Abstract
Research on language and aging typically shows that language comprehension is preserved across the life span. Recent neuroimaging results suggest that this good performance is underpinned by age-related neural reorganization [e.g., Tyler, L. K., Shafto, M. A., Randall, B., Wright, P., Marslen-Wilson, W. D., & Stamatakis, E. A. Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cerebral Cortex, 20, 352–364, 2010]. The current study examines how age-related reorganization affects the balance between component linguistic processes by manipulating semantic and phonological factors during spoken word recognition in younger and older adults. Participants in an fMRI study performed an auditory lexical decision task where words varied in their phonological and semantic properties as measured by degree of phonological competition and imageability. Older adults had a preserved lexicality effect, but compared with younger people, their behavioral sensitivity to phonological competition was reduced, as was competition-related activity in left inferior frontal gyrus. This was accompanied by increases in behavioral sensitivity to imageability and imageability-related activity in left middle temporal gyrus. These results support previous findings that neural compensation underpins preserved comprehension in aging and demonstrate that neural reorganization can affect the balance between semantic and phonological processing.
INTRODUCTION
It is well-established that language comprehension remains relatively preserved across the adult life span (Tyler et al., 2010; also see Burke & Shafto, 2008; Burke, MacKay, & James, 2000, for recent reviews), with the ability to construct various types of linguistic (e.g., phonological and semantic) representations remaining intact as we age (e.g., Taylor & Burke, 2002; James & Burke, 2000). This preserved language comprehension in the context of widespread age-related neural declines (e.g., Raz et al., 2005) raises the fundamental question of how adults maintain good comprehension across the life span as the brain undergoes extensive structural changes. In the current study, we address this question in the context of spoken word comprehension by relating behavioral measures of spoken word recognition to neural activation in younger and older adults.
Language comprehension is known to engage a network of regions including bilateral superior and middle temporal gyrus (STG/MTG), angular gyri, supramarginal gyri, and left (L) inferior frontal gyrus (IFG; Hickok & Poeppel, 2007; Scott & Johnsrude, 2003). This language network is differentially modulated as a function of lexical, morphological, semantic, and syntactic processes (Tyler & Marslen-Wilson, 2008). The role of the L IFG is critical but controversial: Although it plays a central role in a variety of linguistic processes (Tyler & Marslen-Wilson, 2008; Moss et al., 2005), it is also engaged in domain-general executive functions (e.g., Dobbins & Wagner, 2005), memory (Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997), and selection among competing representations (Moss et al., 2005; Thompson-Schill et al., 1997). Regions in bilateral MTG and STG are associated with semantic processing (Binder, Desai, Graves, & Conant, 2009) including sensitivity to conceptual imageability and concreteness (e.g., Binder et al., 2009; Noppeney, Phillips, & Price, 2004).
Because the L IFG shows marked age-related gray matter declines (Raz et al., 2005), the functions that it subserves may be prime candidates for age-related decrements. Many studies have shown age-related changes in IFG activation, which are generally thought to be a consequence of structural decline. Some of these age-related changes in frontal activity may reflect compensatory recruitment (Langenecker, Nielson, & Rao, 2004; Morcom, Good, Frackowiak, & Rugg, 2003; Cabeza, Anderson, Locantore, & McIntosh, 2002). The recruitment observed in these studies often involves bilateral activity under conditions where younger adults have lateralized activity during tasks tapping into memory (Morcom et al., 2003; Cabeza et al., 2002) and executive functions (Langenecker et al., 2004). The relationship between age-related neural changes and language comprehension has received less attention, possibly because of a focus on the causes of age-related cognitive declines rather than preserved performance. However, recent research suggests that preserved syntactic comprehension involves recruitment of bilateral IFG when younger adults’ activity is primarily left lateralized (Tyler et al., 2010). This additional right hemisphere activity is accompanied by preserved syntactic comprehension and decreased gray matter density in L IFG (Tyler et al., 2010). However, whereas L IFG activity correlated with syntactic performance, right (R) IFG activity did not, indicating that the R IFG supports the functionality of the L IFG but does not replace it.
In this study, we focus on another aspect of language comprehension that is typically preserved across the life span, spoken word comprehension (e.g., Tree & Hirsh, 2003; Lazzara, Yonelinas, & Ober, 2002; also see Burke & Shafto, 2008 for a review). We ask whether the processing of spoken words—the mapping from sound onto meaning—is preserved as we age and, if so, whether this preservation is associated with age-related neural flexibility, as has been shown for syntax (Tyler et al., 2010). In particular, we ask whether preserved word comprehension is associated with age-related changes in the relationship between the component phonological and semantic processes involved in word recognition and whether this is underpinned by neural changes.
Recognizing spoken words involves mapping from sound onto meaning representations, and a variety of models aim to specify the processes and representations involved in this mapping (Marslen-Wilson, 1987). Here, we assume a model in which speech input maps directly onto phonological and semantic representations within a distributed system (Gaskell & Marslen-Wilson, 1997). In this type of model, word recognition is an interactive process with both semantics and phonology activated at an early stage. Word-initial speech sounds activate a cohort of phonologically related candidates that compete with each other (e.g., Marslen-Wilson, 1987), so that large compared with small cohorts generate greater competition among activated candidates. The early activation of word candidates involves the activation of not only their phonological properties but also their semantic properties. Moreover, words with rich semantic representations gain an additional boost of activation (Pexman, Lupker, & Hino, 2002), which has a greater effect on words in larger cohorts where discriminability between similar-sounding word candidates is most difficult. This has been demonstrated in behavioral studies using both written (Strain, Patterson, & Seidenberg, 1995) and, most relevant to the current study, spoken words (Tyler, Voice, & Moss, 2000). Tyler et al. (2000) found that, during spoken word recognition, semantics (as indexed by imageability) facilitated performance when words were members of large cohorts but not when they were members of small cohorts. These results suggest that word recognition processes are modulated according to the cohort environment in which a word occurs, such that the effect of semantics varies according to the cohort context. The current study tests whether normal aging affects the role of phonological competition and, in particular, the degree to which semantic effects vary as a function of the phonological competitor environment.
The processes of phonological competition and selection during spoken word recognition have been shown to modulate activity within the neural language system in healthy young volunteers (Prabhakaran, Blumstein, Myers, Hutchison, & Britton, 2006). Frontal activity is also sensitive to semantic competition and selection during single word processing (Thompson-Schill, Bedny, & Goldberg, 2005; Thompson-Schill et al., 1997), and age-related reduction in selection-related L IFG activity is accompanied by performance deficits (Nessler, Johnson, Bersick, & Friedman, 2006). In other tasks manipulating selection demands, including Stroop task and verb generation, when selection demands are high, age-equivalent performance is often accompanied by increased recruitment of IFG and other regions (Langenecker et al., 2004; Persson et al., 2004). Of particular relevance to this study, there is behavioral evidence suggesting that older adults may be worse at recognizing words in phonologically dense neighborhoods (Sommers & Danielson, 1999).
Thus, when performance is primarily dependent on phonological competition, older adults demonstrate declines in performance or compensatory increases in frontal activity. However, the trade-off between competition and imageability demonstrated by Tyler et al. (2000) illustrates that the efficiency of word recognition is not only determined by the degree of phonological competition. If older adults are less responsive to phonological competition, they may demonstrate a compensatory increase in reliance on semantic information. In the current study, we test for age-related compensation by using a task in which both phonological and semantic factors can contribute flexibly to performance on a spoken word recognition task.
We carried out an fMRI study with younger and older adults, manipulating phonological competition and imageability as continuous variables in a lexical decision task. We expected younger adults to show the same effects reported in Tyler et al. (2000), such that (1) word recognition will be sensitive to both phonological competition and imageability, and (2) imageability will have a greater influence when phonological competition is high. We asked whether word recognition is preserved across the life span using both behavioral and fMRI data to test for age-related changes in (1) the effect of phonological competition or imageability on word recognition and (2) the degree to which the level of phonological competition affects sensitivity to imageability.
METHODS
Participants
Fourteen younger (M = 23.86, SD = 4.14) and 16 older (M = 75.75, SD = 4.99) adults participated in this study. All participants completed background screening tasks including the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975), which was above clinical cut-off scores for both younger (M = 29.36, SD = 0.84) and older (M = 28.44, SD = 1.36) participants. Vocabulary knowledge was assessed with a standardized multiple-choice vocabulary test (Shipley, 1946), wherein there was no age difference between younger (M = 36.5, SD = 2.93) and older (M = 37.7, SD = 2.01; t(28) = −1.38, p > .10) adults. Audiometer scores confirmed that no participant had either “moderate” or “severe” hearing loss (all average thresholds were under 40 dB), although younger participants had lower dB thresholds (M = 7.01, SD = 3.76) compared with older participants (M = 21.17, SD = 8.12; t(23) = −4.91, p < .001).
Experimental Materials and Procedure
Materials consisted of 80 words and 60 nonwords (see Table 1 for descriptive statistics). Real words included a range of word frequencies (M = 59.10, SD = 89.59, R = 1–538) and levels of cohort competition, obtained from the CELEX database (Baayen, Pipenbrook, & Gulikers, 1995). Cohort environments were defined as all words sharing the same first two phonemes as the target word (Marslen-Wilson, 1987). Two key lexical variables were measured for each word: cohort competition and imageability. Cohort competition was calculated by taking the ratio of each target’s lemma frequency to the total lemma frequency of all members of the target word’s cohort. Lower ratio scores represent high cohort competition because of either greater numbers of or more frequent cohort members, and higher ratio scores indicate low competition from fewer or less frequent cohort members (Zhuang, Randall, Stamatakis, Marslen-Wilson, & Tyler, 2011). To provide units that are easy to interpret, ratio scores are multiplied by 100. For analyses requiring separate testing of high and low competition words, a median split was performed, so that half of the real words were low competition condition (competition ratio scores: M = 22.68, SD = 27.96), and half were high competition (competition ratio scores: M = 0.74, SD = 0.69). For ease of interpretation in behavioral and imaging analyses involving correlation, the direction of ratio scores were reversed, so that higher “cohort competition” values indicate increased phonological competition. As a measure of semantics, we obtained imageability ratings on a scale of 1 (difficult to image) to 7 (easy to image) for each word from 15 participants who did not participate in the main experiment (see Table 1 for summary scores; mean imageability ratings are multiplied by 100 for ease of interpretation). High and low competition words did not differ on imageability scores or length in duration ( ps > .10). Nonwords did not differ from real words in either duration or cohort size ( ps > .10).
Table 1.
Descriptive Statistics for Real Words (n = 80) and Nonwords (n = 60) in the Lexical Decision Task
| Real Words |
Nonwords |
|||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| Duration (msec) | 469 | 734 | 601 | 69 | 473 | 777 | 619 | 82 |
| Cohort size | 3 | 823 | 164 | 192 | 3 | 823 | 155 | 149 |
| Cohort competition | .02 | 93.91 | 11.71 | 22.54 | ||||
| Imageability | 162 | 661 | 446 | 136 | ||||
We modified the traditional lexical decision task to make it appropriate for the fMRI scanner by including a baseline condition to control for nonlinguistic auditory processing. This baseline consisted of 40 stimuli sharing the complex auditory properties of speech without triggering phonetic interpretation. In this envelope-shaped “musical rain” (MuR; Uppenkamp, Johnsrude, Norris, Marslen-Wilson, & Patterson, 2006), the long-term spectro-temporal distribution of energy is matched to that of the corresponding speech stimuli. Half of the MuR items were low-pass filtered, and half were not, so that half of the MuR trials had a higher tone and half had a lower tone. Participants made a high/low tone decision for MuR trials to provide a similar task-related response to yes/no responses required in the lexical decision task. We also included 40 trials of silence, which did not require a response and were interspersed among the other trial types to jitter the SOA, improving the detectability of the hemodynamic response (Burock, Buckner, Woldorff, Rosen, & Dale, 1998). We used a single trial order, pseudorandomized with a maximum of three consecutive trials of any one condition. Participants listened to the stimuli through etymotic headphones worn underneath ear-protecting headphones and used an MRI-compatible button box to respond yes/no to words and nonwords and high/low to MuR trials.
Image Acquisition and Preprocessing
Participants were scanned at the MRC Cognition and Brain Sciences Unit, Cambridge, with a Siemens 3-T Tim Trio MRI scanner (Siemens Medical Solutions, Camberley, UK). We used a fast sparse imaging design to avoid scanner noise while participants were listening to the spoken stimuli (Hall et al., 1999). We acquired 2-sec scans separated by 1.4 sec of silence, and stimuli were presented within the silent period so that scanning started 1020–1300 msec after stimulus. Each functional volume consisted of 32 oblique axial slices, 3 mm thick with interslice gap of 0.75 mm and in-plane resolution of 3 mm. Slices were angled such that those covering MTG passed superior to the eyes to prevent eye motion from obscuring activation in language areas (field of view = 192 × 192 mm, repetition time = 3.4 sec, acquisition time = 2 sec, echo time = 30 msec, flip angle = 78°). fMRI data were preprocessed and analyzed using SPM5 software (SPM5, Wellcome Institute of Cognitive Neurology, London, UK). Preprocessing comprised within-subject realignment, spatial normalization of images to a template in standard space, and spatial smoothing using an 8-mm Gaussian kernel.
Imaging Analyses
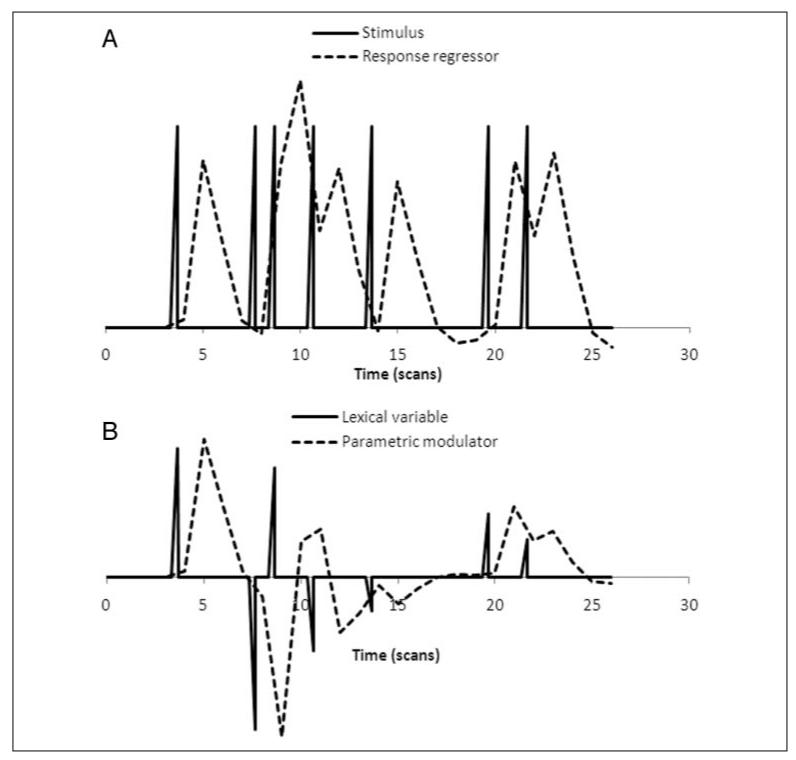
Following preprocessing, task-related responses were localized for each participant using a voxelwise general linear model (GLM). The fMRI response to each stimulus was modeled by defining the stimulus onsets as a stick function and convolving the stick function with a canonical hemodynamic response function (HRF) to give the response regressor (Figure 1A). For contrasts between conditions, the contrast image comprised the weighted sum of parameter estimates for the two conditions (e.g., Words–MuR).
Figure 1.
Examples of regressors used in the fMRI GLM analysis. (A) Main response regressor. (B) Parametric modulator based on lexical variable. Solid lines show the stick function representing stimulus onsets (A) and modulated by the lexical properties of stimuli (B). Dotted lines show each stick function convolved with a canonical HRF. Convolved regressors were used in the GLM.
We assess the relationship between activity and the level of cohort competition or imageability by modulating the size of the stick function for each trial by the value of either competition or imageability for the corresponding word and then convolving the modulated stick function with the HRF. Each resulting parametric modulator (Figure 1B) represents an fMRI response to words, modulated by the words’ lexical properties. The parametric modulators were made orthogonal to their corresponding response regressor by adjusting modulators to a mean of 0. The full GLM for each lexical variable comprised the response regressor, the parametric modulator, response regressors for nonwords and MuR, and nuisance regressors (movement parameters and constant). Low-frequency physiological noise was removed by applying a high-pass filter with a period of 128 sec.
These parametric modulator models were then used to separately examine the effects of competition and imageability and to test for the predicted differences in sensitivity to imageability for high versus low competition words. For models separately assessing the relevance of cohort competition and imageability, the effect of each lexical variable was calculated in a separate GLM, each with a single modulator. The GLM produced parameter estimates for each regressor at each voxel, resulting in “contrast images” for each effect of interest. The contrast images for the parametric modulators indicated the strength of the relationship between the fMRI response and the lexical variable across the brain. For models used to test the prediction that imageability will only affect activity when cohort competition is high, real words were split into high and low cohort competition sets, and each set was modeled as a separate regressor, each with a corresponding parametric modulator.
Contrast images for each participant were entered into group random effects analyses. Group random effects analyses first contrasted words to MuR to reveal the language network with nonspeech auditory processing removed in each group. We entered contrast images for words–MuR into one-sample t tests to identify regions where this contrast differed significantly from zero. We identified regions with a consistent response across both age groups using a conjunction analysis in which each voxel takes the minimum statistical value from the two groups. Only voxels passing the statistical threshold in both groups were included.
We first assessed age differences on the effects of cohort competition and imageability by separately examining activity for each age group and lexical variable using one-sample t tests. We then directly compared age groups and sensitivity indices using a factorial analysis, implemented in SPM5 using the full factorial design. The analysis used two factors, each with two conditions: age (young and older) and type of modulator (cohort competition and imageability). The two conditions of the modulator factor comprised the contrast images for the parametric modulators for cohort competition and imageability.
We then tested for age differences in how the level of competition modulates the effect that imageability has on activity. We used two-sample t tests in each age group to compare imageability sensitivity for low versus high competition. The results of these t tests along with behavioral results motivated a second factorial analysis to test for specific age differences in the trade-off between responsiveness to competition and imageability. This factorial analysis involved splitting words into low and high competition conditions and calculating imageability effects for low and high competition words separately. These effects were then used to compare sensitivity to imageability across age (young and older) and level of competition (low and high). Both factorial designs were implemented using the same settings in SPM5. We applied nonsphericity correction to the age factor for unequal variance and to the lexical factors for nonindependence. Effects within each analysis were examined using planned t contrasts based on our theoretical predictions and the behavioral results. We report Montreal Neurological Institute coordinates and significant voxels at a threshold of p < .005, cluster-level corrected at p < .05, unless otherwise stated. To identify anatomical regions within clusters and cluster maxima, Montreal Neurological Institute coordinates were confirmed using Automatic Anatomic Labeling (Tzourio-Mazoyer et al., 2002) and the Brodmann’s area atlas implemented in MRIcron (www.MRicro.com/MRicron).
RESULTS
Behavioral Results
We removed errors (proportion for real words: M = 0.09, SD = 0.06; nonwords: M = 0.15, SD = 0.17) and timeouts (number for real words M = 0.53, SD = 1.14; nonwords M = 0.53, SD = 1.72) before analysis of RT data. RTs were inversely transformed to control for outliers (Ratcliff, 1993), and we report retransformed RTs below for ease of interpretation.
Both age groups showed robust lexicality effects, with responses to real words faster than to nonwords (younger: words, M = 912 msec, SD = 101; nonwords, M = 996 msec, SD = 116; t(13) = 7.05, p < .001; older: words, M = 1039 msec, SD = 130; nonwords, M = 1200 msec, SD = 159; t(15) = 5.94, p < .001). Although no older participants had severe hearing deficits, within the older group, correlations with age revealed the well-documented age-related increase in average hearing thresholds (r = .59, p < .05). However, correlations of older adults’ hearing thresholds with RTs for all words and for low and high competition words separately revealed that hearing thresholds were not related to performance (all ps > .10).
Effects of Modulators: Competition and Imageability
To measure the effect of the modulator variables on word recognition, we correlated the inversely transformed RTs for each word with imageability ratings and cohort competition scores. The resulting Pearson correlation coefficients were converted to Z scores using Fisher’s Z transform and reflect each participant’s sensitivity to imageability and competition. We averaged these sensitivity scores within each age group and subjected them to one-sample t tests to evaluate the average sensitivity to competition or imageability (see Table 2). Younger adults had longer RTs when imageability was lower and when cohort competition was higher. Older adults were faster when imageability was higher, but average sensitivity to cohort competition was not significantly different from zero.
Table 2.
Behavioral Sensitivity to Lexical Variables
| Imageability |
||||||||
|---|---|---|---|---|---|---|---|---|
| Cohort Competition |
All |
High Competition Cohort |
Low Competition |
|||||
| Mean | t | Mean | t | Mean | t | Mean | t | |
| Younger | .09 | 5.31*** | −.12 | 6.23*** | −.24 | 6.64*** | .01 | −.204 |
| Older | .04 | 1.52 | −.13 | 3.90** | −.16 | 2.67* | −.11 | 2.86* |
Scores reflect the group means for individual subject correlations between lexical variables and RTs.
p < .05.
p < .01.
p < .001.
Sensitivity scores were used to test for age effects on the relative contribution of cohort competition and imageability in a two-way mixed ANOVA comparing the effect of age (young, older) and type of modulator (cohort competition, imageability). A main effect of Modulator reflected an overall stronger sensitivity of RTs to imageability compared with cohort competition, F(1, 28) = 4.87, MSE = 0.011, p < .05. Although this main effect may be, in part, because of older adults’ numerically weaker sensitivity to competition (see Table 2), neither the main effect of Age nor the interaction between Age and Type of Modulator reached significance ( ps > .10).
Imageability Effects in the Context of High versus Low Competition
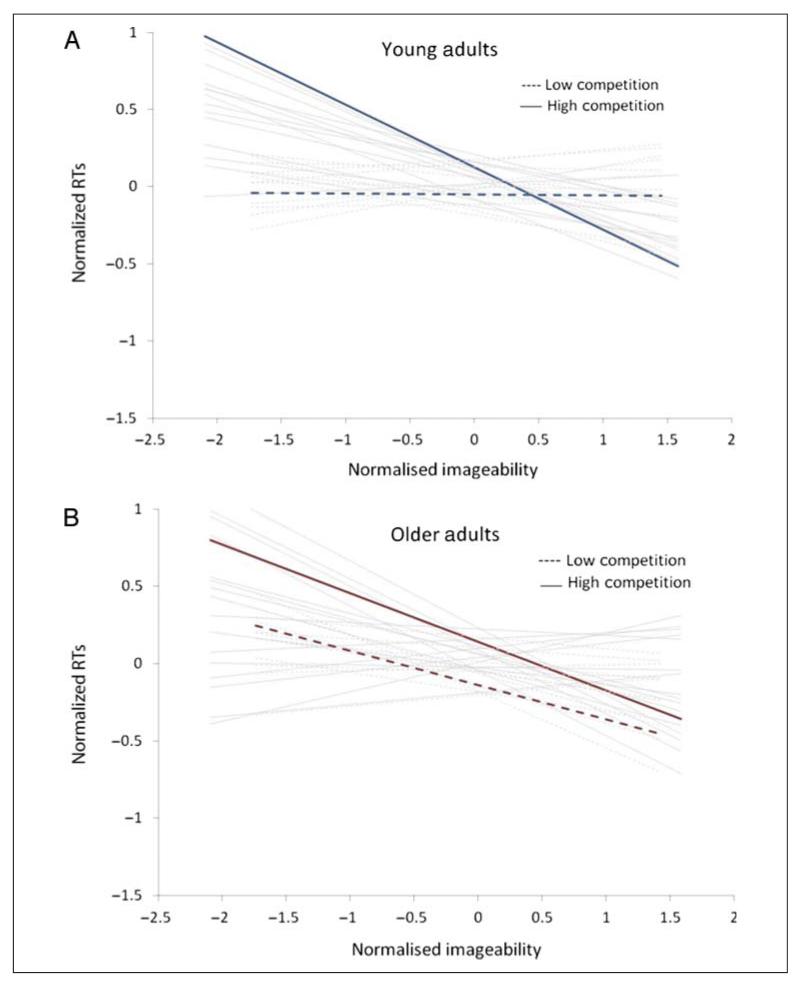
Although the above examination of competition and imageability sensitivity provides only equivocal evidence of age differences, it does not address the question of whether age affects the trade-off between competition and imageability. To test whether imageability affects RTs more when competition is high, we calculated imageability sensitivity for high and low competition words separately. Younger adults were faster when imageability is higher but only for high competition words (r = −.24), replicating Tyler et al. (2000). In contrast, older adults were faster when imageability is higher for both low (r = −.16) and high competition words (r = −.11). Next, we directly examined age effects on imageability sensitivity in low versus high competition environments. We entered imageability sensitivity scores into a 2 (Competition: low vs. high) × 2 (Age: young vs. older) mixed ANOVA. There was no main effect of Age on sensitivity to imageability ( p > .10), but a main effect of Competition Level indicated overall stronger effect of imageability for high than low competition words, F(1, 28) = 9.67, MSE = 0.033, p < .01. A significant interaction between Age and Competition Level, F(1, 28) = 4.59, MSE = 0.033, p < .05, demonstrated that the degree of competition influenced younger adults’ imageability effect, t(13) = 4.57, p < .01, but not older adults’ ( p > .10; see Figure 2).
Figure 2.
Correlations for each participant between normalized imageability scores and normalized retransformed RTs, presented by level of competition and age group. (A) Each younger participant’s correlation between imageability and RT shown in gray lines; mean correlation across younger participants shown in blue lines. (B) Each older participant’s correlation between imageability and RT shown in gray lines; mean correlation across older participants shown in red lines.
To summarize the behavioral data, both younger and older adults demonstrate the well-documented lexicality effect in their word recognition RTs, supporting overall preserved word comprehension in old age. Sensitivity scores confirmed previous findings that responses are faster to words with fewer phonological competitors and higher imageability. There was no age difference in the overall sensitivity to cohort competition or imageability, although older adults appear to have weaker sensitivity to competition (see Table 2). However, when words were split into low and high competition words, younger adults replicated previous findings: RTs were only sensitive to imageability when competition was high, but older adults’ RTs were sensitive to imageability regardless of the cohort competitor environment. Taken together, these results suggest an age-related decline in some aspect of the activation or selection processes underpinning the response to phonological competition, with a corresponding sensitivity to imageability that is independent of the competitor environment. Older adults’ generally preserved lexical access in the current study suggests that the increased reliance in imageability may be a compensatory response, and we next examined whether there is evidence for a similar age-related change in the fMRI data.
fMRI Results
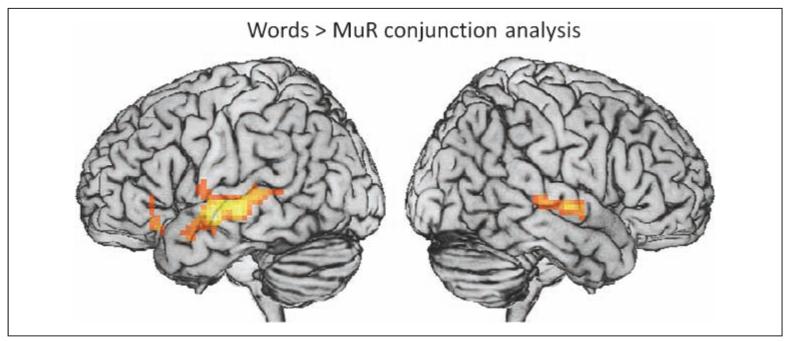
The contrast of words versus the control condition of MuR (words–MuR) revealed language regions active during lexical access after removing nonlinguistic auditory processing and task-related decision making. Young adults activated a network of regions including bilateral middle, superior, and inferior temporal cortex; L IFG (BA 45/47); and bilateral superior frontal and bilateral precentral cortex (see Table 3). Older adults activated a region in L MTG/STG, which extended into L IFG (BA 44), and had a smaller region of activity in R STG/MTG (see Table 3). A conjunction analysis of younger and older groups confirmed that both groups activate a primarily left-lateralized network during word comprehension, including L IFG, L MTG/STG, and R MTG/STG (see Figure 3 and Table 4). These results are consistent with previous studies of spoken word processing (Zhuang et al., 2011; Tyler, Marslen-Wilson, & Stamatakis, 2005; Scott & Johnsrude, 2003).
Table 3.
Significantly Activated Clusters in the Words–MuR Contrast
| Cluster |
Peak Voxel |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age | Region | BA | Pcorrected | Extent | Z | x | y | z |
| Younger | L temporal/IFG | 37/21/20/47/45 | <.001 | 1042 | 4.55 | −42 | −42 | −15 |
| L fusiform | 4.18 | −39 | −54 | −9 | ||||
| L ITG | 4.17 | −45 | −60 | −9 | ||||
| L MTG | 4.11 | − 42 | −24 | −18 | ||||
| L IFG | 3.69 | −45 | 30 | −6 | ||||
| R fusiform/STG | 37/19/21 | <.001 | 842 | 4.86 | 30 | −66 | 12 | |
| R STG | 4.17 | 66 | −9 | 0 | ||||
| R MTG | 3.98 | 63 | −15 | −15 | ||||
| L/R Su F | 10/11/32 | <.001 | 560 | 4.56 | 0 | 57 | 21 | |
| L rectus | 4.00 | −12 | 24 | −15 | ||||
| R precentral | 4/6/3 | <.001 | 349 | 4.36 | 36 | −27 | 54 | |
| R postcentral | 4.23 | 63 | −9 | 36 | ||||
| R STG | 4.13 | 57 | −6 | 45 | ||||
| L/R SMA | 6/24 | <.001 | 232 | 3.88 | 12 | 3 | 45 | |
| L mid-cingulum | 3.56 | −12 | −21 | 45 | ||||
| L paracentral | 3.56 | −12 | −30 | 51 | ||||
| L precentral/postcentral | 4/6 | <.05 | 123 | 3.96 | −51 | −15 | 51 | |
| L postcentral | 3.17 | −39 | −18 | 48 | ||||
| R rolandic operculum | 48 | <.05 | 108 | 3.87 | 39 | −24 | 21 | |
| R rolandic operculum | 3.55 | 45 | −18 | 21 | ||||
| R rolandic operculum | 3.43 | 60 | −3 | 12 | ||||
| Older | L MTG/L IFG | 22/21/44 | <.001 | 389 | 5.40 | −60 | −6 | −6 |
| L MTG | 5.06 | −57 | −15 | −3 | ||||
| L IFG | 3.78 | −57 | 12 | 12 | ||||
| R STG | 21/22 | <.05 | 112 | 4.12 | 63 | −9 | −3 | |
| R STG | 3.53 | 63 | −24 | 0 | ||||
| R MTG | 3.19 | 48 | −33 | 0 | ||||
Abbreviation: Su F = superior frontal.
Figure 3.
Whole-brain conjunction analysis of young and older groups for the words–MuR contrast. Results are presented for p < .005 (uncorrected) threshold.
Table 4.
Conjunction Analysis Showing Regions of Overlap between Young and Older Adults for the Words–MuR Contrast
| Peak Voxel |
||||||
|---|---|---|---|---|---|---|
| Region | BA | Cluster Extent | Z | x | y | z |
| L MTG/L STG | 22/21 | 238 | 5.08 | −60 | −6 | −6 |
| L MTG | 4.32 | −63 | −21 | −3 | ||
| R STG/MTG | 21/22 | 81 | 4.06 | 63 | −9 | −3 |
| R STG | 3.61 | 63 | −24 | 0 | ||
| R MTG | 3.04 | 48 | −33 | 0 | ||
| L fusiform/ITG | 37/20 | 44 | 3.95 | −42 | −42 | −15 |
| L IFG | 47/45 | 37 | 3.61 | −42 | 27 | −15 |
Clusters represent significant voxels at a threshold of p < .005 larger than 30 voxels, uncorrected at the cluster level.
Effects of Modulators: Competition and Imageability
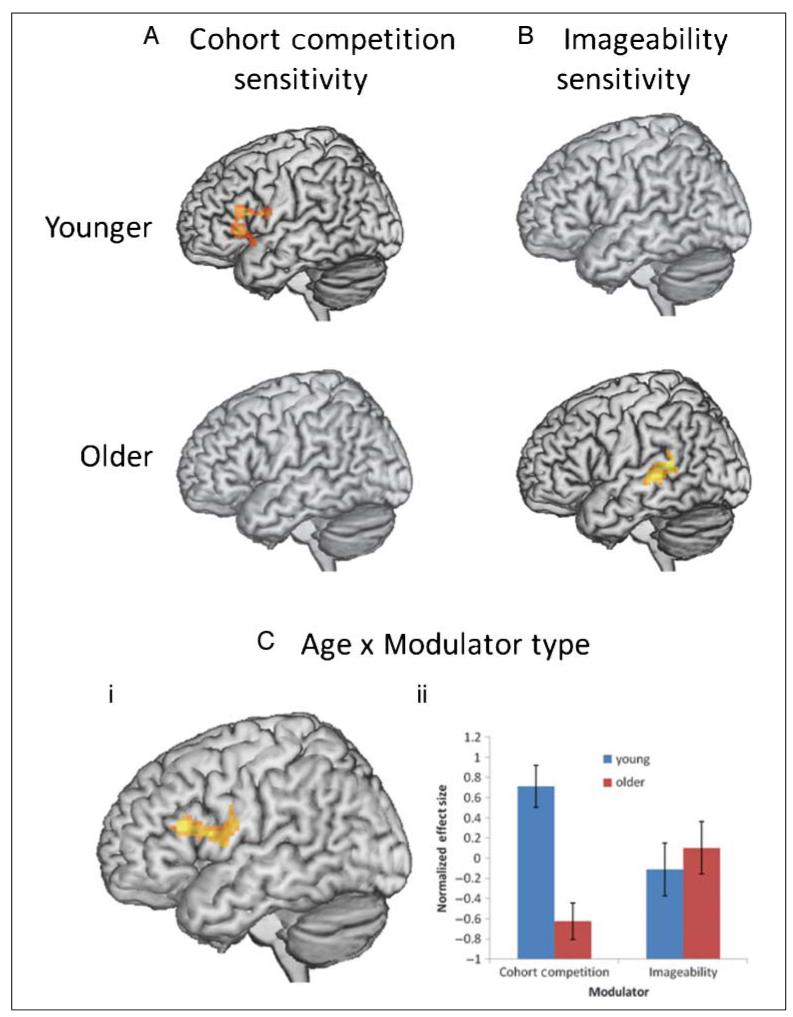
As in the behavioral analyses, we tested age effects on (1) the correlation of activity with cohort competition or imageability during word recognition and (2) the predicted difference in imageability sensitivity for low versus high competition words. We first examined cohort competition and imageability sensitivity using one-sample t tests for each age group separately. For cohort competition, these analyses revealed that younger adults had increased activity in L IFG (BA 45/47; x = −42, y = 18, z = 15; Z = 4.26) when competition was higher (see Figure 4A). This result supports the notion that L IFG (BA 45/47) is involved in selecting among phonological competitors and is consistent with similar studies of spoken word recognition (Zhuang et al., 2011; Bozic, Tyler, Ives, Randall, & Marslen-Wilson, 2010). Younger adults did not have an overall sensitivity to imageability. Older adults’ activity was not sensitive to competition but was sensitive to imageability (see Figure 4B), with increased activity for lower imageability words in L MTG/STG (BA 22; x = −63, y = −42, z = 9; Z = 3.89), a region known to be involved in semantic processing (Binder et al., 2009; Noppeney et al., 2004). When older adults’ hearing levels were entered as a covariate, the cluster became marginal at a clustercorrected level ( p = .051) but had a very similar maximum Z score (x = −66, y = −42, z = 9; Z = 3.77), suggesting that sensitivity to imageability in L MTG/STG did not simply reflect the effects of hearing thresholds (see supplementary figure for rendered image).
Figure 4.
Factorial analysis testing for the interaction of age and modulator. (A) Cohort competition sensitivity for young and older adults (significant only for the younger group) and (B) Imageability sensitivity for young and older adults (significant only for the older group). (C) Interaction of age and modulator (cohort competition vs. imageability) in L IFG, shown as part of a whole-brain analysis (i) and plot of the peak significant voxel (ii).
To directly examine age-related changes in sensitivity to imageability and competition, we tested for regions with an interaction between age (young, older) and type of modulator (cohort competition, imageability) using a planned comparison (Y[I]−Y[C])−(O[I]−O[C]). Although the behavioral interaction between age and type of modulator was nonsignificant, the fMRI data revealed an interaction in L IFG (BA 45; x = −42, y = 3, z = 15; Z = 4.41) and a smaller cluster in R superior and middle frontal gyrus (BA 9; x = 18, y = 36, z = 27; Z = 3.69; see Figure 4Ci). The nature of this interaction was evidenced in the plot of the effect size extracted at the peak voxel in L IFG (BA 45; see Figure 4Cii), which revealed that younger adults showed similar sensitivity to competition as in the within-group analyses, with more activity in the L IFG as cohort competition increased. Younger adults demonstrated a stronger sensitivity to competition than older adults, but there was no age difference in imageability (see Figure 4Cii). Although only older adults were sensitive to imageability in the separate group analyses (Figure 4B), the direct comparison of groups did not reveal any regions with a stronger effect of imageability for older than younger adults (see Figure 4Cii). However, as with the behavioral analyses, a critical question was whether there were age differences in the trade-off between the influence of competition and imageability.
Imageability Effects in the Context of High versus Low Competition
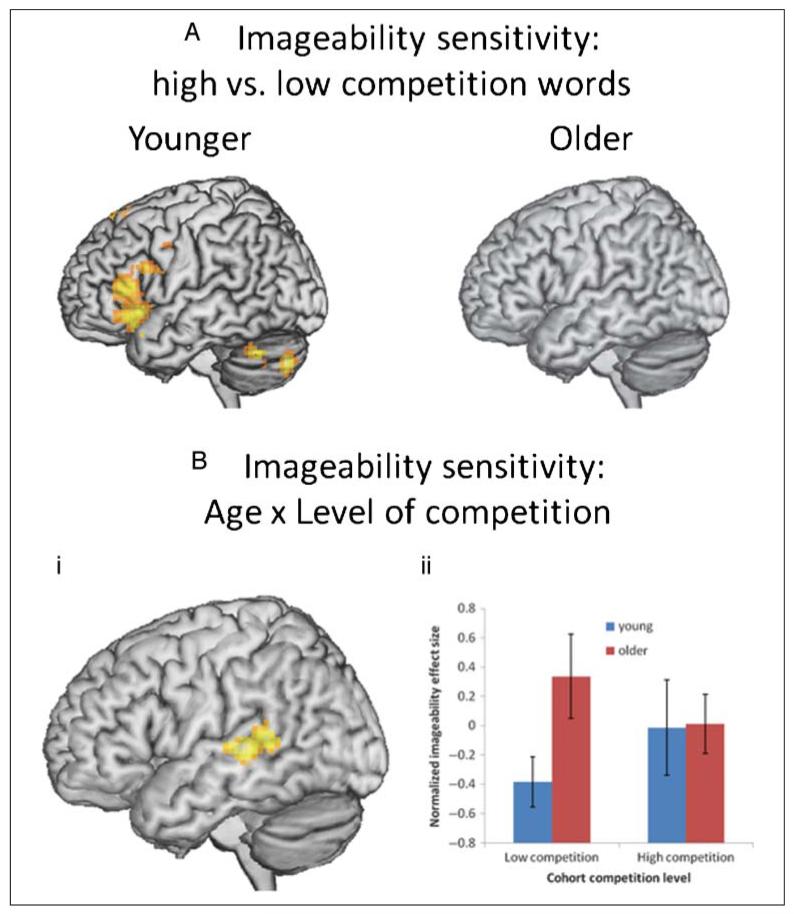
To examine how the competitor environment modulates the impact of imageability, we compared sensitivity to imageability for high and low competition words in each age group. Younger adults revealed a stronger sensitivity to imageability for high compared with low competition words (see Figure 5A) in L IFG (BA 47/45; x = −42, y = 6, z = −24; Z = 3.77) with additional clusters in R cerebellum (x = 21, y = −72, z = −27; Z = 4.21), L cerebellum (x = −36, y = −78, z = −39; Z = 3.95), and L SMA (BA 8; x = −6, y = 24, z = 42; Z = 3.83). The maximum of this contrast was in the same area of L IFG (BA 47/45) sensitive to competition in younger adults (see Figure 4A), suggesting that, for younger adults, the competitor environment is a critical determiner of how imageability affects activity. By contrast, older adults did not demonstrate any regions where sensitivity to imageability was different for high versus low competition words. This finding is in keeping with the behavioral evidence that competition influences younger adults’ imageability effect, but not older adults’ (see Figure 2).
Figure 5.
Factorial analysis testing for the interactive effect of age and cohort competition level on sensitivity to imageability. (A) Two-sample t tests for young and older adults, comparing imageability sensitivity for low versus high competition (significant only for young adults). (B) Interaction of age and competition in L MTG/STG, shown as a whole-brain analysis (i) and plot of the peak significant voxel (ii).
To directly compare age groups, we constructed t contrasts examining sensitivity to imageability across age (young, older) and competition level (low, high). We first tested for a crossover Age × Competition interaction (Y[L]−Y[H])−(O[H]−O[L]) with a model similar to the test for an Age × Type of modulator interaction; this analysis did not yield any regions showing a significant interaction. Next, following our initial predictions, the results of the behavioral interaction, and the evidence from fMRI for the groups separately, we tested the prediction that sensitivity to imageability depends on having a high competition environment for younger but not older adults. We constructed a t contrast that sets the Y[L] cell equal to zero and examined the effect of the contrast (Y[H] + O[H] + O[L]). This analysis revealed a significant cluster in L MTG/STG (BA 22/21; x = −54, y = −30, z = 3; Z = 4.06), a region in keeping with older adults’ sensitivity to imageability (see Figure 4B). Figure 5Bi shows the results of the whole-brain analysis; a plot of the peak voxel (see Figure 5Bii) confirms the pattern in which older adults are sensitive to imageability in L MTG/STG for both low and high competition words. Moreover, older adults’ imageability effect is stronger than younger adults’ for low competition words only, a finding in keeping with the pattern of behavioral data (see Figure 2).
DISCUSSION
The current study addresses critical aspects of how cognitive abilities including word recognition remain preserved across the adult life span. First, we confirmed that older adults maintained good accuracy and a robust lexicality effect in the lexical decision task, a finding in keeping with previous studies demonstrating maintained lexical processing across the life span (Taylor & Burke, 2002; James & Burke, 2000). Second, both behavioral and fMRI data suggest that semantic feature availability (as measured by imageability) becomes increasingly critical for older adults’ word recognition in response to declining sensitivity to phonological competition.
Younger adults were behaviorally sensitive to cohort competition, and fMRI analyses revealed a neural competition effect in L IFG (BA 47/45), an area associated with competition and selection processes in a number of language and nonlanguage contexts (e.g., Zhuang et al., 2011; Bozic et al., 2010; Tyler & Marslen-Wilson, 2008; Moss et al., 2005; Langenecker et al., 2004; Persson et al., 2004; Thompson-Schill et al., 1997). Moreover, in a direct comparison, younger adults had a stronger sensitivity to competition effects in L IFG (BA 47/45) than older adults. The current behavioral and neural results from younger adults are in keeping with the findings of Tyler et al. (2000) and support models in which word recognition involves a process of competition and selection among phonologically related cohort members (e.g., Marslen-Wilson, 1987).
Older adults, on the other hand, showed evidence of weaker sensitivity to cohort competition both behaviorally and in the fMRI analyses. Instead, older adults demonstrated a behavioral and neural sensitivity to imageability in both low and high competition environments. Neural effects centered around L MTG/STG, where we found increased activity in response to low imageability words, a pattern in keeping with previous findings with younger adults (e.g., Binder et al., 2009; Noppeney et al., 2004).
In the context of older adults’ good word recognition performance, the age-related increase in sensitivity to imageability is likely to reflect a compensatory reaction in response to an age-related decrease in sensitivity to phonological competition. This explanation leaves open the question of why older adults are less sensitive to cohort competition in this task. The cohort competition measure characterizes the processes of activating phonologically related competitors and selecting the target from among them, and there is evidence that age may affect both cohort activation and selection from among cohort members. A selection-based explanation is consistent with previous findings that older adults have increased difficulty recognizing words from dense phonological environments (e.g., Sommers & Danielson, 1999; Sommers, 1996). Moreover, these declines correlate with measures of inhibition (Sommers & Danielson, 1999), a finding interpreted as being because of age-related declines in inhibitory function. More general support comes from fMRI evidence of age-related increases in Stroop interference accompanied by increased IFG recruitment (Langenecker et al., 2004) and by models of cognitive aging that posit age-related deficits in inhibitory function (Hasher & Zacks, 1988).
However, not all behavioral findings support age-related increases in phonological-competitor interference (Takayangi, Dirks, & Moshfegh, 2002; Carter & Wilson, 2001), and age-related increases in interference may depend on semantic in addition to phonological overlap (Taylor & Burke, 2002). Moreover, selection impairments predict a pattern of activity that we did not find, namely more rather than less sensitivity to cohort competition in the older group. Thus, older adults’ decreased sensitivity to competition may reflect changes in the activation of cohort competitors rather than an inability to select among them. If older adults variably activate onset phonology, they may be less accurate in activating phonologically appropriate cohort competitors, with a resulting difficulty in differentiating between competitors in the process of identifying the target. This would impact target words that are members of both large and small cohorts, resulting in increased reliance on semantic relative to phonological information. Although age-related declines in phonological activation have been reported in word production studies (James & Burke, 2000; Burke, MacKay, Worthley, & Wade, 1991), phonological access during comprehension is typically thought to be preserved in old age, as indicated by age-equivalent phonological facilitation (Taylor & Burke, 2002; James & Burke, 2000). However, there is some evidence that phonological facilitation may start to become less effective during naming, starting in the mid-70s (White & Abrams, 2002). Our study suggests that there may be greater similarity between production and comprehension in terms of processing phonology than what was previously thought to be the case.
One of the difficulties in differentiating between the role of age-related selection deficits on one hand and weakened cohort activation on the other is that many results can be explained by either mechanism (see Burke & Shafto, 2008; Burke et al., 2000, for discussion). The current findings cannot adjudicate between the contribution of age-related deficits in phonological activation and lexical selection, but our results do suggest that neural reorganization underpins preserved word recognition across the life span. Previous research on language comprehension has primarily identified two patterns of age-related recruitment: bilateral IFG activity to support a typically left-lateralized function (Tyler et al., 2010) or recruitment of general resources to support a domain-specific task (Peelle, Troiani, Wingfield, & Grossman, 2010). The current paradigm identifies a different type of recruitment, namely an age-related shift in the contributions of and interactions between component processes that underpin successful language comprehension.
Conclusions
The current results suggest that much of the fundamental nature of lexical access is preserved in old age: Younger and older adults employ largely similar neural networks during word recognition, and both groups demonstrate robust sensitivity to lexicality. However, although performance remains good across the life span, the current paradigm reveals that normal aging affects the component processes of spoken word recognition. Although aging decreases sensitivity to cohort competition, there is a compensatory increase in sensitivity to semantic image-ability. Finally, the nature of neural recruitment in older adults demonstrates that, although prefrontal function clearly plays a key role in age-related neural compensation (e.g., Tyler et al., 2010; Langenecker et al., 2004; Morcom et al., 2003; Cabeza et al., 2002), bilateral frontal recruitment is not the only pattern of neural reorganization relevant for preserved language comprehension.
Supplementary Material
Acknowledgments
This work was supported by a Medical Research Council (UK) program grant (grant no. G0500842) to L. K. T. and a grant from the Dunhill Medical Trust to L. K. T. and M. A. S.
Footnotes
Publisher's Disclaimer: Citation: Age-related Neural Reorganization during Spoken Word Recognition: The Interaction of Form and Meaning
Meredith Shafto, Billi Randall, Emmanuel A. Stamatakis, Paul Wright, L. K. Tyler, Journal of Cognitive Neuroscience June 2012, Vol. 24, No. 6: 1434–1446.
Copyright belongs to MIT Press Journals, Journal of Cognitive Neuroscience
Journal home page: http://www.mitpressjournals.org/loi/jocn
REFERENCES
- Baayen RH, Pipenbrook R, Gulikers L. The CELEX lexical database. Philadelphia Linguistic Data Consortium, University of Pennsylvania; Philadelphia: 1995. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic M, Tyler LK, Ives DT, Randall B, Marslen-Wilson WD. Bihemispheric foundations for human speech comprehension. Proceedings of the National Academy of Sciences, U.S.A. 2010;107:17439–17444. doi: 10.1073/pnas.1000531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, James LE. Theoretical approaches to language and aging. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford University Press; Oxford, UK: 2000. pp. 204–237. [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, Wade E. On the tip-of-the-tongue: What causes word finding failures in young and older adults? Journal of Memory and Language. 1991;30:542–579. [Google Scholar]
- Burke DM, Shafto MA. Language and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Psychology Press; New York: 2008. pp. 373–443. [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carter AS, Wilson RH. Lexical effects on dichotic word recognition in young and elderly listeners. Journal of the American Academy of Audiology. 2001;12:86–100. [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaskell MG, Marslen-Wilson WD. Integrating form and meaning: A distributed model of speech perception. Language and Cognitive Processes. 1997;12:613–656. [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, et al. “Sparse” temporal sampling in auditory fMRI. Human Brain Mapping. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Academic Press; San Diego, CA: 1988. pp. 193–225. [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- James LE, Burke DM. Phonological priming effects on word retrieval and tip-of-the-tongue experiences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1378–1391. doi: 10.1037//0278-7393.26.6.1378. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Lazzara MM, Yonelinas AP, Ober BA. Implicit memory in aging: Normal transfer across semantic decisions and stimulus format. Aging Neuropsychology and Cognition. 2002;9:145–156. [Google Scholar]
- Marslen-Wilson WD. Functional parallelism in spoken word-recognition. Cognition. 1987;25:71–102. doi: 10.1016/0010-0277(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RSJ, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, et al. Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cerebral Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessler D, Johnson R, Bersick M, Friedman D. On why the elderly have normal semantic retrieval but deficient episodic encoding: A study of left inferior frontal ERP activity. Neuroimage. 2006;30:299–312. doi: 10.1016/j.neuroimage.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C. The neural areas that control the retrieval and selection of semantics. Neuropsychologia. 2004;42:1269–1280. doi: 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults’ comprehension of spoken sentences: Age differences in resource allocation and connectivity. Cerebral Cortex. 2010;20:773–782. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester CYC, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: Differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Pexman PM, Lupker SJ, Hino Y. The impact of feedback semantics in visual word recognition: Number-of-features effects in lexical decision and naming tasks. Psychonomic Bulletin & Review. 2002;9:542–549. doi: 10.3758/bf03196311. [DOI] [PubMed] [Google Scholar]
- Prabhakaran R, Blumstein SE, Myers EB, Hutchison E, Britton B. An event-related fMRI investigation of phonological–lexical competition. Neuropsychologia. 2006;44:2209–2221. doi: 10.1016/j.neuropsychologia.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychological Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends in Neurosciences. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Institute of living scale. Western Psychological Services; Los Angeles: 1946. [Google Scholar]
- Sommers MS. The structural organization of the mental lexicon and its contribution to age-related declines in spoken-word recognition. Psychology and Aging. 1996;11:333–341. doi: 10.1037//0882-7974.11.2.333. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Danielson SM. Inhibitory processes and spoken word recognition in young and older adults: The interaction of lexical competition and semantic context. Psychology and Aging. 1999;14:458–472. doi: 10.1037//0882-7974.14.3.458. [DOI] [PubMed] [Google Scholar]
- Strain E, Patterson K, Seidenberg M. Semantic effects in single word naming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:1140–1154. doi: 10.1037//0278-7393.21.5.1140. [DOI] [PubMed] [Google Scholar]
- Takayangi S, Dirks DD, Moshfegh A. Lexical and talker effects on word recognition among native and non-native listeners with normal and impaired hearing. Journal of Speech, Language and Hearing Research. 2002;45:585–597. doi: 10.1044/1092-4388(2002/047). [DOI] [PubMed] [Google Scholar]
- Taylor JK, Burke DM. Asymmetric aging effects on semantic and phonological processes: Naming in the picture–word interference task. Psychology and Aging. 2002;17:662–676. doi: 10.1037//0882-7974.17.4.662. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences, U.S.A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree JJ, Hirsh KW. Sometimes faster, sometimes, slower: Associative and competitor priming in picture naming with young and elderly participants. Journal of Neurolinguistics. 2003;16:489–514. [Google Scholar]
- Tyler LK, Marslen-Wilson WD. Fronto-temporal brain systems supporting spoken language comprehension. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2008;363:1037–1054. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Stamatakis EA. Differentiating lexical form, meaning, and structure in the neural language system. Proceedings of the National Academy of Sciences, U.S.A. 2005;102:8375–8380. doi: 10.1073/pnas.0408213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cerebral Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Voice JK, Moss HE. The interaction of meaning and sound in spoken word recognition. Psychonomic Bulletin & Review. 2000;7:320–326. doi: 10.3758/bf03212988. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uppenkamp S, Johnsrude IS, Norris D, Marslen-Wilson W, Patterson RD. Locating the initial stages of speech-sound processing in human temporal cortex. Neuroimage. 2006;31:1284–1296. doi: 10.1016/j.neuroimage.2006.01.004. [DOI] [PubMed] [Google Scholar]
- White KK, Abrams L. Does priming specific syllables during tip-of-the-tongue states facilitate word retrieval in older adults? Psychology and Aging. 2002;17:226–235. [PubMed] [Google Scholar]
- Zhuang J, Randall B, Stamatakis EA, Marslen-Wilson W, Tyler LK. The interaction of lexical semantics and cohort competition in spoken word recognition: An fMRI study. Journal of Cognitive Neuroscience. 2011;23:3778–3790. doi: 10.1162/jocn_a_00046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.