Abstract
Earlier studies have shown that activation of adenosine A1 receptors on peripheral pain fibers contributes to acupuncture-induced suppression of painful input. In addition to adenosine, acupuncture triggers the release of other purines, including ATP and ADP that may bind to purine receptors on nearby fibroblasts. We here show that purine agonists trigger increase in cytosolic Ca 2+ signaling in a cultured human fibroblasts cell line. The profile of agonist-induced Ca2+ increases indicates that the cells express functional P2yR2 and P2yR4 receptors, as well as P2yR1 and P2xR7 receptors. Unexpectedly, purine-induced Ca2+ signaling was associated with a remodeling of the actin cytoskeleton. ATP induced a transient loss in F-actin stress fiber. The changes of actin cytoskeleton occurred slowly and peaked at 10 min after agonist exposure. Inhibition of ATP-induced increases in Ca2+ by cyclopiazonic acid blocked receptor-mediated cytoskeleton remodeling. The Ca2+ ionophore failed to induce cytoskeletal remodeling despite triggering robust increases in cytosolic Ca2+. These observations indicate that purine signaling induces transient changes in fibroblast cytoarchitecture that could be related to the beneficial effects of acupuncture.
Keywords: Fibroblasts, Calcium signaling, Purinergic receptors, Cytoskeleton
1. Introduction
Emerging evidence suggests that adenosine released during manual acupuncture needle stimulation binds to adenosine A1 receptors expressed by peripheral pain fibers, which locally suppresses the transmission of painful input. ATP is present in millimolar concentration within the cytosol of all cell types and increases extraellularly as it leaks out when the plasma membrane is damaged during the mild tissue injury associated with acupuncture. [1, 2] Alternatively, or in addition, ATP can be actively released by local cells [3]. HPLC analysis of microdialysis samples collected near the location of the needle has shown that the concentrations of all purines are increased in tissue close to the stimulated area in human volunteers receiving traditional acupuncture [4]. Potent ectonucleotidases present in the interstitial space degrade ATP to adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP), and adenosine, each of which has their own respective sets of receptors [5]. In particular the adenosine A1 receptor has been shown to suppress the conductance of painful input by activating A1 receptors on peripheral pain fibers [6-9]. In support of a key role of A1 receptors in the peripheral mechanisms by which acupuncture reduces chronic pain is the observation that A1 receptor knockout mice do not benefit from acupuncture or from local injection of adenosine A1 receptor agonists [1].
Another line of work conducted in parallel has shown that fibroblasts within whole areolar connective tissue expand and develop larger cross-sectional areas in response to acupuncture [10, 11]. We here thought to combine these lines of work by asking whether purines released during acupuncture elicit structural changes in fibroblasts by activating P2 purinergic receptors (Fig. 1A). We found that several purinergic receptor agonists induced a transient remodeling of the actin cytoskeleton of cultured fibroblasts, detected as a transient loss of stress fibers. These structural changes of the cytoarchitecture of cultured fibroblasts share similarities with the increase in cross-sectional area of fibroblasts in situ, in response to acupuncture.
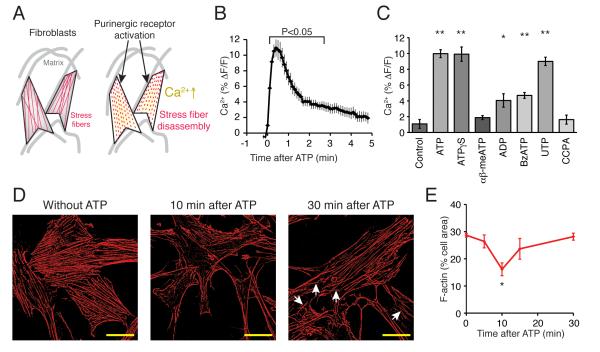
Fig. 1. Fibroblasts respond to purinergic agonists with intracellular Ca2+ increase consistent with expression of P2yR2, P2yR4, P2yR1, and P2xR7.
(A) Experimental model. Fibroblast cells express purinergic receptors and their activation triggers intracellular Ca2+ signaling. Consequently, disassembly of polymerized actin, manifested as a transient loss of stress fibers. The stress fibers are eventually re-assembled. (B) Ca2+ increase by ATP (100μM) application. N = 8, repeated measures ANOVA with Dunnett test compared to 0 min. (C) Responses of fibroblast Ca2+ by purinergic agonists, ATPγS (100 μM), αβ-meATP (100 μM), ADP (100 μM), BzATP (10 μM), UTP (100 μM), and CCPA (10 μM). N = 6-7. *, p < 0.05, **, p < 0.01, ANOVA with Tukey-Kramer test compared to Control. (D) Purinergic stimulation initiates temporal stress fiber rearrangement. F-actins of the fibroblasts stained with phalloidin (red) before, 10 min after, and 30 min after application of ATP (100 μM). White arrows indicate F-actins that are not along the primary axis of the cells. Scale bars, 30 μm. (E) Summary histograms of actin disassembly over time by ATP (100 μM). N = 4-8. *, p < 0.05, ANOVA with Tukey-Kramer test compared to 0 min.
2. Methods
2.1. Cell culture and calcium measurement
Human foreskin fibroblast BJ cells were obtained from American Type Culture Collection (ATCC, CRL -2522) and grown in Eagle’s Minimum Essential Medium (EMEM; ATCC #30-2003) with 10% fetal bovine serum (Hyclone, #SH3007103) [12]. Cells were plated at 5,000 – 10,000 cells / well to 8-chamber cover glass (Labtek II, Nunc) coated with 0.01% poly-L-ornithine (Sigma P4957) and 5 μg/ml laminine (BD Biosciences, #354232) and grown for 48-72 hours, with 60-80% confluency. The cultured cells were loaded with the Ca2+ indicator rhod-2 AM (Life Technologies, 2 μM, 60 min) in serum-free EMEM. Purinergic receptor agonist ATP (Sigma), adenosine-5′-(γ-thio)-triphosphate (ATPγS, Sigma) uridine 5′-triphosphate (UTP, Sigma), ADP (Sigma), 2′(3′)-O-(4-Benzoylbenzoyl)adenosine-5′-triphosphate (BzATP, Sigma), alpha,beta-methylene-ATP (αβ-meATP, Sigma) or 2-chloro-N(6)-cyclopentyladenosine (CCPA, Tocris) were applied to the cells while imaging rhod-2 fluorescence with 543 nm excitation laser and 560 nm long pass emission filter using a confocal microscope with 10× objective lens (FV-300, Olympus) every 3-6 sec [13-15]. Cyclopiazonic acid (CPA, 20 μM, Sigma) was applied to the cells 10-30 min prior to the agonist application.
2.2. Cytoskeleton measurement
Cells in the chambers were fixed with 4% paraformaldehyde for 10 min in room temperature after the exposure to the agonists for 0, 5, 15, or 30 min. Actin fibers were labeled with Alexa Fluor 594-conjugated phalloidin (Life Technologies, 5 U/ml) according to the manufacturer’s procedure, and imaged with 543 nm excitation laser and 560 nm long pass emission filter using a confocal microscope with 60× objective lens [16]. The images were analyzed by ImageJ software (NIH) by measuring area stained with phalloidin, and the data from each image were expressed as the percent of area covered by the cells in the field of view.
2.3. Statistics
All data were expressed as means ± standard errors of the mean. Normality of the data was verified by Shapiro-Wilk test. Statistical comparison tests were carried out with t tests for 2 sample comparisons, and ANOVA with Tukey-Kramer or Dunnett post-hoc tests for multiple comparisons. P values less than 0.05 were considered significantly different.
3. Results
3.1. Fibroblasts express functional purinergic receptors
We first analyzed the functional expression of purine receptors on the cultured fibroblasts. ATP consistently evoked increases in cytosolic Ca2+, albeit of low amplitude. The max peak was around 9.98 ± 0.50% of baseline fluorescence (N = 7, p < 0.01, Tukey-Kramer test) (Fig. 1B,C). The agonist-induced Ca2+ response peaked at 30-50 sec and cytosolic Ca2+ returned to near baseline at 2.8 min (N = 8, p > 0.05, Repeated measures one way ANOVA with Dunnett test) (Fig. 1B). To determine the functional expression of purine receptor, rhod-2 AM-loaded cells were next exposed to an array of purine agonists. This analysis showed that the fibroblasts displayed robust increases in cytosolic Ca2+ when exposed to non-hydrolyzable ATP analogue ATPγS, (100 μM, 9.91 ± 0.91%, N = 7, p < 0.01) and UTP (100 μM, 9.00 ± 0.53%, N = 6, p < 0.01), and more modest increases in cytosolic Ca2+ to ADP (100 μM, 4.05 ± 0.85%, N = 6, p < 0.05) and BzATP (10 μM, 4.69 ± 0.37%, N = 6, p < 0.01) (Fig. 1C). On the other hand, αβ-meATP (100 μM) failed to induce a significant increase (1.88 ± 0.25%, N = 6, p > 0.05) (Fig. 1C). This pattern of in Ca2+ responses suggest that fibroblasts express functional P2yR2 and P2yR4 (UTP), P2yR1 (ADP), P2xR7 (BzATP), but not P2xR1 or P2xR4 (αβ-meATP). This pattern of receptor expression is consistent with earlier reports describing that fibroblasts of various origin express P2 receptors[17-20]. Adenosine A1 receptor agonist CCPA (10 μM) did not induce a Ca2+ increase (1.63 ± 0.57%, N = 7, p > 0.05) (Fig. 1C) [1].
3.2. Purinergic activation induces transient cytoskeleton rearrangement
We next asked whether purinergic receptor activation is associated with changes in the cytoarchitecture of cultured fibroblasts. Phalloidin staining of actin showed that the polymerized actin cytoskeleton was organized into classical stress fibers (Fig. 1D). The phalloidin labeling indicated that the stress fibers were organized in evenly distributed parallel bundles that were oriented in one direction within the individual cells (Fig. 1D). Upon exposure to ATP (100 μM), the classical stress fibers underwent a partial disassembly, detected as a decrease in the intensity of phalloidin staining of F-actin of parallel stress fibers (Fig. 1D). The disassembly was quantified by measuring the area occupied by F-actin within the cells. F-actin initially occupied 28.7 ± 0.7% of the cells, which decreased to 16.1 ± 2.4% at 10 min after the application of ATP (N = 5-7, p < 0.05, repeated measures one way ANOVA with Tukey-Kramer test) (Fig. 1E). This partial disassembly of the cytoskeleton was followed by a relatively rapid restoration of stress fibers: At 30 min F-actin rose back to the initial levels (28.2 ± 1.3%, N = 4, p > 0.05), providing additional evidence for a transient disassembly of stress fibers in fibroblast in response to purine receptor activation (Fig. 1E). Although the coverage of stress fibers returned to pre-stimulation values within 15-30 min after agonist exposure, in most cells the F-actin fibers were less strictly organized into evenly distributed, parallel oriented stress fibers. Post-agonist recovered stress fibers typically displayed F-actin fibers running in other directions than along primary axis of the cell (Fig. 1D, arrows).
3.3. Cytoskeleton rearrangement requires Ca2+ mobilization and purinergic activation
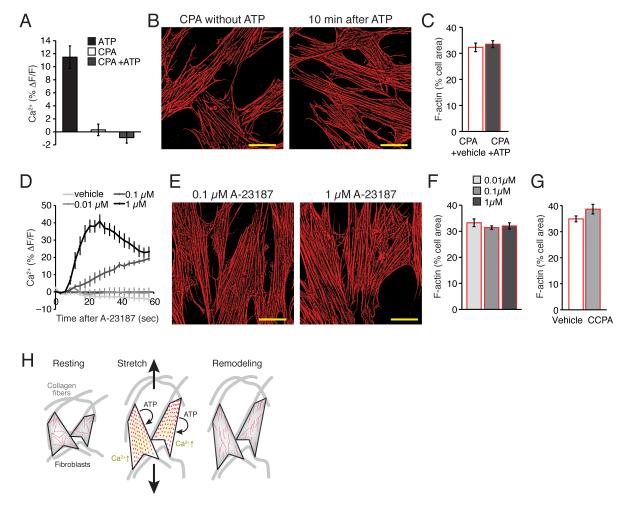
As cytoskeletal rearrangement of fibroblasts has been shown to be induced by mechanical stimulation of acupuncture needles ex vivo [21], we next assessed the roles of the molecular components by asking whether purine-induced disassembly of the cytoskeleton was a Ca2+ dependent process. Preloading with the inhibitor of Ca2+-ATPase pump in intracellular Ca2+ storage, CPA (20 μM) [22] effectively blocked Ca2+ increases in the cultured fibroblast (™0.88 ± 0.84%, N = 6, p > 0.05, one way ANOVA with Tukey-Kramer test) (Fig. 2A). ATP-induced stress fiber disassembly was also eliminated (Fig. 2B). The amounts of F-actin were unchanged by ATP application (33.5 ± 1.3%, N = 6, p > 0.05, t test) (Fig. 2C). This observation indicates that an increase in intracellular Ca2+ was critical for ATP-induced stress fiber disassembly. CPA loading alone had no effect on cytoskeleton organization (32.3 ± 1.6%, N = 6, p > 0.05, t test) (Fig. 2B,C). We next asked whether increases in cytosolic Ca2+ in the absence of agonist stimulation were sufficient to evoke a disassembly of F-actin stress fibers. To our surprise the Ca2+ ionophore A-23187 (0.01, 0.1, and 1 μM, Sigma) [22] had no effect on F-actin organization (N = 11, p > 0.5, one way ANOVA) despite triggering robust and long-lasting increases in cytosolic Ca2+ (N = 7, p < 0.05, one way ANOVA with Tukey-Kramer test) (Fig. 2D-F). Adenosine A1 receptor stimulation by CCPA (10 μM) did not result in stress fiber disassembly (N = 8, p > 0.05, t test compared to vehicle control at 10 min) (Fig. 2G). Combined, these observations suggest that purine receptor stimulated increases in cytosolic Ca2+ is required for reorganization of the actin cytoskeleton in cultured fibroblasts.
Fig. 2. Increase in cytosolic Ca 2+ is required, but not sufficient for purine-induced cytoskeletal reorganization.
(A) Ca2+ increase by ATP (100 μM) is completely blocked by a pretreatment of CPA (20 μM). N = 6, p = 0.35, t test between CPA and CPA+ATP. (B) The presence of CPA (20 μM) blocks F-actin (red) disassembly by ATP. Scale bars, 30 μm. (C) A summary histogram of actin disassembly at 10 min after vehicle or ATP (100 μM) application in the presence of CPA (20 μM). N = 6, p > 0.1, t tests. (D) Ca2+ increases by A-23187 (0, 0.01, 0.1, & 1 μM). N = 6, p < 0.001, ANOVA. (E) A Ca2+ ionophore A-23187 failed to induce F-actin (red) disassembly. Scale bars, 30 μm. (F) A summary histogram of F-actin disassembly at 10 min after application of A-23187 (0, 0.01, 0.1, & 1 μM). N = 11, p > 0.5, ANOVA. (G) A summary histogram of F-actin disassembly at 10 min after application of CCPA (10 μM). N = 7-8, p > 0.05 t tests compared to vehicle. (H) Proposed model. Fibroblast cells in situ are attached to collagen fibers of extracellular matrix. Increase of mechanical tension by tissue stretch causes ATP release, which in autocrine fashion activates own purinergic receptors. Purinergic activation triggers Ca2+ signaling and disassembly of polymerized actin. This transient disassembly enables the cells to undergo longer lasting morphological remodeling.
4. Discussion
This study showed that several purine agonists, including ATP, ADP, UTP and the P2X7 receptor agonist BzATP induced Ca2+ signaling and a transient loss of actin stress fibers in cultured human fibroblasts. The loss of actin stress fibers was visualized as a reduction in the relative area of parallel F-actin fibers stained by phalloidin [16]. The cytoskeleton changes peaked at 5 min after agonist-exposure or several minutes after the peak increase in cytosolic Ca2+ (Fig. 1D-E). Although the cytoskeleton was rebuilt within 15 -30 min after agonist exposure, the actin bundles were less organized in parallel bundles demonstrating a long-lasting effect of purine stimulation (Fig. 1D). Additional observations suggest that purine receptor-induced Ca2+ increases are required for remodeling of the actin cytoskeleton, since pretreatment with CPA suppressed both receptor-mediated Ca2+ increases and cytoskeletal remodeling (Fig. 2A-C). Ca2+ increases in themselves were, however, not sufficient to trigger cytoskeleton remodeling. Exposure to the Ca2+ionophore, ionomycin failed to evoke changes in the actin organization changes despite evoking robust increases in Ca2+ (Fig. 2D-F). Combined, these observations provide new critical insight into the mechanisms by which fibroblast respond to purine signaling and potentially extend prior analysis on how tissue remodeling may contribute to acupuncture induced suppression of chronic pain.
Earlier studies have shown that rotation of an inserted acupuncture needle stretches nearby connective tissue by pulling collagen fibers from the periphery toward the needle [23]. Both acupuncture needle rotation and simple tissue stretching cause fibroblasts to increase their cross-sectional area, as their cell bodies expand and spread out [10, 21, 24]. Tissue stretching is associated with a transient increase in tissue tension, but the viscoelastic properties of the tissue return to pre-stretching level within minutes, which occurs in parallel with active remodeling of the cytoskeleton of fibroblasts [25]. Tissue tension is likely sensed by fibroblast by their adhesion to collagen fibers [26]. Interestingly, pre-treatment with rho kinase inhibitors [27] or colchicine (inhibitor of microtubule polymerization) is linked to a 60–80% greater resting tissue tension after tissue stretching and prevents the expansion of fibroblasts [25]. These observations suggest that the remodeling of fibroblast in response to mechanical stimulation dampens the increase in tissue tension induced by tissue stretch and thereby is important for maintaining stable viscoelastic properties of the tissue [28]. Thus, the cytoskeleton of fibroblast plays an important role in dynamic tissue remodeling.
Is it possible that purinergic receptors are key players in the dynamic remodeling of the actin cytoskeleton of fibroblasts induced by mechanical stimulation? An extensive literature indicates that mechanical stimulation of fibroblast and other cell types is linked to ATP release [3]. Mechanosensitive channels open in response to physical stimulation of the plasma membrane resulting in release of cytosolic ATP [29]. HPLC measurement of microdialysis samples collected during acupuncture shows that purines are released in large quantities in both human volunteers and mice receiving manual acupuncture treatment [1, 4]. Thus, fibroblasts located close to the location of needle stimulation are not only exposed to the changes in tissue tension induced by needle rotations, but also to ATP [19]. Our current findings suggest that activation of purinergic receptors will in turn trigger a transient disassembly of polymerized actin (Fig. 2H). In cultured fibroblasts grown on a two-dimensional substrate, this actin depolymerization is manifested as a decrease in stress fibers without a marked change in the shape of the cell. In three-dimensional whole tissue, the same mechanism of purinergic signaling and actin bundle depolymerization would not be expected to cause a decrease in fibroblast stress fibers (because visible stress fibers are not present under these conditions) but could, nevertheless contribute to the rapid cytoskeletal remodeling and cell body expansion induced by tissue stretch.
An important remaining question is whether purine receptor-mediated remodeling of fibroblasts contribute to long-lasting pain relief following acupuncture. It has long been known that scar tissue is a common cause of chronic pain [30, 31]. Novel lines of work also suggest that connective tissue may become thicker and less compliant in patients with chronic pain, possibly as a result of chronic inflammation and fibrosis [32-34]. We propose that acupuncture-induced purine signaling triggers fibroblast cytoskeletal remodeling that counteracts fibrosis. In addition, adenosine has several anti-inflammatory actions that may contribute to the long-term reduction of chronic pain following acupuncture [35, 36].
Earlier studies have shown that the elastic property of connective tissue is a function of not only the extracellular matrix, but also of cellular actin and microtubuli cytoskeleton [25]. Fibroblasts respond to mechanical stress by cytoskeletal remodeling that contribute to adjusting the tissue viscoelastic properties to pre-existing levels [25]. Our observations reported here suggest that purinergic signaling via increases in cytosolic Ca2+ may contribute to such dynamic changes of the actin cytoskeleton. Thus, purinergic signaling may potentially play a role in connective tissue remodeling and thereby in the long-term benefits of acupuncture or physical therapy involving mechanical manipulation of connective tissue.
Acknowledgements
This study was supported by NIH (R01DE022743 and NS075177). We thank Ben Kress for valuable comments on the manuscript
Footnotes
Conflict of Interest: Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sawynok J. Caffeine and pain. Pain. 2011;152:726–729. doi: 10.1016/j.pain.2010.10.011. [DOI] [PubMed] [Google Scholar]
- [3].Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- [4].Takano T, Chen X, Luo F, Fujita T, Ren Z, Goldman N, Zhao Y, Markman JD, Nedergaard M. Traditional acupuncture triggers a local increase in adenosine in human subjects. J Pain. 2012;13:1215–1223. doi: 10.1016/j.jpain.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- [6].Lynch ME, Clark AJ, Sawynok J. Intravenous adenosine alleviates neuropathic pain: a double blind placebo controlled crossover trial using an enriched enrolment design. Pain. 2003;103:111–117. doi: 10.1016/s0304-3959(02)00419-0. [DOI] [PubMed] [Google Scholar]
- [7].Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- [8].Inoue K, Tsuda M, Koizumi S. ATP- and adenosine-mediated signaling in the central nervous system: chronic pain and microglia: involvement of the ATP receptor P2X4. J Pharmacol Sci. 2004;94:112–114. doi: 10.1254/jphs.94.112. [DOI] [PubMed] [Google Scholar]
- [9].Zylka MJ. Needling adenosine receptors for pain relief. Nat Neurosci. 2010;13:783–784. doi: 10.1038/nn0710-783. [DOI] [PubMed] [Google Scholar]
- [10].Langevin HM, Bouffard NA, Churchill DL, Badger GJ. Connective tissue fibroblast response to acupuncture: dose-dependent effect of bidirectional needle rotation. J Altern Complement Med. 2007;13:355–360. doi: 10.1089/acm.2007.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Langevin HM, Storch KN, Cipolla MJ, White SL, Buttolph TR, Taatjes DJ. Fibroblast spreading induced by connective tissue stretch involves intracellular redistribution of alpha- and beta-actin. Histochem Cell Biol. 2006;125:487–495. doi: 10.1007/s00418-005-0138-1. [DOI] [PubMed] [Google Scholar]
- [12].Jeong HJ, Park SW, Kim H, Park SK, Yoon D. Coculture with BJ fibroblast cells inhibits the adipogenesis and lipogenesis in 3T3-L1 cells. Biochem Biophys Res Commun. 2010;392:520–525. doi: 10.1016/j.bbrc.2009.12.184. [DOI] [PubMed] [Google Scholar]
- [13].Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nedergaard M. Extracellular Ca(2)(+) acts as a mediator of communication from neurons to glia. Sci Signal. 2012;5:ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem. 2002;277:44638–44650. doi: 10.1074/jbc.M203012200. [DOI] [PubMed] [Google Scholar]
- [18].Neary JT, Kang Y, Shi YF. Cell cycle regulation of astrocytes by extracellular nucleotides and fibroblast growth factor-2. Purinergic Signal. 2005;1:329–336. doi: 10.1007/s11302-005-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Furuya K, Sokabe M, Furuya S. Characteristics of subepithelial fibroblasts as a mechano-sensor in the intestine: cell-shape-dependent ATP release and P2Y1 signaling. J Cell Sci. 2005;118:3289–3304. doi: 10.1242/jcs.02453. [DOI] [PubMed] [Google Scholar]
- [20].Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol. 2011;300:F62–70. doi: 10.1152/ajprenal.00473.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Langevin HM, Bouffard NA, Badger GJ, Churchill DL, Howe AK. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006;207:767–774. doi: 10.1002/jcp.20623. [DOI] [PubMed] [Google Scholar]
- [22].Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+ Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Langevin HM, Churchill DL, Cipolla MJ. Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. FASEB J. 2001;15:2275–2282. doi: 10.1096/fj.01-0015hyp. [DOI] [PubMed] [Google Scholar]
- [24].Langevin HM, Bouffard NA, Badger GJ, Iatridis JC, Howe AK. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physiol Cell Physiol. 2005;288:C747–756. doi: 10.1152/ajpcell.00420.2004. [DOI] [PubMed] [Google Scholar]
- [25].Langevin HM, Bouffard NA, Fox JR, Palmer BM, Wu J, Iatridis JC, Barnes WD, Badger GJ, Howe AK. Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J Cell Physiol. 2011;226:1166–1175. doi: 10.1002/jcp.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- [27].Zhou C, Petroll WM. Rho Kinase Regulation of Fibroblast Migratory Mechanics in Fibrillar Collagen Matrices. Cell Mol Bioeng. 2010;3:76–83. doi: 10.1007/s12195-010-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abbott RD, Koptiuch C, Iatridis JC, Howe AK, Badger GJ, Langevin HM. Stress and matrix-responsive cytoskeletal remodeling in fibroblasts. J Cell Physiol. 2013;228:50–57. doi: 10.1002/jcp.24102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl- transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol. 2008;586:2779–2798. doi: 10.1113/jphysiol.2008.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Langevin HM, Cornbrooks CJ, Taatjes DJ. Fibroblasts form a body-wide cellular network. Histochem Cell Biol. 2004;122:7–15. doi: 10.1007/s00418-004-0667-z. [DOI] [PubMed] [Google Scholar]
- [31].Shin TM, Bordeaux JS. The role of massage in scar management: a literature review. Dermatol Surg. 2012;38:414–423. doi: 10.1111/j.1524-4725.2011.02201.x. [DOI] [PubMed] [Google Scholar]
- [32].Langevin HM, Stevens-Tuttle D, Fox JR, Badger GJ, Bouffard NA, Krag MH, Wu J, Henry SM. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC musculoskeletal disorders. 2009;10:151. doi: 10.1186/1471-2474-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Langevin HM, Fox JR, Koptiuch C, Badger GJ, Greenan-Naumann AC, Bouffard NA, Konofagou EE, Lee WN, Triano JJ, Henry SM. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC musculoskeletal disorders. 2011;12:203. doi: 10.1186/1471-2474-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Langevin HM, Sherman KJ. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses. 2007;68:74–80. doi: 10.1016/j.mehy.2006.06.033. [DOI] [PubMed] [Google Scholar]
- [35].da Rocha Lapa F, da Silva MD, de Almeida Cabrini D, Santos AR. Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal. 2012;8:693–704. doi: 10.1007/s11302-012-9299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nakav S, Chaimovitz C, Sufaro Y, Lewis EC, Shaked G, Czeiger D, Zlotnik M, Douvdevani A. Anti-inflammatory preconditioning by agonists of adenosine A1 receptor. PLoS One. 2008;3:e2107. doi: 10.1371/journal.pone.0002107. [DOI] [PMC free article] [PubMed] [Google Scholar]