan et al101 found that a low-carbohydrate, high-protein diet also reduced the abundance of Bacteroidetes.
Gut Microbes, Endotoxemia, Systemic Inflammation, Innate Immunity, and Obesity
Endotoxin (lipopolysaccharide [LPS]), derived from the cell wall of gram-negative bacteria, circulates at low concentrations in the blood of healthy individuals. The presence of genetic and diet-induced obesity and other metabolic disorders has been associated with a substantial increase in LPS concentrations in the blood, a condition termed metabolic endotoxemia.102,103 Obesity and a number of other metabolic disorders are characterized by chronic, systemic, low-grade inflammation, possibly related to this metabolic endotoxemia.
Consumption of a high-fat meal in both animals and humans results in both changes to the gut microbiota composition, as previously described, and significant increases in endotoxin concentrations.104,105 It has been suggested that the increases in systemic endotoxin levels may result from increased intestinal permeability caused by the compositional changes in the microbiota.103 Endotoxemia may then contribute to the low-grade inflammation, insulin resistance, adipocyte hyperplasia, and decreased β-cell function that characterize the metabolic syndrome. Further supporting this hypothesis is a recent study in which antibiotics were administered to both high-fat-fed and ob/ob mice.106 Reduced levels of endotoxemia and cecal LPS content were found as were a decrease in intestinal permeability, reductions in glucose intolerance, body weight gain and fat mass development, and a decrease in markers of inflammation, oxidative stress, and infiltration of macrophages into visceral adipose tissue. A recent report suggests that these effects may be mediated, at least in part, by interactions between LPS and adipose tissue metabolism through endocannabinoid-driven adipogenesis.107
The innate immune system also appears to play a role in both regulating the gut microbiota and, by extension, influencing the development of metabolic disorders. Toll-like receptors (TLRs) are highly expressed transmembrane proteins in the innate immune system that recognize structurally conserved molecules derived from microbes. TLR4, which recognizes LPS, knockout mice have been shown to be resistant to LPS and diet-induced weight gain.106 Similarly, loss of function mutations in TLR4 prevent diet-induced obesity and insulin resistance.108 Mice genetically deficient for TLR5, which recognizes bacterial flagellin, exhibit hyperphagia, features of metabolic syndrome, and significant changes in the composition of the gut microbiota.109 Furthermore, transfer of the gut microbiota from TLR5-deficient mice to wild-type, germ-free mice was shown to confer features of the metabolic syndrome. Finally, removal of the expression of TLR2, which recognizes a number of microbial products, was shown to protect mice from diet-induced adiposity, insulin resistance, hypercholesterolemia, and hepatic steatosis and was also associated with attenuation of adipocyte hypertrophy.110 Notably, members of the phyla Bacteroidetes, Proteobacteria, and Actinobacteria were more abundant in mutant mice than in wild-type mice,111 consistent with previous studies suggesting that the relative proportion of Bacteroidetes is reduced in obese mice and humans.
Weight Reduction and Gut Microorganisms
The gut microbiome is responsive to weight loss.90 Weight loss programs involve not only calorie restriction and changes in the macronutrient composition of the diet but are also generally accompanied by an increase in physical exercise, another factor influencing gut microbiota composition and further complicating our knowledge of the direct effects of diet changes on the gut microbiota. Following a 10-week weight loss program supplemented with exercise, adolescents who achieved both high weight loss and low weight loss demonstrated increased counts of Bacteroides fragilis and Lactobacillus and decreased counts of Clostridium coccoides and Bifidobacterium longum.112 Another study from the same group showed reduced counts of Clostridium histolyticum, E rectale, and C coccoides and increased Bacteroides-Prevotella associated with weight loss >4 kg.113
Gastric Bypass Surgery and Gut Microorganisms
The mechanisms of action of surgical weight loss procedures are incompletely understood but clearly are more complex than their typical division into restrictive and malabsorptive. The alterations in bowel anatomy and physiology that occur following certain operations such as the Roux-en-Y gastric bypass (RYGB) cause changes to the gut microbiota that may have relevance for energy harvest and storage postoperatively,43,114,115 and these bariatric operations may represent good models to study adaptations of the intestinal microbiota to dietary variations, metabolism, and systemic inflammation.93
Quantification of bacteria based on culture-dependent methods in the bypassed stomach and proximal gastric pouch revealed bacterial overgrowth and an increase in the pH in the functioning proximal stomach following RYGB.114 Using both Sanger sequencing and high-throughput sequencing, our group demonstrated altered microbial composition of the distal gut following RYGB.43 In comparison with obese and normal-weight control groups, individuals who underwent bariatric surgery and achieved successful weight loss harbored proportionally more Gammaproteobacteria and less Clostridia and Verrucomicrobia. In another recent study, when microbiome composition changes following RYGB were monitored using quantitative polymerase chain reaction (qPCR)–based methods over 6 months at 3-month intervals, Bacteroides and Prevotella counts decreased and approached the counts from healthy, normal-weight control individuals even though the RYGB individuals had not achieved normal body weight.93 In contrast, at 3 and 6 months after the surgery, Bifidobacterium and Lactobacillus/Leuconostoc/Pediococcus counts decreased. Bifidobacterium levels in RYGB patients were lower at 6 months than the normal-weight control group,93 a finding that corresponds well with the lower abundance of Bifidobacterium generally found in obese individuals.95
Microorganisms With Antiobesity Effects
Recent studies in mice and humans suggest that the dietary inclusion of beneficial bacteria (ie, probiotic agents) with antiobesity effects may also help to reduce body weight.116–118 Lactobacillus species, Lactobacillus rhamnosus PL60 and Lactobacillus plantarum PL62, were reported to reduce the fat content of mice adipose tissue based on their ability to produce conjugated linoleic acid (CLA).119,120 CLA has been shown to reduce body fat in animal and human studies121–123; however, humans do not produce significant levels of CLA from linoleic acid.119 Thus, CLA needs to be provided by direct uptake of dietary sources of CLA such as dairy products124 or probiotic bacteria, such as Lactobacillus species, that continuously digest dietary linoleic acid to CLA in the large intestine.119 Moreover, because dietary CLA is mainly absorbed in the small intestine, gut microbe-generated CLA may also contribute to mucosal homeostasis in the large intestine because of CLA’s anti-inflammatory effects.125 Rosberg-Cody et al126 obtained a significantly higher CLA level in adipose tissue in mice when a recombinant Lactobacillus strain that harbors the linoleic acid isomerase gene was administered. Lactobacillus gasseri and L plantarum have also been shown to reduce adipose tissue mass and size in mice.118,127
Bifidobacterium species appear to have a similar antiobesity role as Lactobacillus species.128–130 Bifidobacterium breve and Bifidobacterium dentium are efficient CLA producers,128,131 and mice fed a high-fat diet showed a suppression of body weight accumulation when administrated B breve.117 Isolated Bifidobacterium L66-5 also induced weight loss in mice.132 Yin et al132 observed that 4 strains of Bifidobacterium reduced liver lipid deposition but, interestingly, each species caused a different pattern of body weight change. In addition to Lactobacillus and Bifidobacteria species, other CLA producers of the genera Propionibacterium, Enterococcus, and Pediococcus warrant testing for possible antiobesity effects.133,134 Roseburia and Bacteroides/Prevotella species also appear to be candidates for further study based on findings from a study that revealed an inverse correlation between those species and obesity-related metabolic parameters.116
Modifying the Gut Microbiome: Insights on Host Energy Regulation
Gut microorganisms are capable of extracting energy from nondigested food, interfering with gene expression, and ultimately changing host body weight and adiposity. Theoretically, energy extraction from dietary intake may be regulated by manipulation of the gut microbiome during malnutrition. A critical aspect in the regulation of energy through microorganisms involves the need for a better understanding of host-microbe and microbe-microbe relationships. Interspecies interactions are of particular importance in energy regulation. For instance, germ-free mice colonized with B theta with and without M smithii showed increased energy harvest and greater adiposity in the mice co-colonized with B theta and M smithii.42 Samuel and Gordon,42 Samuel et al,96 and Hansen et al135 suggested that targeting hydrogen-consuming species, especially M smithii, with the goal of increasing SCFA production, could be one adjunctive approach to the treatment of undernutrition by helping the host extract the most possible energy from the limited diet.96 Manipulating the abundance of targeted groups of microorganisms using probiotics and prebiotics or their combination (ie, synbiotics) has the potential to shift the abundance of microbial groups and, therefore, to control host body weight.
Probiotics are classically defined as living, nonpathogenic microbes that, when ingested in sufficient amounts, stimulate beneficial effects in the host, typically by multifactorial mechanisms.136,137 The impact of probiotic supplementation on host energy regulation remains insufficiently investigated at present and often has demonstrated conflicting results. A clinical study conducted with infants who received either a mixture of pre/pro-biotic or placebo showed weight gain only in infants who received the pro/prebiotic mixture.138 In contrast, identification of lipid-lowering properties of many Lactobacillus127,139 and B breve140,141 strains during diet-induced obesity suggests that pro-biotics may lower host adiposity and weight. Delzenne and Reid142 suggest there is not enough evidence to suggest a link between obesity and probiotics, and the results attained from animal studies do not always correspond to human metabolism.
Prebiotics are nondigestible substances that, when ingested, stimulate microbial growth of specific bacteria within the colon, particularly bifidobacteria and lactobacilli, that are associated with health benefits to the host.137,143 The prebiotics most commonly studied in the area of weight regulation are oligofructose and inulin. In addition to the prebiotic dosage, differing degrees of polymerization (DP) appear to affect the degree of microbial community and metabolic changes—changes that seem to be based on differences in fermentability within particular colon segments.144 Inulin has a high DP between 3 and 60 and is produced by extraction from chicory roots, whereas oligofructose has a low DP between 2 and 20 and results from enzymatic degradation of inulin. The ingestion of oligofructose has been demonstrated to result in a reduction of food intake145 and a decrease in body weight146 in mice, as well as weight loss and satiety in humans.147,148 Weight loss due to oligofructose supplementation has been associated with colonic fermentation of oligofructose and production of peptide YY146,147 and glucagon-like peptide 1 (GLP-1).149,150
Inulin consumption has been shown to lead to an enrichment of Bifidobacterium in infants149 and, in particular, Bifidobacterium adolescentis in adults.151 In infants, inulin consumption showed a reduction of Clostridia and coliforms.150 Unfortunately, the reports of the impact of inulin administration on gut metabolism have not been readily reproducible. In vivo experiments showed inulin stimulated the production of SCFAs in adults152 and also in infants150; however, it has also been reported that inulin does not change SCFA concentration.153 Inulin administration to healthy subjects has been shown to result in a reduction of lipogenesis and plasma triacylglycerol concentrations.154 Clearly, further study in this area is needed.
The effects of dietary fiber on energy regulation remain controversial, and the use of dietary fiber to treat obesity appears to have modest benefit.155 The beneficial effects of dietary fiber, both soluble and insoluble forms, have generally been attributed to increases in satiation and satiety. A microbial mechanism has also been postulated. Although not considered to be prebiotic in the classical sense, dietary fiber consumption has been shown to result in the production of colonic SCFAs and recently was demonstrated to have beneficial effects on endotoxemia, inflammatory cytokine production, and obesity-related inflammation as well as glucose and lipid metabolism.156
Conclusion
Malnutrition may manifest as either obesity or undernutrition. Microorganisms play an important role in nutrient and energy extraction and energy regulation. To date, the specific roles that individual gut microbes play in energy harvest remain uncertain. A better understanding of host-microbe and microbe-microbe interactions may lead to the development of novel adjunctive treatment strategies for obesity and undernutrition. This will undoubtedly be an important area of nutrition research in the years to come.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Financial disclosure: Supported in part by grant R01DK090379 (to RKB and JKD) from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Macfarlane S, Macfarlane GT. Bacterial diversity in the human gut. Adv Applied Microbiol. 2004;54:261–289. doi: 10.1016/S0065-2164(04)54010-8. [DOI] [PubMed] [Google Scholar]
- 2.Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLOS Biol. 2007;5(7):1556–1573. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson J, Jones RC, Cortes C, et al. Community-associated methicillin-resistant Staphylococcus aureus infection among healthy newborns—Chicago and Los Angeles County, 2004 [Reprinted from MMWR. 2006;55:329–332) JAMA. 2006;296(1):36–38. [PubMed] [Google Scholar]
- 8.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disesase: meta-analyses. Clin Exp Allergy. 2008;38(4):634–642. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 9.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4(11):430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 12.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 13.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jumpertz R, Duc Son L, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94(1):58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiihonen K, Ouwehand AC, Rautonen N. Human intestinal microbiota and healthy ageing. Ageing Res Rev. 2010;9(2):107–116. doi: 10.1016/j.arr.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Clin Nutr. 2002;41(suppl 1):32–37. doi: 10.1007/s00394-002-1105-4. [DOI] [PubMed] [Google Scholar]
- 20.Zoetendal EG, Akkermans ADL, Akkermansvan Vliet WM, de Visser JAGM, de Vos WM. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health Dis. 2001;13(3):129–134. [Google Scholar]
- 21.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–487. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome (vol 473, pg 174, 2011) Nature. 2011;474(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chacko A, Cummings JH. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut. 1988;29(6):809–815. doi: 10.1136/gut.29.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70(6):443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 26.Bergman EN. Energy contribution of volatile fatty-acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70(2):567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 27.Parker DS. Measurement of production-rates of volatile fatty-acids in cecum of conscious rabbit. Br J Nutr. 1976;36(1):61–70. doi: 10.1079/bjn19760058. [DOI] [PubMed] [Google Scholar]
- 28.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Ann Rev Food Science Tech. 2010;1:363–395. doi: 10.1146/annurev.food.102308.124101. [DOI] [PubMed] [Google Scholar]
- 30.Gibson GR, Macfarlane GT, Cummings JH. Sulfate reducing bacteria and hydrogen metabolism in the human large-intestine. Gut. 1993;34(4):437–439. doi: 10.1136/gut.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 32.Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Murphy EF, Cotter PD, Healy S, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59(12):1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JM, Fahey GC, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127(1):130–136. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- 35.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2009;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 36.Lewis SJ, Heaton KW. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut. 1997;41(2):245–251. doi: 10.1136/gut.41.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker AW, Duncan SH, Leitch ECM, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71(7):3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72(1):57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 39.Marthinsen D, Fleming SE. Excretion of breath and flatus gases by human consuming high-fiber diets. J Nutr. 1982;112(6):1133–1143. doi: 10.1093/jn/112.6.1133. [DOI] [PubMed] [Google Scholar]
- 40.Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR. Abundance and diversity of mucosa-associated hydrogenotrophic microbes in the healthy human colon. ISME J. 2012;6(1):57–70. doi: 10.1038/ismej.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS One. 2009;4(9):e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA. 2006;103(26):10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernalier A, Willems A, Leclerc M, Rochet V, Collins MD. Ruminococcus hydrogenotrophicus sp nov, a new H-2/CO2-utilizing acetogenic bacterium isolated from human feces. Arch Microbiol. 1996;166(3):176–183. doi: 10.1007/s002030050373. [DOI] [PubMed] [Google Scholar]
- 45.Liu CX, Finegold SM, Song YJ, Lawson PA. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov and description of Blautia wexlerae sp nov., isolated from human faeces. Int J Syst Evol Microbiol. 2008;58:1896–1902. doi: 10.1099/ijs.0.65208-0. [DOI] [PubMed] [Google Scholar]
- 46.Robert C, Del’Homme C, Bernalier-Donadille A. Interspecies H-2 transfer in cellulose degradation between fibrolytic bacteria and H-2-utilizing microorganisms from the human colon. FEMS Microbiol Lett. 2001;205(2):209–214. doi: 10.1111/j.1574-6968.2001.tb10949.x. [DOI] [PubMed] [Google Scholar]
- 47.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 48.Fuchs G. CO2 Fixation in acetogenic bacteria-variations on a theme. Fems Microbiology Rev. 1986;39(3):181–213. [Google Scholar]
- 49.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PC, Gordon JI. Molecular analysis of commensal host-microbial relations hips in the intestine. Science. 2001;291(5505):881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 50.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12(6):499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 52.McNeil NI, Cummings JH, James WPT. Short chain fatty-acid absorption by human large intestine. Gut. 1978;19(9):819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubinstein R, Howard AV, Wrong OM. In vivo dialysis of faeces as a method of stool analysis, IV: organic anion component. Clin Sci. 1969;37(2):549–564. [PubMed] [Google Scholar]
- 54.Fleming SE, Choi SY, Fitch MD. Absorption of short-chain fatty-acids from the rat cecum in vivo. J Nutr. 1991;121(11):1787–1797. doi: 10.1093/jn/121.11.1787. [DOI] [PubMed] [Google Scholar]
- 55.Reilly KJ, Rombeau JL. Metabolism and potential clinical-application of short-chain fatty-acids. Clin Nutr. 1993;12:97–105. [Google Scholar]
- 56.Venter CS. Effects of dietary propionate on carbohydrate and lipid-metabolism in healthy-volunteers. Am J Gastroenterol. 1990;85(5):549–553. [PubMed] [Google Scholar]
- 57.McNeil NI. The contribution of the large-intestine to energy supplies in man. Am J Clin Nutr. 1984;39(2):338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- 58.Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- 59.Arora T, Sharma R. Fermentation potential of the gut microbiome: implications for energy homeostasis and weight management. Nutr Rev. 2011;69(2):99–106. doi: 10.1111/j.1753-4887.2010.00365.x. [DOI] [PubMed] [Google Scholar]
- 60.Payne AN, Chassard C, Zimmermann M, Muller P, Stinca S, Lacroix C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr Diabetes. 2011;1:e12. doi: 10.1038/nutd.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 62.Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology. 2009;136(5):1476–1483. doi: 10.1053/j.gastro.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 63.Sonnenburg JL, Xu J, Leip DD, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307(5717):1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Bjursell MK, Himrod J, et al. A genomic view of the human–Bacteroides thetaiotaomicron symbiosis. Science. 2003;299(5615):2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 65.Robert C, Bernalier-Donadille A. The cellulolytic microflora of the human colon: evidence of microcrystalline cellulose-degrading bacteria in methane-excreting subjects. FEMS Microbiol Ecol. 2003;46(1):81–89. doi: 10.1016/S0168-6496(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 66.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 67.Towle HC. Glucose and cAMP: adversaries in the regulation of hepatic gene expression. Proc Natl Acad Sci USA. 2001;98(24):13476–13478. doi: 10.1073/pnas.251530798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown AJ, Goldsworthy SM, Barnes AA, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 69.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 70.Xiong YM, Miyamoto N, Shibata K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101(4):1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dass NB, John AK, Bassil AK, et al. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19(1):66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 72.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cuche G, Cuber JC, Malbert CH. Ileal short-chain fatty acids inhibit gastric motility by a humoral pathway. Am J Physiol Gastrointest Liver Physiol. 2000;279(5):G925–G930. doi: 10.1152/ajpgi.2000.279.5.G925. [DOI] [PubMed] [Google Scholar]
- 74.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong YH, Nishimura Y, Hishikawa D, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146(12):5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 76.Preiss-Landl K, Zimmermann R, Hammerle G, Zechner R. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr Opin Lipidol. 2002;13(5):471–481. doi: 10.1097/00041433-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Greenwood MRC. The relationship of enzyme-activity to feeding-behavior in rats: lipoprotein-lipase as the metabolic gatekeeper. Int J Obes (Lond) 1985;9:67–70. [PubMed] [Google Scholar]
- 78.Kern PA. Potential role of TNF alpha and lipoprotein lipase as candidate genes for obesity. J Nutr. 1997;127(9):S1917–S1922. doi: 10.1093/jn/127.9.1917S. [DOI] [PubMed] [Google Scholar]
- 79.Coppack SW, Evans RD, Fisher RM, et al. Adipose-tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metab Clin Exp. 1992;41(3):264–272. doi: 10.1016/0026-0495(92)90269-g. [DOI] [PubMed] [Google Scholar]
- 80.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 82.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 83.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 84.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 85.Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56(5):1055–1068. doi: 10.1016/j.pcl.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Bryce J, Boschi-Pinto C, Shibuya K, Black RE Refer WHOCHE. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 87.Bhutta ZA, Ahmed T, Black RE, et al. Maternal and child undernutrition 3—what works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 88.Roseboom TJ, Painter RC, van Abeelen AFM, Veenendaal MVE, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141–145. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 89.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 91.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32(11):1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 92.Mai V, Draganov PV. Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J Gastroenterol. 2009;15(1):81–85. doi: 10.3748/wjg.15.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Furet J-P, Kong L-C, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AMS, Ramakrishna BS. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr. 2010;103(3):335–338. doi: 10.1017/S0007114509992182. [DOI] [PubMed] [Google Scholar]
- 95.Million M, Maraninchi M, Henry M, et al. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes (Lond) 2011 Aug 9; doi: 10.1038/ijo.2011.153. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Samuel BS, Hansen EE, Manchester JK, et al. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci USA. 2007;104(25):10643–10648. doi: 10.1073/pnas.0704189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turnbaugh PJ, Baeckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr. 2009;101(10):1493–1502. doi: 10.1017/S0007114508094658. [DOI] [PubMed] [Google Scholar]
- 101.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73(4):1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 103.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 104.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86(5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 105.Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87(5):1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 106.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 107.Muccioli GG, Naslain D, Backhed F, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56(8):1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 109.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24(3):731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kellermayer R, Dowd SE, Harris RA, et al. Colonic mucosal DNA methylation, immune response, and microbiome patterns in Toll-like receptor 2-knockout mice. FASEB J. 2011;25(5):1449–1460. doi: 10.1096/fj.10-172205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santacruz A, Marcos A, Warnberg J, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity. 2009;17(10):1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 113.Nadal I, Santacruz A, Marcos A, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes. 2009;33(7):758–767. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 114.Ishida RK, Faintuch J, Paula AMR, et al. Microbial flora of the stomach after gastric bypass for morbid obesity. Obes Surg. 2007;17(6):752–758. doi: 10.1007/s11695-007-9139-6. [DOI] [PubMed] [Google Scholar]
- 115.Woodard GA, Encarnacion B, Downey JR, et al. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13(7):1198–1204. doi: 10.1007/s11605-009-0891-x. [DOI] [PubMed] [Google Scholar]
- 116.Neyrinck AM, Possemiers S, Druart C, et al. Prebiotic effects of wheat arabinoxylan related to the increase in Bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One. 2011;6(6):e20944. doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kondo S, Xiao JZ, Satoh T, et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010;74(8):1656–1661. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 118.Takemura N, Okubo T, Sonoyama K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp Biol Med. 2010;235(7):849–856. doi: 10.1258/ebm.2010.009377. [DOI] [PubMed] [Google Scholar]
- 119.Lee HY, Park JH, Seok SH, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta Mol Cell Biol Lipids. 2006;1761(7):736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 120.Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobesity effect of trans-10,cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol. 2007;103(4):1140–1146. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 121.Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr. 2000;130(12):2943–2948. doi: 10.1093/jn/130.12.2943. [DOI] [PubMed] [Google Scholar]
- 122.Smedman A, Vessby B. Conjugated linoleic acid supplementation in humans: metabolic effects. Lipids. 2001;36(8):773–781. doi: 10.1007/s11745-001-0784-7. [DOI] [PubMed] [Google Scholar]
- 123.West DB, Delany JP, Camet PM, Blohm F, Truett AA, Scimeca J. Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am J Physiol Regul Integr Comp Physiol. 1998;275(3):R667–R672. doi: 10.1152/ajpregu.1998.275.3.R667. [DOI] [PubMed] [Google Scholar]
- 124.Chin SF, Liu W, Storkson JM, Ha YL, Pariza MW. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J Food Compost Anal. 1992;5(3):185–197. [Google Scholar]
- 125.Bassaganya-Riera J, Reynolds K, Martino-Catt S, et al. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127(3):777–791. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 126.Rosberg-Cody E, Stanton C, O’Mahony L, et al. Recombinant lactobacilli expressing linoleic acid isomerase can modulate the fatty acid composition of host adipose tissue in mice. Microbiology (UK) 2011;157:609–615. doi: 10.1099/mic.0.043406-0. [DOI] [PubMed] [Google Scholar]
- 127.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48(5):712–714. doi: 10.1007/s12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 128.Coakley M, Ross RP, Nordgren M, Fitzgerald G, Devery R, Stanton C. Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J Appl Microbiol. 2003;94(1):138–145. doi: 10.1046/j.1365-2672.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- 129.Rosberg-Cody E, Ross RP, Hussey S, et al. Mining the microbiota of the neonatal gastrointestinal tract for conjugated linoleic acid-producing bifidobacteria. Appl Environ Microbiol. 2004;70(8):4635–4641. doi: 10.1128/AEM.70.8.4635-4641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barrett E, Ross RP, Fitzgerald GF, Stanton C. Rapid screening method for analyzing the conjugated linoleic acid production capabilities of bacterial cultures. Appl Environ Microbiol. 2007;73(7):2333–2337. doi: 10.1128/AEM.01855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wall R, Ross RP, Shanahan F, et al. Metabolic activity of the enteric microbiota influences the fatty acid composition of murine and porcine liver and adipose tissues. Am J Clin Nutr. 2009;89(5):1393–1401. doi: 10.3945/ajcn.2008.27023. [DOI] [PubMed] [Google Scholar]
- 132.Yin YN, Yu QF, Fu NA, Liu XW, Lu FG. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol. 2010;16(27):3394–3401. doi: 10.3748/wjg.v16.i27.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oh DK, Hong GH, Lee Y, Min SG, Sin HS, Cho SK. Production of conjugated linoleic acid by isolated Bifidobacterium strains. World J Microbiol Biotechnol. 2003;19(9):907–912. [Google Scholar]
- 134.Sieber R, Collomb M, Aeschlimann A, Jelen P, Eyer H. Impact of microbial cultures on conjugated linoleic acid in dairy products: a review. Int Dairy J. 2004;14(1):1–15. [Google Scholar]
- 135.Hansen EE, Lozupone CA, Rey FE, et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc Natl Acad Sci USA. 2011;108:4599–4606. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics: approaching a definition. Am J Clin Nutr. 2001;73(2):361S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 137.Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69(5):1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 138.Underwood MA, Salzman NH, Bennett SH, et al. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J Pediatr Gastroenterol Nutr. 2009;48(2):216–225. doi: 10.1097/MPG.0b013e31818de195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee SJ, Cho SJ, Park EA. Effects of probiotics on enteric flora and feeding tolerance in preterm infants. Neonatology. 2007;91(3):174–179. doi: 10.1159/000097449. [DOI] [PubMed] [Google Scholar]
- 140.Kondo S, Xiao J-Z, Satoh T, et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010;74(8):1656–1661. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 141.Choi I, Kim Y, Park Y, Seog H, Choi H. Anti-obesity activities of fermented soygerm isoflavones by Bifidobacterium breve. Biofactors. 2007;29(2–3):105–112. doi: 10.1002/biof.552029201. [DOI] [PubMed] [Google Scholar]
- 142.Delzenne N, Reid G. No causal link between obesity and probiotics. Nat Rev Microbiol. 2009;7(12):901. doi: 10.1038/nrmicro2209-c2. [DOI] [PubMed] [Google Scholar]
- 143.Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of Bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108(4):975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 144.van de Wiele T, Boon N, Possemiers S, et al. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J Appl Microbiol. 2007;102:452–460. doi: 10.1111/j.1365-2672.2006.03084.x. [DOI] [PubMed] [Google Scholar]
- 145.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr. 2006;60(5):567–572. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- 146.Delmee E, Cani PD, Gual G, et al. Relation between colonic proglucagon expression and metabolic response to oligofructose in high fat diet-fed mice. Life Sci. 2006;79(10):1007–1013. doi: 10.1016/j.lfs.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 147.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89(6):1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 149.Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr. 2006;93(suppl 1):S157–S161. doi: 10.1079/bjn20041342. [DOI] [PubMed] [Google Scholar]
- 150.Yap WKW, Mohamed S, Jamal MH, Diederick M, Manap YA. Changes in infants faecal characteristics and microbiota by inulin supplementation. J Clin Biochem Nutr. 2008;43(3):159–166. doi: 10.3164/jcbn.2008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101(4):541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 152.van Nuenen MHMC, Meyer PD, Venema K. The effect of various inulins and Clostridium difficile on the metabolic activity of the human colonic microbiota in vitro. Microb Ecol Health Dis. 2003;15(2–3):137–144. [Google Scholar]
- 153.Kruse HP, Kleessen B, Blaut M. Effects of inulin on faecal bifidobacteria in human subjects. Br J Nutr. 1999;82(5):375–382. doi: 10.1017/s0007114599001622. [DOI] [PubMed] [Google Scholar]
- 154.Letexier D, Diraison F, Beylot M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am J Clin Nutr. 2003;77(3):559–564. doi: 10.1093/ajcn/77.3.559. [DOI] [PubMed] [Google Scholar]
- 155.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59:129–139. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 156.Al-Lahham S, Roelofsen H, Rezaee F, et al. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest. 2011 Aug 23; doi: 10.1111/j.1365-2362.2011.02590.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 157.Mahowald MA, Rey FE, Seedorf H, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci USA. 2009;106(14):5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]