Abstract
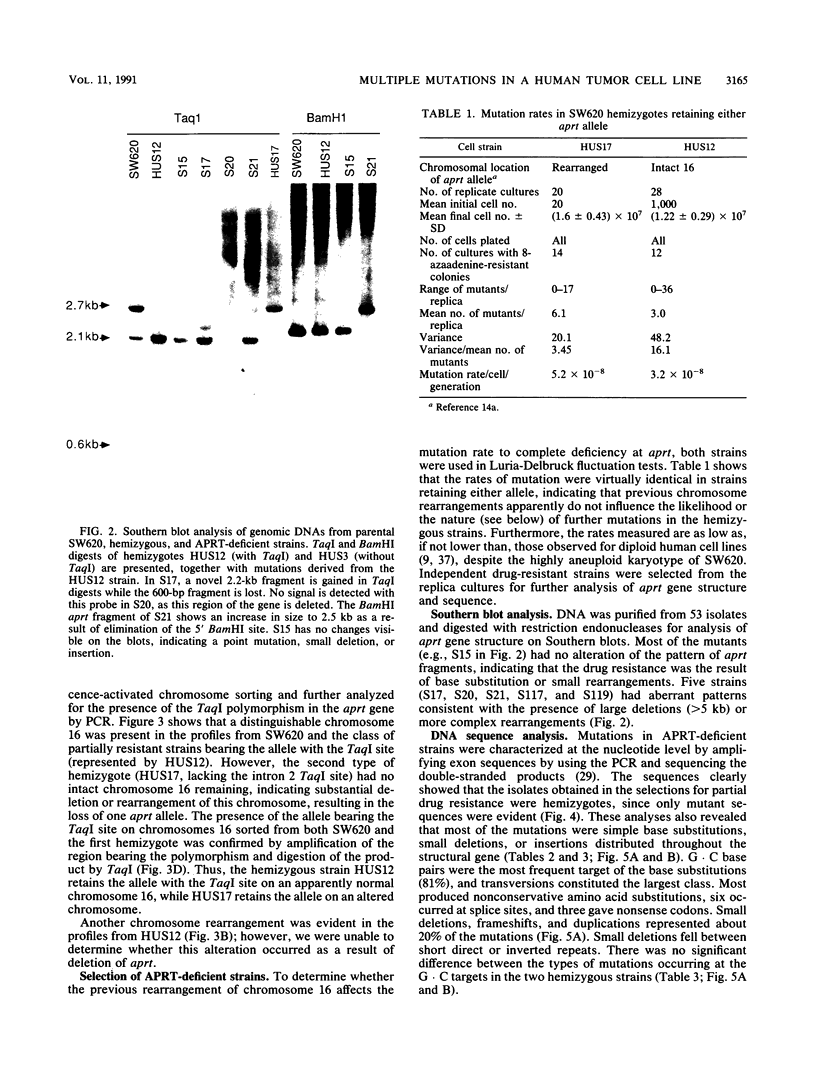
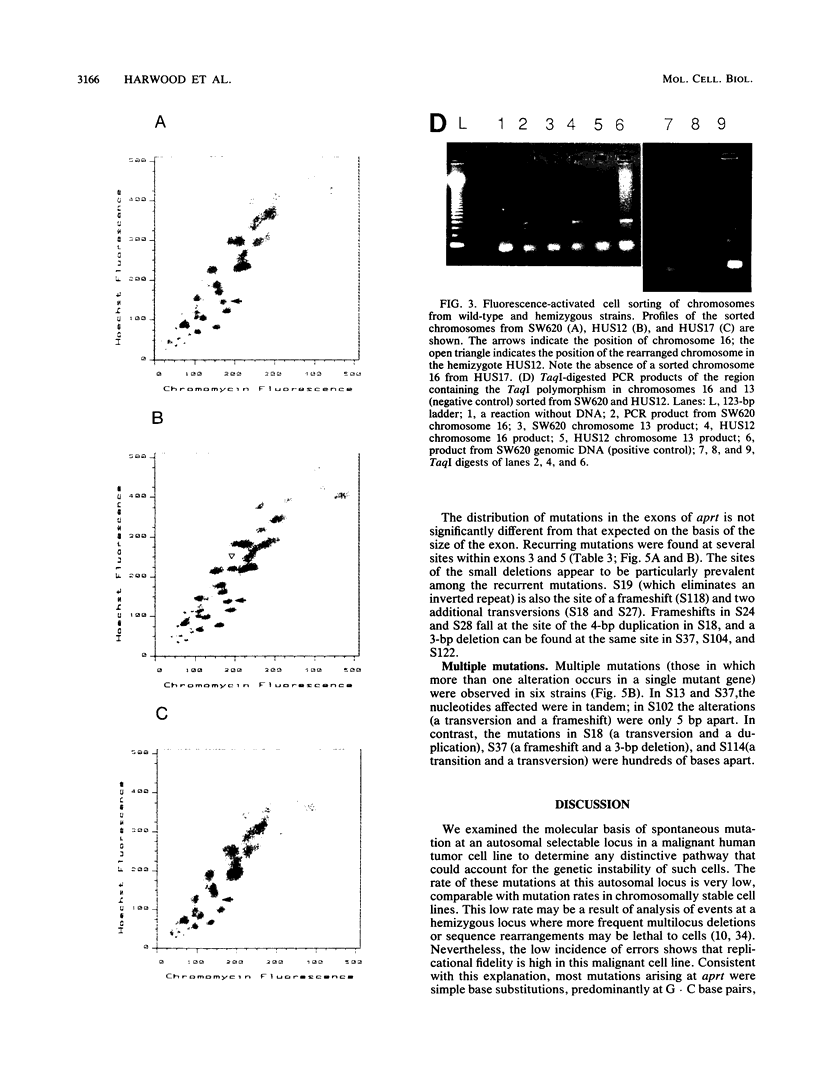
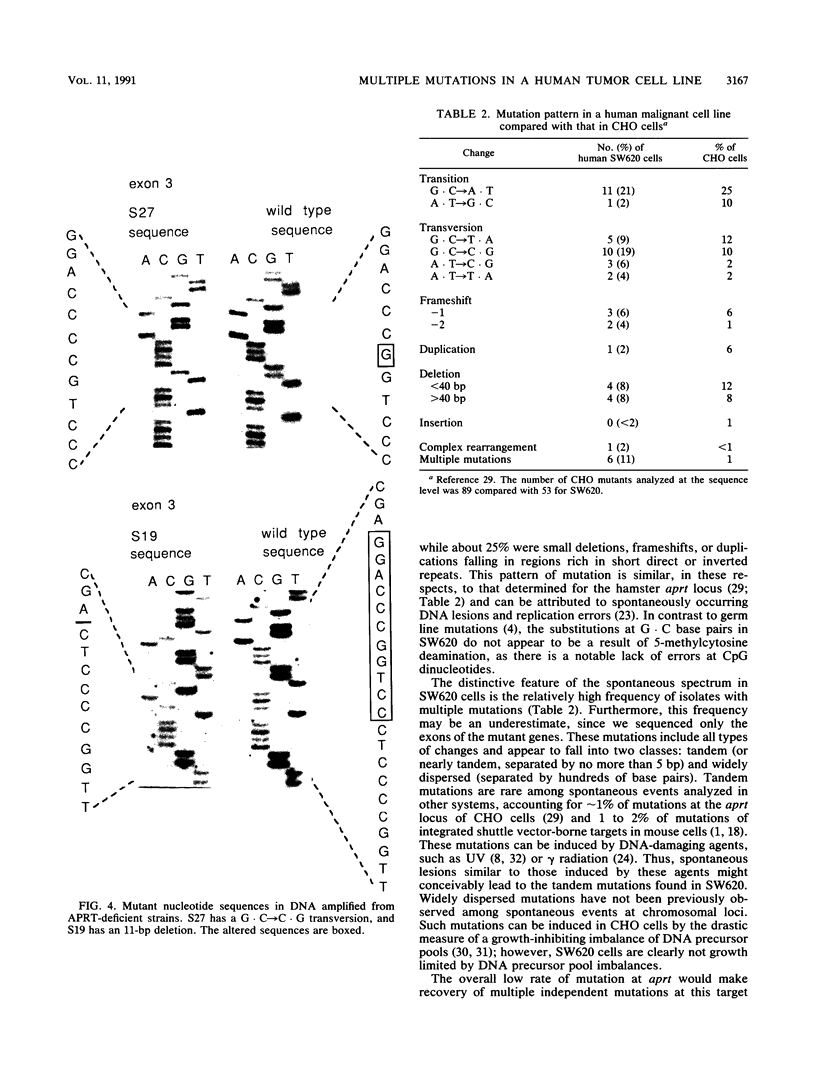
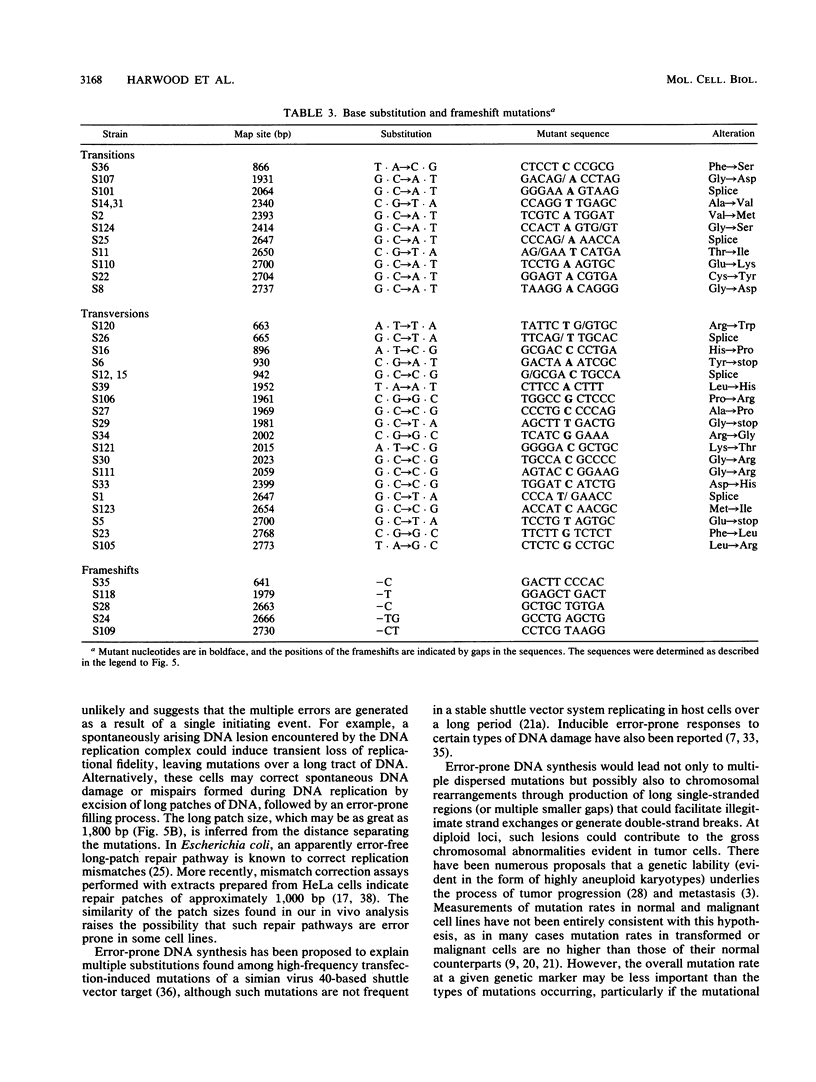
We analyzed the nature of spontaneous mutations at the autosomal locus coding for adenine phosphoribosyltransferase in the human colorectal carcinoma cell line SW620 to establish whether distinctive mutational pathways exist that might underlie the more complex genome rearrangements arising in tumor cells. Point mutations occur at a low rate in aprt hemizygotes derived from SW620, largely as a result of base substitutions at G.C base pairs to yield transversions and transitions. However, a novel pathway is evident in the form of multiple dispersed mutations in which two errors, separated by as much as 1,800 bp, fall in the same mutant gene. Such mutations could be the result of error-prone DNA synthesis occurring during normal replication or during long-patch excision-repair of spontaneously arising DNA lesions. This process could also contribute to the chromosomal instability evident in these tumor cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman C. R., Davidson R. L. Sequence analysis of spontaneous mutations in a shuttle vector gene integrated into mammalian chromosomal DNA. Proc Natl Acad Sci U S A. 1987 May;84(10):3354–3358. doi: 10.1073/pnas.84.10.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Campbell G. Mutations in human breast cancer: an overview. J Natl Cancer Inst. 1989 Dec 6;81(23):1780–1786. doi: 10.1093/jnci/81.23.1780. [DOI] [PubMed] [Google Scholar]
- Cifone M. A., Fidler I. J. Increasing metastatic potential is associated with increasing genetic instability of clones isolated from murine neoplasms. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6949–6952. doi: 10.1073/pnas.78.11.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Cotter F., Nasipuri S., Lam G., Young B. D. Gene mapping by enzymatic amplification from flow-sorted chromosomes. Genomics. 1989 Oct;5(3):470–474. doi: 10.1016/0888-7543(89)90011-6. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Role of chromosome translocations in human neoplasia. Cell. 1987 Apr 24;49(2):155–156. doi: 10.1016/0092-8674(87)90552-6. [DOI] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobetsky E. A., Grosovsky A. J., Glickman B. W. The specificity of UV-induced mutations at an endogenous locus in mammalian cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9103–9107. doi: 10.1073/pnas.84.24.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore E., Kakunaga T., Barrett J. C. Comparison of spontaneous mutation rates of normal and chemically transformed human skin fibroblasts. Cancer Res. 1983 Apr;43(4):1650–1655. [PubMed] [Google Scholar]
- Evans H. H., Mencl J., Horng M. F., Ricanati M., Sanchez C., Hozier J. Locus specificity in the mutability of L5178Y mouse lymphoma cells: the role of multilocus lesions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4379–4383. doi: 10.1073/pnas.83.12.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Hidaka Y., Tarlé S. A., O'Toole T. E., Kelley W. N., Palella T. D. Nucleotide sequence of the human APRT gene. Nucleic Acids Res. 1987 Nov 11;15(21):9086–9086. doi: 10.1093/nar/15.21.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J., Jr, Clark S., Modrich P. Strand-specific mismatch correction in nuclear extracts of human and Drosophila melanogaster cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5837–5841. doi: 10.1073/pnas.87.15.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehata H., Akagi T., Kimura H., Akasaka S., Kato T. Spectrum of spontaneous mutations in a cDNA of the human hprt gene integrated in chromosomal DNA. Mol Gen Genet. 1989 Nov;219(3):349–358. doi: 10.1007/BF00259606. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Kaden D., Gadi I. K., Bardwell L., Gelman R., Sager R. Spontaneous mutation rates of tumorigenic and nontumorigenic Chinese hamster embryo fibroblast cell lines. Cancer Res. 1989 Jun 15;49(12):3374–3379. [PubMed] [Google Scholar]
- Kendal W. S., Frost P. Metastatic potential and spontaneous mutation rates: studies with two murine cell lines and their recently induced metastatic variants. Cancer Res. 1986 Dec;46(12 Pt 1):6131–6135. [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Meuth M. The structure of mutation in mammalian cells. Biochim Biophys Acta. 1990 Jun 1;1032(1):1–17. doi: 10.1016/0304-419x(90)90009-p. [DOI] [PubMed] [Google Scholar]
- Miles C., Meuth M. DNA sequence determination of gamma-radiation-induced mutations of the hamster aprt locus. Mutat Res. 1989 Oct;227(2):97–102. doi: 10.1016/0165-7992(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Modrich P. Methyl-directed DNA mismatch correction. J Biol Chem. 1989 Apr 25;264(12):6597–6600. [PubMed] [Google Scholar]
- Nalbantoglu J., Goncalves O., Meuth M. Structure of mutant alleles at the aprt locus of Chinese hamster ovary cells. J Mol Biol. 1983 Jul 5;167(3):575–594. doi: 10.1016/s0022-2836(83)80099-0. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Phear G., Armstrong W., Meuth M. Molecular basis of spontaneous mutation at the aprt locus of hamster cells. J Mol Biol. 1989 Oct 20;209(4):577–582. doi: 10.1016/0022-2836(89)90595-0. [DOI] [PubMed] [Google Scholar]
- Phear G., Meuth M. The genetic consequences of DNA precursor pool imbalance: sequence analysis of mutations induced by excess thymidine at the hamster aprt locus. Mutat Res. 1989 Oct;214(2):201–206. doi: 10.1016/0027-5107(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Phear G., Nalbantoglu J., Meuth M. Next-nucleotide effects in mutations driven by DNA precursor pool imbalances at the aprt locus of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4450–4454. doi: 10.1073/pnas.84.13.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romac S., Leong P., Sockett H., Hutchinson F. DNA base sequence changes induced by ultraviolet light mutagenesis of a gene on a chromosome in Chinese hamster ovary cells. J Mol Biol. 1989 Sep 20;209(2):195–204. doi: 10.1016/0022-2836(89)90272-6. [DOI] [PubMed] [Google Scholar]
- Ronai Z. A., Lambert M. E., Weinstein I. B. Inducible cellular responses to ultraviolet light irradiation and other mediators of DNA damage in mammalian cells. Cell Biol Toxicol. 1990 Jan;6(1):105–126. doi: 10.1007/BF00135030. [DOI] [PubMed] [Google Scholar]
- Sargent G., Phear G., Meuth M. Deletion formation in mammalian cells: molecular analysis of breakpoints and junctions in the hamster aprt locus. New Biol. 1989 Nov;1(2):205–213. [PubMed] [Google Scholar]
- Sarkar S., Dasgupta U. B., Summers W. C. Error-prone mutagenesis detected in mammalian cells by a shuttle vector containing the supF gene of Escherichia coli. Mol Cell Biol. 1984 Oct;4(10):2227–2230. doi: 10.1128/mcb.4.10.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Bredberg A., Seetharam S., Kraemer K. H. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. doi: 10.1073/pnas.84.14.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri R., Kutlaca R. J., Trainor K., Matthews C., Morley A. A. Mutation rate of normal and malignant human lymphocytes. Cancer Res. 1987 Jan 15;47(2):407–409. [PubMed] [Google Scholar]
- Thomas D. C., Roberts J. D., Kunkel T. A. Heteroduplex repair in extracts of human HeLa cells. J Biol Chem. 1991 Feb 25;266(6):3744–3751. [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Young B. D., Ferguson-Smith M. A., Sillar R., Boyd E. High-resolution analysis of human peripheral lymphocyte chromosomes by flow cytometry. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7727–7731. doi: 10.1073/pnas.78.12.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]