Abstract
Imaging of the eye plays an important role in ocular therapeutic discovery and evaluation in preclinical models and patients. Advances in ophthalmic imaging instrumentation have enabled visualization of the retina at an unprecedented resolution. These developments have contributed toward early detection of the disease, monitoring of disease progression, and assessment of the therapeutic response. These powerful technologies are being further harnessed for clinical applications by configuring instrumentation to detect disease biomarkers in the retina. These biomarkers can be detected either by measuring the intrinsic imaging contrast in tissue, or by the engineering of targeted injectable contrast agents for imaging of the retina at the cellular and molecular level. Such approaches have promise in providing a window on dynamic disease processes in the retina such as inflammation and apoptosis, enabling translation of biomarkers identified in preclinical and clinical studies into useful diagnostic targets. We discuss recently reported and emerging imaging strategies for visualizing diverse cell types and molecular mediators of the retina in vivo during health and disease, and the potential for clinical translation of these approaches.
Introduction
Ocular imaging is valuable as a clinical tool for assessing retinal pathology, tracking disease progression, and monitoring the response to therapeutic interventions in the patient. Imaging of the retina has been performed for over a century, allowing direct observation of ocular structures in health and disease. Digital fundus cameras and scanning laser ophthalmoscopes are used in the clinic for morphological analysis of the vasculature using white light illumination, and can be utilized in conjunction with barrier filters and injection of blood-pooling fluorescent organic dyes, such as fluorescein and indocyanine green, for assessing disease pathology. Fluorescein and indocyanine green-guided angiography have been particularly useful for determining the progression of retinal vascular diseases by enhancing imaging of vascular irregularities, ocular angiogenesis in proliferative diabetic retinopathy, and neovascular age-related macular degeneration, as well as blood–retina barrier integrity.1,2 In angiography, blood pooling agents are injected intravenously to illuminate major vessels and capillary beds for analysis of the vascular structure. Quantitative ocular fluorophotometry, a technique that quantifies albumin-bound Evans Blue dye leakage from retinal vessels, has been used in preclinical and clinical settings for analysis of retinal vascular integrity.3 In addition, imaging of the eye using optical coherence tomography (OCT) is now routinely performed in the clinic for imaging of retinal tissue and pathology, including macular edema, with a high lateral and axial resolution.4–6 OCT is an interferometric, near-infrared light illumination-based technique that allows for a histological-type z-axis resolution and volumetric measurement capabilities.4 Continued advancements in imaging instrumentation, such as the adaptive optics scanning laser ophthalmoscope, provide the means for imaging of the retina with a single-cell resolution and for imaging important hallmarks of retinal disease initiation and progression such as rod-cone photoreceptors,7,8 ganglion cell morphology,9 and single leukocyte flow in capillaries.9,10 Therefore, imaging instrumentation is capable of enhancing clinical diagnostic capabilities such that cellular and molecular events may be characterized in the patient.
While ophthalmic imaging instrumentation is capable of imaging retinal morphology with unprecedented resolution, a challenge remains to detect subclinical retinal changes, which occur before advanced retinal disease. Subtle cellular and molecular changes may occur in the retina before the point at which disease can be detected by current ophthalmic diagnostic imaging. Furthermore, development of strategies for imaging events, which occur at the molecular level, may aid efforts to more closely track disease progression and evaluate a response to therapeutics. Toward this objective, a number of preclinical and clinical studies using a number of molecular profiling techniques such as mass spectrometry and sequencing have confirmed that molecular changes can precede retinal structural changes in a number of diseases responsible for vision loss, including age-related macular degeneration, glaucoma, diabetic retinopathy, and retinopathy of prematurity.11–20 Therefore, there is a strong and clinically unmet need to translate imaging strategies capable of detecting disease biomarkers in the patient.
The development of strategies, which bridge the discovery of retinal molecular biomarkers associated with disease susceptibility [e.g., mitochondrial dysfunction in retinal ganglion cells (RGCs) before apoptosis], advancement [e.g., the dry to wet age-related macular degeneration (AMD) transition], and regression due to effective treatment (e.g., responsiveness to antivascular endothelial growth factor therapy) with advances in imaging methods hold the potential to revolutionize ophthalmology. In this review, we will discuss strategies for interrogating the retina at the cellular and molecular level using ophthalmic imaging instrumentation and targeted contrast agents as they pertain to imaging specific disease-relevant cell types, along with their potential for clinical translation.
Molecular Imaging of RGCs
RGC dysfunction, apoptosis, and/or necrosis have been observed in a number of retinal diseases and insults, including glaucoma, ischemic disease, and ocular injury.21–23 Using conventional imaging techniques, RGC dysfunction cannot be detected before cell death and even RGC death may not be detectable until over 20% of RGCs have been lost.23 Similarly, visual field measurements detect retinal damage following widespread RGC apoptosis, and may not allow for early detection of diseases like glaucoma. Therefore, methods for detection of dysfunctional RGCs before their death, or at the very least detection of enough apoptosing or necrosing RGCs to lead to substantial visual deficits, are needed for improved diagnosis of RGC-related diseases. Identification of RGC deficits earlier in the time course of retinal disease would allow for timely therapeutic interventions with potentially improved outcomes in the patient.
Important progress has been made in the detection of RGC apoptosis with improved sensitivity. Using a technique developed by Cordeiro and colleagues named detection of apoptosing retinal cells (DARC), single-cell detection of RGC apoptosis has been demonstrated in experimental animal models of retinal diseases, and efforts to evaluate DARC in clinical trials have commenced.24–29 This imaging approach takes advantage of plasma membrane redistribution in apoptosis, which involves the translocation of the phosphatidylserine (PS) phospholipid from the inner to outer membrane leaflet, at which point, PS is accessible to extracellular targeting ligands. In DARC, fluorescently labeled Annexin V is administered via intraocular injection, and binds specifically to PS on apoptosing RGCs,30 which can be detected by ophthalmic fluorescence imaging instrumentation (Fig. 1). Another innovative approach for detecting apoptosis with high specificity and sensitivity involves the use of caspase enzyme-activatable fluorophores. Piwnica-Worms and colleagues developed this technique, which utilizes a peptide-based fluorescent probe called TcapQ for in vivo detection of RGC apoptosis.31–33 TcapQ is sensitive to active caspases such as caspase 3 involved in RGC apoptosis, and indicates the presence of active effector caspases. The peptide is administered intravitreally, and contains a modified cell-penetrating Tat peptide demonstrated to exhibit efficient RGC internalization in animal models.34 The peptide also contains a short peptide sequence, which is cleaved by caspases. On one end of the cleavable sequence, a fluorophore is coupled to the peptide, and on the other end, an organic fluorescence quencher is conjugated, which prevents emission of fluorescence from TCapQ unless the peptide is cleaved by caspase, liberating the fluorophore from its quencher. This concept has been further developed into a second-generation near-infrared probe, KcapQ.35 KcapQ has a modified peptide sequence to maintain high signal-to-background ratios due to improved quenching and caspase cleavage efficiencies, as well as reduced potential for toxicity making it a useful probe for imaging RGC death. A highlight of DARC and CapQ techniques are their amenability to quantitative imaging instrumentation and image processing. With appropriate imaging instrumentation, it will be possible to quantify apoptotic RGCs in the retina to standardize diagnosis and monitoring.
FIG. 1.
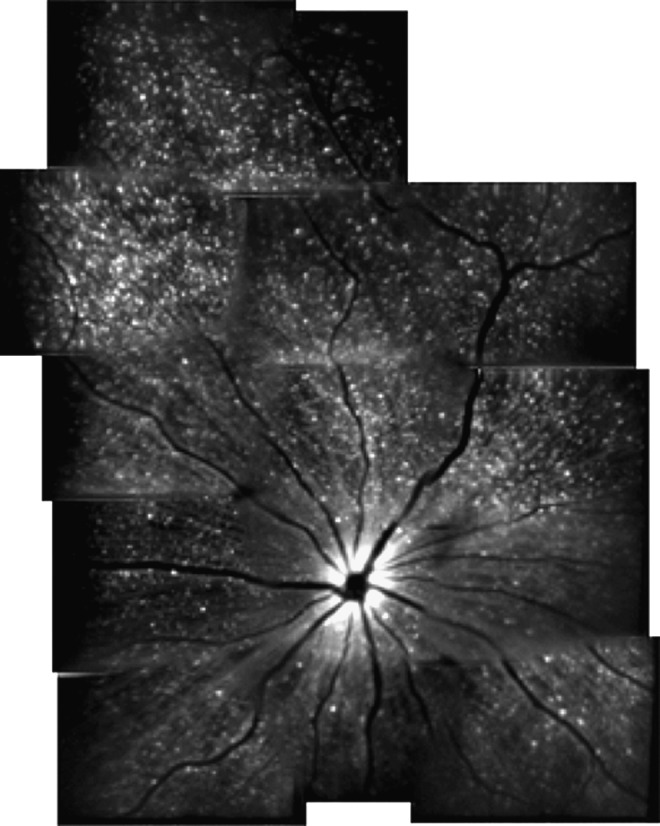
The detection of apoptosing retinal cells imaging approach for detecting retinal ganglion cell (RGC) apoptosis in animal models of ocular disease. Representative montage in vivo image of RGC apoptosis in a rat treated with intravitreally injected staurosporine. Imaging was accomplished using a confocal scanning laser ophthalmoscope with argon laser (488 nm) excitation of intravitreally injected Alexa Fluor 488-Annexin V conjugates, allowing visualization of apoptosing RGCs (hyperfluorescent white dots). Reproduced with permission from Cordeiro.24
The National Eye Institute, along with a number of clinicians and scientists, have collectively underscored the need for earlier diagnostic measurements for assessing RGC health,26 and to that end, a technique for early detection of apoptosis is imperative. The 2 aforementioned approaches, DARC and T- or KCapQ, have potential for clinical translation. DARC is entering clinical trials, and radiolabeled Annexin V has been used in over 30 clinical trials for diseases such as myocardial infarction, ischemic brain injury, and a number of cancers using radiological techniques with no significant indications of tissue toxicity.26 Therefore, the DARC approach has strong potential for clinical translation, and if incorporated into standard ophthalmic practice, this molecular imaging approach will likely pave the way for effective neuroprotective strategies to halt or stabilize RGC dysfunction and death. Likewise, the CapQ approach may prove to be clinically translatable following further assessments of long-term potential for toxicity and off-target effects due to the lack of cell-type specificity for the cationic Tat peptide,34 as well as biodistribution and clearance, due to the observation that TCapQ can be transported to the optic nerve.34 Both approaches are amenable for imaging by widely adopted retinal imaging instrumentation, which will facilitate translation and address an important need in the field by detecting RGC death early in disease.
A promising opportunity for molecular imaging to play a role in RGC-related diseases lies in the imaging of RGC dysfunction before cell death. The aforementioned techniques utilizing Annexin V and CapQ only enable imaging of RGC apoptosis, and do not enable detection of sick RGCs before cell death. Methods for detection of molecular processes associated with dysfunctional RGCs would provide an opportunity for therapeutic intervention to prevent cell death and subsequent visual deficits. Oxidative stress has been implicated as a possible contributor to RGC death in injury and glaucoma attributable to generation of reactive oxygen (ROS) and nitrogen species,36 and several approaches from other fields used to image ROS products may be useful in detection of early RGC dysfunction. For example, molecular probes are available for imaging singlet oxygen (Singlet oxygen sensor green37–40), superoxide (dihydroethidium41), and hydrogen peroxide (peroxalate nanoparticles and numerous other probes42–44). Of these probes, only hydrogen peroxide has been successfully imaged in vivo using targeted imaging probes, as demonstrated using a mouse model of lipopolysaccharide-induced inflammation.42 Therefore, while these imaging agents have not been tested for their utility in the retina or even in vivo in many cases, their potential compatibility with ophthalmic imaging equipment warrant their further consideration for detecting molecular insults in RGC-related diseases that contribute to cell death. Other potential molecular imaging targets for consideration in these diseases are RGC axonal transport deficits, mitochondrial dysfunction, as well as glutamate and calcium levels, as abnormalities involving these targets have been implicated in RGC dysfunction and death.45–48 Several imaging probes described in the literature may be useful for interrogating these targets in vivo. Fluorescently labeled proteins, such as the brain-derived neurotrophic factor, cholera toxin subunit, dextrans, and lectins, can be utilized to monitor the efficiency of RGC uptake and transport, although specialized imaging equipment and/or multiple modalities may be required for such approaches, and the species used for axonal tracing have not been extensively tested for their toxicity.47,49–51 The mitochondria-selective JC-1 dye has been used to monitor changes in mitochondrial membrane potential, which is a function of the dye's fluorescence emission spectra.52 Novel sensors for glutamate have been developed, including the E glutamate optical sensor, which contains a glutamate-binding receptor subunit and a fluorophore, which changes its intensity upon a glutamate interaction.53,54 Calcium sensor dyes, such as Fura2, calcium green, and indo-1, have been developed for imaging intracellular calcium and can be adapted for in vivo applications.55 These dyes generally feature an acetoxymethyl ester, which enables their intracellular uptake, and through ratiometric optical imaging techniques and specially configured imaging instrumentation such as 2 photon microscopy, intracellular, and tissue Ca2+ levels can be determined.56 Therefore, an abundance of molecular imaging probes yet to be applied toward ocular disease diagnosis for glaucoma and other RGC-related diseases hold great promise in improving the level of detail achieved in ophthalmic diagnosis. An important hurdle to implementation of these approaches in the clinic will be to determine quantitative baseline metrics for the imaging parameters in healthy patients, such that disease thresholds for events such as defective axonal transport, mitochondrial dysfunction, and ROS generation and concentrations in the retina can be objectively defined for diagnostic procedures.
Molecular Imaging of the Retinal Pigment Epithelium
The retinal pigment epithelium (RPE) is an important cell layer for retinal health that is located between the retinal photoreceptor outer segments and choriocapillaris. The RPE is a postmitotic monolayer that serves as the outer portion of the blood–retina barrier, which is necessary to prevent diffusion of harmful components in circulating blood into the retina. Additionally, the RPE is involved in clearing the photoreceptor outer segments of toxins produced during the visual cycle, transporting nutrients and metabolites from the choroid, and absorbing light energy. Defects in the RPE structure and function can lead to a number of retinal degenerative and aging diseases such as retinitis pigmentosa,57 Stargardt disease,58 and AMD.59 The spectral domain OCT and associated image processing techniques have been critical in improving our understanding of the RPE structure and function in health and disease. Regarding other imaging modalities used for imaging the RPE, in vivo reflectance imaging of the RPE is difficult due to the overlapping photoreceptor layer, which obscures it, but the RPE can be visualized noninvasively on account of intrinsic autofluorescence of RPE molecules, and changes in this autofluorescence profile over time can be used in diagnosis and monitoring of RPE-related diseases.60,61 Recently, the adaptive optics scanning laser ophthalmoscope (SLO) was modified for imaging the RPE mosaic with the cellular and subcellular resolution.62,63 Therefore, imaging instrumentation is well-suited for imaging the RPE and is poised to make a transition whereby it could accommodate molecular imaging contrast agents. Although targeted molecular imaging agents for interrogating the RPE have yet to be extensively studied, some areas of exploration in this arena are discussed.
Autofluorescence imaging techniques for imaging intrinsic RPE molecules will continue to play an important role in preclinical studies as well as diagnosis of RPE-related diseases.64–67 One of the major imaging targets in this area, lipofuscin, is a byproduct of photoreceptor phagocytosis that is stored in granules on the basal side of the RPE and is composed of digested lipid and protein aggregates.64 In normal human RPE, lipofuscin constitutes 20%–33% of the cells total cytoplasmic space, while in disease conditions such as geographic atrophy, there is found to be elevated amounts of lipofuscin accumulation directly adjacent to the area of disease.64,68 The presence of lipofuscin in RPE can be detected as early as 1 year of age, and has been shown to progressively increase with age.69 A2E, a bisretinoid from the accumulation of all-trans retinal product of the visual processing cycle of the photoreceptors, is an abundant, specific component of lipofuscin useful for targeted molecular imaging.64,69,70 This bisretinoid is increased in disease and has been shown to disable RPE function through photo-oxidative damage, complement activation, and membrane degradation.67 A2E is an intrinsically fluorescent protein that can be imaged by direct optical imaging at an excitation wavelength of 488 nm and an emission ranging from 565 to 725 nm.69 On the apical side of the RPE, melanin, another intrinsically fluorescent protein, accumulates and can be used for fluorescence imaging. Melanin serves as a protective function against light scattering, radiation, oxidative stress, and photo-toxic damage.67,71 Near-infrared autofluoresence (NIA), with an excitation wavelength of 787 nm, is used to image melanin changes with an emission of >800 nm.71 NIA shows less change with age as compared to lipofuscin and has a more widespread distribution, whereas lipofuscin has a more focal appearance at lesion sites.71 Nonetheless, melanin-based NIA can provide some insight into RPE health since NIA decreases have been detected in genetic retinal degenerative diseases.71–73 To continue to implement autofluorescence imaging techniques in the clinic, it will be valuable to establish baseline and threshold autofluorescence levels (e.g., quantitative intensity values for a given optical imaging instrument) for standardized illumination and detection wavelengths with defined cohorts of patients and healthy controls to define uniform procedures. For example, spectral imaging has been utilized in a limited group of AMD patients to demonstrate that quantitative ratiometric imaging of Bruch's membrane autofluorescence, sub-RPE deposits, and lipofuscin using distinct illumination and detection settings may reveal disease-specific biomarker signatures, which may be useful in AMD diagnosis and monitoring.74
Beyond autofluorescence imaging, several RPE-related molecular targets may warrant further consideration for development of clinical diagnostic approaches, including biomarkers of oxidative stress as discussed for RGCs, which have long been utilized in vitro in conjunction with microscopy to characterize RPE dysfunction and may be useful as in vivo imaging tools.75,76 Oxidative stress has been shown to contribute to many age-related diseases, such as AMD, and to light-induced damage of the RPE.76 In vitro studies have shown that inhibiting mitochondrial electron transport chain action can reduce ROS and preserve the normal RPE phenotype.76 This suggests that ROS may be a good early indicator of disease-related changes in the RPE. A key diagnostic target for RPE-related diseases, primarily AMD, may lie in the formation of drusen between Bruch's membrane and the RPE with aging and other factors. Drusen is an extracellular heterogeneous aggregation of proteins and lipids that form with age. Its accumulation is seen in almost all aged eyes; however, drusen can change in its appearance and is thought to be directly involved in AMD pathogenesis. Drusen is classified clinically by its size and appearance. Hard drusen is any drusen less than 63 μm in size, however, if it appears hard and flat in a fundus measurement, it can be up to 125 μm.77–79 Soft drusen is greater than 63 μm in size and can be further characterized as distinct and indistinct. Distinct soft drusen has a uniform density appearance, whereas indistinct appears to have decreasing density from the center outwards.77,79 Using size, number, and the extent of confluency of drusen, as determined by fundus imaging, risk can be determined for AMD pathogenesis.77–79
Drusen can be identified as areas of hyperfluorescence in a fluorescein angiogram and components of drusen may be a potential target for molecular imaging modalities, for possibly enhancing molecular characterization of AMD and identifying additional risk factors, for example, drusen types and components, which hasten transition from dry to wet AMD. Since drusen develops with age, it is thought that its accumulation could be an age-related deficit of RPE filtration.79 Farkas et al. stated that some components of drusen include denatured mitochondria, cytoplasmic debris, pigment granules, and photoreceptor remnants.80,81 Additionally, esterified cholesterol and carbohydrate moieties such as N-acetylglucosamine and sialic acid have been identified in drusen, and might be targeted by small molecule synthetic probes.78 Protein components that have been identified include ubiquitin, integrins, Tissue Inhibitors of Metalloproteinases, Advanced Glycation End-Product, and β amyloid, which might be targets for antibody-coupled imaging contrast agents.78 Of equal potential for molecular imaging approaches involving RPE, complement system activation is altered, resulting in reduced cellular C-reactive protein-binding affinity, which disturbs extracellular RPE clearance, leading to build up of drusen.82 These changes are all potential targets for molecular imaging probes, and may be beneficial for determining disease progression in AMD or identify areas of risk related to drusen. One major issue in developing probes to drusen components is the lack of animal models for testing. Dry AMD, which constitutes 85%–90% of AMD cases, is characterized by focal areas of soft drusen at the macula.83 However, rodents do not have a macula and do not form focal regions of drusen build up. Wang and Neufield identified a potential animal model, the smoking mouse, which has similar components of drusen.83 In the smoking mouse, drusen-like deposits can be found at 8 months in a more uniformly distributed pattern than the human drusen.83 It is hoped that this model and others will be used to speed up the development of imaging probes for the molecular imaging of RPE. A major question to be addressed before advancing this line of investigation will be to examine contrast agent accessibility to RPE and drusen, and to evaluate classical measures of biodistribution and toxicity in an already compromised tissue microenvironment. Furthermore, as drusen characteristics (morphology, frequency) can be highly variable between patients, it may be difficult to establish quantitative thresholds to aid in diagnostic procedures, although efforts to standardize drusen detection, instrumentation, and imaging processing procedures have been reported.84
Molecular Imaging of Endothelial Cells
Retinal and/or choroidal endothelial cell dysfunction occurs in a number of diseases, including retinopathy of prematurity, diabetic retinopathy, and choroidal neovascularization, all leading causes of blindness. In these diseases, endothelial cells participate in a number of changes, such as expression of inflammatory proteins and loss of tight junction molecules, which hold blood vessel linings together, as well as proliferation and migration to form abnormal neovascular structures (i.e., ocular angiogenesis).85 Vascular leakage resulting from poor retinal endothelial barrier integrity and angiogenesis are significant retinal complications, and imaging techniques such as fluorescence angiography (FA), indocyanine green angiography (ICGA), and OCT are routinely used in the clinic to monitor these complications.86,87 However, these structural imaging techniques may often detect pathology only beyond subclinical disease stages, at which therapies might be more effective in preventing vision loss. Therefore, molecular imaging of endothelial cells could be utilized to identify molecular targets that are expressed in dysfunctional endothelial cells before advancement of complications and pathology.
Endothelial cell surface biomarkers of inflammation and/or angiogenesis are promising candidates for development of targeted contrast agents for ophthalmic imaging approaches, as these targets are accessible to injected contrast agents from the bloodstream and can be imaged in conjunction with FA or ICGA, thus integrating well into current ophthalmic imaging techniques. In addition, many such biomarkers are expressed focally on inflammatory endothelial cells and not adjacent, healthy endothelial cells, providing higher signal to noise. Furthermore, many of these targets have been extensively investigated using molecular imaging contrast agents in other fields, such as cardiovascular medicine and oncology. The C-C chemokine receptor 3 (CCR3) is a potentially important biomarker of choroidal neovascularization (CNV) in neovascular AMD, as demonstrated by CCR3 expression on choroidal neovascular endothelial cells in animal models and human CNV specimens.88,89 CCR3 binds to a number of chemokines and is typically associated with eosinophils, but in neovascular AMD receptor, expression was observed specifically on choroidal neovascular endothelial cells as detected in vivo using quantum dot nanocrystals linked to CCR3-targeted antibody fragments. Importantly, CCR3 imaging in the choroid revealed subclinical CNV at a stage before which it could be detected by FA, underscoring the power of molecular imaging to complement and enhance conventional ophthalmic imaging strategies.88 Another useful target for imaging retinal vascular disease, endoglin (CD105), is an endothelial cell surface glycoprotein upregulated on proliferating endothelial cells in preretinal or choroidal neovascular lesions, and can be targeted using antibodies.90–93 Endoglin-targeted antibodies have already shown promise in targeting proliferating endothelial cells in animal models of laser-induced choroidal neovascularization as well as patient specimens ex vivo.94,95 Integrins such as αVβ3 have been successfully imaged in cardiovascular disease96 and cancer,97–101 and should prove useful should they be investigated in retinal diseases using similar antibody and peptide-targeted approaches, due to the established role of integrins in retinal vascular biology.102 The intercellular adhesion molecule 1 and the vascular cell adhesion molecule 1 (VCAM-1) can be targeted using a number of nanoparticle-, antibody-, and peptide-mediated approaches for vascular imaging in a number of diseases.103–106 Jayagopal et al. have developed fluorescent quantum dot nanocrystals conjugated to targeting antibodies or peptides to interrogate VCAM-1 expression and other proteins in mouse models of laser-induced choroidal neovascularization (Fig. 2) and other experimental models of disease, and imaging these targets with sufficient signal to noise is feasible in a number of approaches using magnetic resonance imaging (MRI), optical imaging, positron emission tomography, and radiography.107,108 VCAM-1 is an especially relevant target for imaging inflammation due to focally increased expression on inflammatory or neovascular endothelial cells, with lack of detectable surface expression on healthy or quiescent endothelial cells in diseases such as neovascular AMD and proliferative diabetic retinopathy.109,110 A limitation of endothelial cell-targeted imaging approaches is the inability of the currently used imaging probes for accessing intracellular endothelial targets. However, Jayagopal et al. have recently developed gold nanoparticles for imaging intracellular RNA biomarkers, such as mRNA and miRNA, in mammalian cells,111 and are adapting their approach for imaging of RNA biomarkers in retinal endothelial cells in vivo. This approach may address limitations of current approaches such that currently inaccessible, but clinically relevant molecular targets can be interfaced for imaging and/or therapeutic purposes.
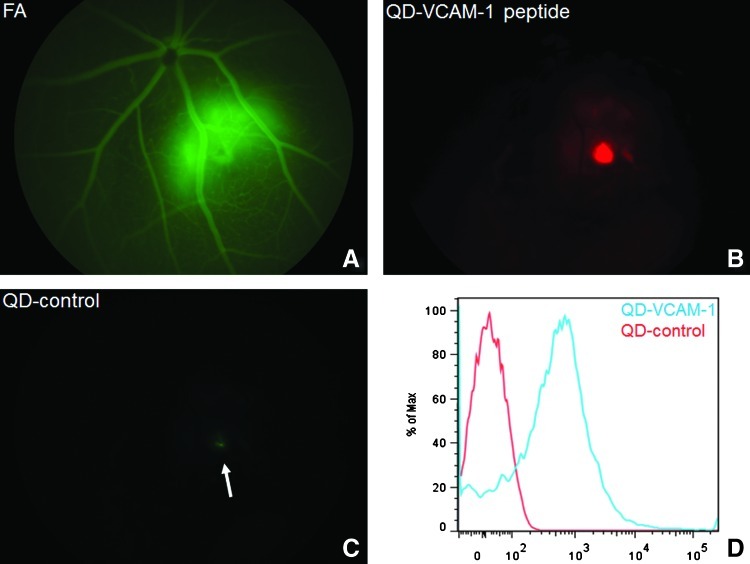
FIG. 2.
In vivo imaging of vascular cell adhesion molecule 1 (VCAM-1) expression in a mouse model of laser-induced choroidal neovascularization (LCNV) using custom VCAM-1 targeting peptides conjugated to quantum dot (QD) fluorescent semiconducting nanocrystals. (A) Fluorescein angiogram indicating CNV lesion 1 day postlaser injury. (B) Same retina as (A) in QD emission channel, indicating VCAM-1 expression in CNV lesion. (C) A scrambled peptide conjugated to spectrally distinct QD did not emit significant fluorescence from the lesion (arrow). (D) Flow cytometric analysis of QD conjugates incubated with (10 ng/mL) lipopolysaccharide-stimulated mouse retinal microvascular endothelial cells to induce VCAM-1 expression. QD-VCAM-1 conjugates were specifically internalized by VCAM-1+ endothelial cells in vitro.
Several other endothelial cell-related targets in the retina are useful targets for direct or indirect molecular imaging, including hypoxic biomarkers, matrix metalloproteinases (MMPs), and tight junction molecules. Hypoxia is an important biomarker associated with ocular angiogenesis in a number of diseases. The deprivation of local tissue oxygen supply can be optically imaged either by targeting relevant biomarkers, such as carbonic anhydrases, using antibodies or small molecules related to sulfonamides,112,113 can be imaged indirectly by using novel spectral imaging techniques using phosphorescence lifetime-based imaging agents,114–118 or can be imaged in conjunction with fluorescent nitroimidazole derivatives.119 Nitroimidazoles, such as pimonidazole, form adducts with intracellular proteins preferentially in hypoxic cells due to bioreduction with nitroreductases, causing their intracellular retention, subsequently enabling visualization of hypoxic cells with high signal to background ratios. MMPs are involved in remodeling of the tissue microenvironment, which enables cell migration in angiogenesis, among other functions, and a number of peptide-based probes have been developed to detect their function using optical imaging. In this approach, peptides containing a fluorophore and fluorophore-matched quencher are coupled on opposite ends of a short peptide, the sequence of which is engineered to be cleaved by (1) specific MMP member(s), thus resulting in fluorescence emission.120–123 This strategy has not been applicable to all MMP members due to lack of available specific cleavage peptide sequences, but the presence and absence of MMPs in the retina may be valuable for diagnosis and monitoring of angiogenesis in retinal neovascular diseases.124 To image tight junction molecules, which are critical components of the blood–retinal barrier,125–127 established and emerging techniques are available for assessment of retinal vascular integrity via imaging of leakage. Several approaches, including vitreous fluorophotometry, FA, and dual tracer fluorescence angiography, use fluorescent tracers of varying molecular weight to monitor leakage in the retina, often using quantitative techniques.128–134 In addition, dynamic contrast-enhanced MRI has been adapted for similar analysis.135,136 By examining leakage of compounds of varying molecular weight, quantitative thresholding can be performed to quantify the extent of disease severity. However, these approaches are indirect imaging approaches examining surrogate endpoints underlying the blood–retinal barrier function, and approaches to image the loss of tight junction molecules directly in the retina may be useful for targeted drug development and diagnostic studies. It is important to note that many endothelial cell-targeted therapies described above are in development or in clinical usage, thus making possible combined imaging and therapy of retinal disease (theranostics).
Molecular Imaging of Leukocytes
Inflammation is thought to be a common component of most retinal diseases, including retinopathy, macular degeneration, glaucoma, and uveitis, as exhibited by cascades of molecular signaling pathways and leukocyte–endothelial–tissue interactions in these diseases.137 As inflamed endothelial cells express surface proteins to recruit leukocytes to the sites of inflammation, which can be detected by molecular imaging as discussed above, much interest has also been directed toward imaging leukocytes and leukocyte subpopulations in retinal diseases as well. Early efforts for imaging leukocytes such as neutrophils in preclinical models of retinal inflammation have involved the DNA-intercalating dye acridine orange (AO), which is used to identify circulating leukocytes as hyperfluorescent dots in the retina, along with endothelial cell linings, using the SLO (Fig. 3).138–141 Studies incorporating AO fluorography of leukocytes have improved our understanding of complex spatiotemporal dynamics of leukocyte–endothelial interactions in retinal inflammatory diseases, but as AO is phototoxic and intercalates with nucleic acids within the cell, the dye is not suitable for clinical usage.142 Efforts to image leukocyte subtypes are being investigated to allow thorough characterization of leukocyte populations in disease. Macrophages play an important role in diseases such as AMD and methods of detecting these cell types specifically have been clinically translated. Typical approaches are MRI- or optical imaging-based, and use lipoproteins, peptides, and/or antibodies to direct nanoparticles, such as superparamagnetic iron oxide particles, which are T2 MRI contrast agents, or fluorescent dyes to resident macrophages within blood vessels or tissue, as demonstrated by a number of studies involving atherosclerosis.107,143–146 Optical imaging of leukostasis through AO fluorography can be used for quantitative analysis (e.g., counting of arrested leukocytes), demonstrating that leukocyte imaging can be useful for standardized clinical diagnostic approaches.
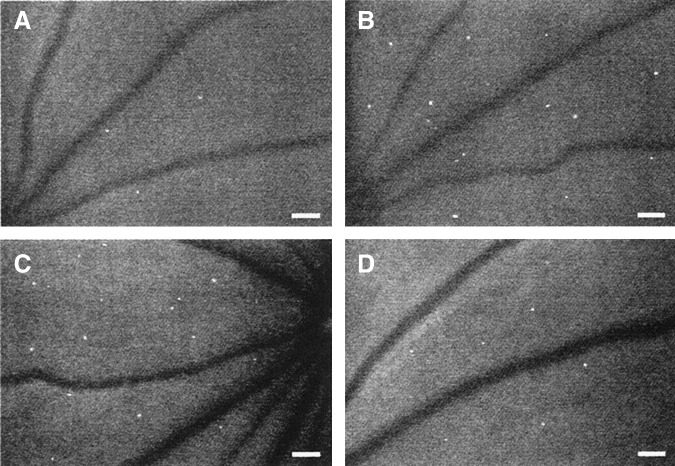
FIG. 3.
In vivo imaging of retinal leukostasis and therapeutic inhibition of leukostasis in mice using acridine orange (AO) fluorography. AO-stained leukocytes appear as hyperfluorescent white dots in the microvasculature. (A) nondiabetic mice, (B) diabetic mice, (C) diabetic mice administered control immunoglobulin G (IgG), (D) diabetic mice administered leukostasis-inhibiting anti-intercellular adhesion molecule-1 IgG. Reproduced with permission from Miyamoto et al.138 Scale Bars=100 microns (μm) 3.2 pixels=1μm.
Other approaches involve imaging of leukocytes using dye or nanocrystal-labeled antibodies or peptides, but the effects of these conjugates on the inflammatory response (i.e., contrast agent-induced dampening or exacerbating inflammation) have not been extensively investigated.107,108,147 Further development of biocompatible, inert imaging agents for imaging of leukocytes in vivo is warranted to enable detection of leukocyte–endothelial interactions, such as arrest, rolling, and transmigration, to develop new therapies and evaluate the effects of current therapies in the patient. Other circulating cell types of interest for imaging applications are endothelial progenitor cells (EPCs), which are thought to play important roles in vessel remodeling in angiogenesis or vessel injury in retinal diseases.148–153 EPC functions are still being elucidated and EPCs are being studied as therapies themselves or as targets for therapy. Therefore, development of targeting strategies similar to those used for leukocytes would be helpful in advancing preclinical and clinical studies involving EPC function. However, a potential limitation of these strategies may involve adverse activation of an already stimulated immune system via introduction of imaging probes and their interfacing with leukocytes and endothelial cell surface proteins. It will be important for clinical translation of leukocyte-directed imaging probes to judiciously select molecular imaging targets in conjunction with utilizing stealth or inert imaging probes to reduce the potential for such effects.
Conclusion
Through preclinical studies and emerging clinical applications, molecular imaging of the retina is poised to play an important role in disease diagnosis and monitoring, as well as assessment of therapeutic efficacy. Key considerations for improving clinical translation and incorporation of these imaging approaches will revolve around minimizing adverse effects of imaging agents, due to potential for off-target biodistribution and activation of the immune system, among many possibilities. Furthermore, imaging agents developed initially for preclinical investigations will need to be scaled up for clinical implementation using appropriate manufacturing and quality control practices used in the industry, which constitutes a major translational hurdle. Nevertheless, molecular imaging has revealed great insight into the mechanisms and mediators governing the retina in health and disease, and many of the technologies discussed in this work will likely evolve into powerful techniques in the ophthalmic practice.
Acknowledgments
This work was supported by the International Retinal Research Foundation, the American Health Assistance Foundation, a Core Grant in Vision Research from the National Institutes of Health (P30-EY008126), the American Diabetes Association and a departmental unrestricted grant from Research to Prevent Blindness.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gass J.D. Sever R.J. Sparks D. Goren J. A combined technique of fluorescein funduscopy and angiography of the eye. Arch. Ophthalmol. 1967;78:455–461. doi: 10.1001/archopht.1967.00980030457009. [DOI] [PubMed] [Google Scholar]
- 2.Bernardes R. Serranho P. Lobo C. Digital ocular fundus imaging: a review. Ophthalmologica. 2011;226:161–181. doi: 10.1159/000329597. [DOI] [PubMed] [Google Scholar]
- 3.Raines M.F. Vitreous fluorophotometry: a review. J. R. Soc. Med. 1988;81:403–406. doi: 10.1177/014107688808100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadler Z. Wollstein G. Ishikawa H. Schuman J.S. Clinical application of ocular imaging. Optom. Vis. Sci. 2012;89:E543–E553. doi: 10.1097/OPX.0b013e31824f164d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabriele M.L. Wollstein G. Ishikawa H. Kagemann L. Xu J. Folio L.S. Schuman J.S. Optical coherence tomography: history, current status, and laboratory work. Invest. Ophthalmol. Vis. Sci. 2011;52:2425–2436. doi: 10.1167/iovs.10-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriele M.L. Wollstein G. Ishikawa H. Xu J. Kim J. Kagemann L. Folio L.S. Schuman J.S. Three dimensional optical coherence tomography imaging: advantages and advances. Prog. Retin. Eye Res. 2010;29:556–579. doi: 10.1016/j.preteyeres.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubra A. Sulai Y. Norris J.L. Cooper R.F. Dubis A.M. Williams D.R. Carroll J. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed. Opt. Express. 2011;2:1864–1876. doi: 10.1364/BOE.2.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godara P. Siebe C. Rha J. Michaelides M. Carroll J. Assessing the photoreceptor mosaic over drusen using adaptive optics and SD-OCT. Ophthalmic Surg. Lasers Imaging. 2010;41(Suppl):S104–108. doi: 10.3928/15428877-20101031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roorda A. Romero-Borja F. Donnelly Iii W. Queener H. Hebert T. Campbell M. Adaptive optics scanning laser ophthalmoscopy. Opt. Express. 2002;10:405–412. doi: 10.1364/oe.10.000405. [DOI] [PubMed] [Google Scholar]
- 10.Eter N. Molecular imaging in the eye. Br. J. Ophthalmol. 2010;94:1420–1426. doi: 10.1136/bjo.2009.158105. [DOI] [PubMed] [Google Scholar]
- 11.Recchia F.M. Xu L. Penn J.S. Boone B. Dexheimer P.J. Identification of genes and pathways involved in retinal neovascularization by microarray analysis of two animal models of retinal angiogenesis. Invest. Ophthalmol. Vis. Sci. 2010;51:1098–1105. doi: 10.1167/iovs.09-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brantley M.A., Jr. Osborn M.P. Sanders B.J. Rezaei K.A. Lu P. Li C. Milne G.L. Cai J. Sternberg P., Jr. Plasma biomarkers of oxidative stress and genetic variants in age-related macular degeneration. Am. J. Ophthalmol. 2012;153:460–467. doi: 10.1016/j.ajo.2011.08.033. e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiggs J.L. Yaspan B.L. Hauser M.A. Kang J.H. Allingham R.R. Olson L.M. Abdrabou W. Fan B.J. Wang D.Y. Brodeur W. Budenz D.L. Caprioli J. Crenshaw A. Crooks K. Delbono E. Doheny K.F. Friedman D.S. Gaasterland D. Gaasterland T. Laurie C. Lee R.K. Lichter P.R. Loomis S. Liu Y. Medeiros F.A. McCarty C. Mirel D. Moroi S.E. Musch D.C. Realini A. Rozsa F.W. Schuman J.S. Scott K. Singh K. Stein J.D. Trager E.H. Vanveldhuisen P. Vollrath D. Wollstein G. Yoneyama S. Zhang K. Weinreb R.N. Ernst J. Kellis M. Masuda T. Zack D. Richards J.E. Pericak-Vance M. Pasquale L.R. Haines J.L. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012;8:e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman A.M. Gallo N.B. Hancox L.S. Miller N.J. Radeke C.M. Maloney M.A. Cooper J.B. Hageman G.S. Anderson D.H. Johnson L.V. Radeke M.J. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012;4:16. doi: 10.1186/gm315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhutto I.A. Baba T. Merges C. Juriasinghani V. McLeod D.S. Lutty G.A. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br. J. Ophthalmol. 2011;95:1323–1330. doi: 10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brantley M.A., Jr. Osborn M.P. Sanders B.J. Rezaei K.A. Lu P. Li C. Milne G.L. Cai J. Sternberg P., Jr. The short-term effects of antioxidant and zinc supplements on oxidative stress biomarker levels in plasma: a pilot investigation. Am. J. Ophthalmol. 2012;153:1104–1109. doi: 10.1016/j.ajo.2011.12.010. e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz S.G. Brantley M.A., Jr. Pharmacogenetics and age-related macular degeneration. J. Ophthalmol. 2011;2011:252549. doi: 10.1155/2011/252549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tezel G. Thornton I.L. Tong M.G. Luo C. Yang X. Cai J. Powell D.W. Soltau J.B. Liebmann J.M. Ritch R. Immunoproteomic analysis of potential serum biomarker candidates in human glaucoma. Invest. Ophthalmol. Vis. Sci. 2012;53:8222–8231. doi: 10.1167/iovs.12-10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tezel G. Yang X. Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest. Ophthalmol. Vis. Sci. 2005;46:3177–3187. doi: 10.1167/iovs.05-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju W.K. Kim K.Y. Lindsey J.D. Angert M. Duong-Polk K.X. Scott R.T. Kim J.J. Kukhmazov I. Ellisman M.H. Perkins G.A. Weinreb R.N. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Invest. Ophthalmol. Vis. Sci. 2008;49:4903–4911. doi: 10.1167/iovs.07-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farkas R.H. Grosskreutz C.L. Apoptosis, neuroprotection, and retinal ganglion cell death: an overview. Int. Ophthalmol. Clin. 2001;41:111–130. doi: 10.1097/00004397-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Qu J. Wang D. Grosskreutz C.L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp. Eye Res. 2010;91:48–53. doi: 10.1016/j.exer.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborne N.N. and Del Olmo-Aguado, S. Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr. Opin. Pharmacol. 2013;13:16–22. doi: 10.1016/j.coph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Cordeiro M.F. Guo L. Luong V. Harding G. Wang W. Jones H.E. Moss S.E. Sillito A.M. Fitzke F.W. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl. Acad. Sci. U. S. A. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordeiro M.F. Guo L. Coxon K.M. Duggan J. Nizari S. Normando E.M. Sensi S.L. Sillito A.M. Fitzke F.W. Salt T.E. Moss S.E. Imaging multiple phases of neurodegeneration: a novel approach to assessing cell death in vivo. Cell Death Dis. 2010;1:e3. doi: 10.1038/cddis.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordeiro M.F. Migdal C. Bloom P. Fitzke F.W. Moss S.E. Imaging apoptosis in the eye. Eye. 2011;25:545–553. doi: 10.1038/eye.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvao J. Davis B.M. Cordeiro M.F. In vivo imaging of retinal ganglion cell apoptosis. Curr. Opin. Pharmacol. 2013;13:123–127. doi: 10.1016/j.coph.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz-Valckenberg S. Guo L. Maass A. Cheung W. Vugler A. Moss S.E. Munro P.M. Fitzke F.W. Cordeiro M.F. Real-time in vivo imaging of retinal cell apoptosis after laser exposure. Invest. Ophthalmol. Vis. Sci. 2008;49:2773–2780. doi: 10.1167/iovs.07-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maass A. von Leithner P.L. Luong V. Guo L. Salt T.E. Fitzke F.W. Cordeiro M.F. Assessment of rat and mouse RGC apoptosis imaging in vivo with different scanning laser ophthalmoscopes. Curr. Eye Res. 2007;32:851–861. doi: 10.1080/02713680701585872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coxon K.M. Duggan J. Cordeiro M.F. Moss S.E. Purification of annexin V and its use in the detection of apoptotic cells. Methods Mol. Biol. 2011;731:293–308. doi: 10.1007/978-1-61779-080-5_24. [DOI] [PubMed] [Google Scholar]
- 31.Bullok K. and Piwnica-Worms, D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J. Med. Chem. 2005;48:5404–5407. doi: 10.1021/jm050008p. [DOI] [PubMed] [Google Scholar]
- 32.Bullok K.E. Maxwell D. Kesarwala A.H. Gammon S. Prior J.L. Snow M. Stanley S. Piwnica-Worms D. Biochemical and in vivo characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. Biochemistry. 2007;46:4055–4065. doi: 10.1021/bi061959n. [DOI] [PubMed] [Google Scholar]
- 33.Barnett E.M. Zhang X. Maxwell D. Chang Q. Piwnica-Worms D. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9391–9396. doi: 10.1073/pnas.0812884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnett E.M. Elangovan B. Bullok K.E. Piwnica-Worms D. Selective cell uptake of modified Tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest. Ophthalmol. Vis. Sci. 2006;47:2589–2595. doi: 10.1167/iovs.05-1470. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell D. Chang Q. Zhang X. Barnett E.M. Piwnica-Worms D. An improved cell-penetrating, caspase-activatable, near-infrared fluorescent peptide for apoptosis imaging. Bioconjug. Chem. 2009;20:702–709. doi: 10.1021/bc800516n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin L.A. Intrinsic survival mechanisms for retinal ganglion cells. Eur. J. Ophthalmol. 1999;9(Suppl 1):S12–S16. doi: 10.1177/112067219900901S08. [DOI] [PubMed] [Google Scholar]
- 37.Lin H. Shen Y. Chen D. Lin L. Wilson B.C. Li B. Xie S. Feasibility study on quantitative measurements of singlet oxygen generation using singlet oxygen sensor green. J. Fluoresc. 2013;23:41–47. doi: 10.1007/s10895-012-1114-5. [DOI] [PubMed] [Google Scholar]
- 38.Gollmer A. Arnbjerg J. Blaikie F.H. Pedersen B.W. Breitenbach T. Daasbjerg K. Glasius M. Ogilby P.R. Singlet Oxygen Sensor Green(R): photochemical behavior in solution and in a mammalian cell. Photochem. Photobiol. 2011;87:671–679. doi: 10.1111/j.1751-1097.2011.00900.x. [DOI] [PubMed] [Google Scholar]
- 39.Ragas X. Jimenez-Banzo A. Sanchez-Garcia D. Batllori X. Nonell S. Singlet oxygen photosensitisation by the fluorescent probe singlet oxygen sensor green. Chem. Commun. 2011:2920–2922. doi: 10.1039/b822776d. [DOI] [PubMed] [Google Scholar]
- 40.Flors C. Fryer M.J. Waring J. Reeder B. Bechtold U. Mullineaux P.M. Nonell S. Wilson M.T. Baker N.R. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, singlet oxygen sensor green. J. Exp. Bot. 2006;57:1725–1734. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 41.Robinson K.M. Janes M.S. Pehar M. Monette J.S. Ross M.F. Hagen T.M. Murphy M.P. Beckman J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee D. Khaja S. Velasquez-Castano J.C. Dasari M. Sun C. Petros J. Taylor W.R. Murthy N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007;6:765–769. doi: 10.1038/nmat1983. [DOI] [PubMed] [Google Scholar]
- 43.Dickinson B.C. Tang Y. Chang Z. Chang C.J. A nuclear-localized fluorescent hydrogen peroxide probe for monitoring sirtuin-mediated oxidative stress responses in vivo. Chem. Biol. 2011;18:943–948. doi: 10.1016/j.chembiol.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van de Bittner G.C. Dubikovskaya E.A. Bertozzi C.R. Chang C.J. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21316–21321. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munemasa Y. Kitaoka Y. Kuribayashi J. Ueno S. Modulation of mitochondria in the axon and soma of retinal ganglion cells in a rat glaucoma model. J. Neurochem. 2010;115:1508–1519. doi: 10.1111/j.1471-4159.2010.07057.x. [DOI] [PubMed] [Google Scholar]
- 46.Kuehn M.H. Fingert J.H. Kwon Y.H. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol. Clin. North Am. 2005;18:383–395. doi: 10.1016/j.ohc.2005.04.002. vi. [DOI] [PubMed] [Google Scholar]
- 47.Crish S.D. Sappington R.M. Inman D.M. Horner P.J. Calkins D.J. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5196–5201. doi: 10.1073/pnas.0913141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calkins D.J. and Horner, P.J. The cell and molecular biology of glaucoma: axonopathy and the brain. Invest. Ophthalmol. Vis. Sci. 2012;53:2482–2484. doi: 10.1167/iovs.12-9483i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takihara Y. Inatani M. Hayashi H. Adachi N. Iwao K. Inoue T. Iwao M. Tanihara H. Dynamic imaging of axonal transport in living retinal ganglion cells in vitro. Invest. Ophthalmol. Vis. Sci. 2011;52:3039–3045. doi: 10.1167/iovs.10-6435. [DOI] [PubMed] [Google Scholar]
- 50.Chidlow G. Wood J.P. Ebneter A. Casson R.J. Interleukin-6 is an efficacious marker of axonal transport disruption during experimental glaucoma and stimulates neuritogenesis in cultured retinal ganglion cells. Neurobiol. Dis. 2012;48:568–581. doi: 10.1016/j.nbd.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Crish S.D. Dapper J.D. Macnamee S.E. Balaram P. Sidorova T.N. Lambert W.S. Calkins D.J. Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience. 2013;229:55–70. doi: 10.1016/j.neuroscience.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vrabec J.P. Lieven C.J. Levin L.A. Cell-type-specific opening of the retinal ganglion cell mitochondrial permeability transition pore. Invest. Ophthalmol. Vis. Sci. 2003;44:2774–2782. doi: 10.1167/iovs.02-1061. [DOI] [PubMed] [Google Scholar]
- 53.Okubo Y. Sekiya H. Namiki S. Sakamoto H. Iinuma S. Yamasaki M. Watanabe M. Hirose K. Iino M. Imaging extrasynaptic glutamate dynamics in the brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6526–6531. doi: 10.1073/pnas.0913154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Namiki S. Sakamoto H. Iinuma S. Iino M. Hirose K. Optical glutamate sensor for spatiotemporal analysis of synaptic transmission. Eur. J. Neurosci. 2007;25:2249–2259. doi: 10.1111/j.1460-9568.2007.05511.x. [DOI] [PubMed] [Google Scholar]
- 55.Grynkiewicz G. Poenie M. Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 56.Stosiek C. Garaschuk O. Holthoff K. Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitamura Y. Mitamura-Aizawa S. Nagasawa T. Katome T. Eguchi H. Naito T. Diagnostic imaging in patients with retinitis pigmentosa. J. Med. Invest. 2012;59:1–11. doi: 10.2152/jmi.59.1. [DOI] [PubMed] [Google Scholar]
- 58.Vasireddy V. Wong P. Ayyagari R. Genetics and molecular pathology of Stargardt-like macular degeneration. Prog. Retin. Eye Res. 2010;29:191–207. doi: 10.1016/j.preteyeres.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinnunen K. Petrovski G. Moe M.C. Berta A. Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90:299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 60.Framme C. Brinkmann R. Birngruber R. Roider J. Autofluorescence imaging after selective RPE laser treatment in macular diseases and clinical outcome: a pilot study. Br. J. Ophthalmol. 2002;86:1099–1106. doi: 10.1136/bjo.86.10.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Ruckmann A. Fitzke F.W. Bird A.C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br. J. Ophthalmol. 1995;79:407–412. doi: 10.1136/bjo.79.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan J.I. Dubra A. Wolfe R. Merigan W.H. Williams D.R. In vivo autofluorescence imaging of the human and macaque retinal pigment epithelial cell mosaic. Invest. Ophthalmol. Vis. Sci. 2009;50:1350–1359. doi: 10.1167/iovs.08-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roorda A. Zhang Y. Duncan J.L. High-resolution in vivo imaging of the RPE mosaic in eyes with retinal disease. Invest. Ophthalmol. Vis. Sci. 2007;48:2297–2303. doi: 10.1167/iovs.06-1450. [DOI] [PubMed] [Google Scholar]
- 64.Delori F.C. Goger D.G. Dorey C.K. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest. Ophthalmol. Vis. Sci. 2001;42:1855–1866. [PubMed] [Google Scholar]
- 65.Imanishi Y. Batten M.L. Piston D.W. Baehr W. Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibbs D. Cideciyan A.V. Jacobson S.G. Williams D.S. Retinal pigment epithelium defects in humans and mice with mutations in MYO7A: imaging melanosome-specific autofluorescence. Invest. Ophthalmol. Vis. Sci. 2009;50:4386–4393. doi: 10.1167/iovs.09-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kellner S. Kellner U. Weber B.H. Fiebig B. Weinitz S. Ruether K. Lipofuscin- and melanin-related fundus autofluorescence in patients with ABCA4-associated retinal dystrophies. Am. J. Ophthalmol. 2009;147:895–902. doi: 10.1016/j.ajo.2008.12.023. 902 e891. [DOI] [PubMed] [Google Scholar]
- 68.Holz F.G. Bellmann C. Margaritidis M. Schutt F. Otto T.P. Volcker H.E. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefe's Arch. Clin. Exp. Ophthalmol. 1999;237:145–152. doi: 10.1007/s004170050209. [DOI] [PubMed] [Google Scholar]
- 69.Grey A.C. Crouch R.K. Koutalos Y. Schey K.L. Ablonczy Z. Spatial localization of A2E in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2011;52:3926–3933. doi: 10.1167/iovs.10-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyer N.P. Tang P.H. Higbee D. Ablonczy Z. Crouch R.K. Koutalos Y. Lipofuscin and A2E accumulate with age in the retinal pigment epithelium of Nrl(−/−) Mice(dagger) Photochem. Photobiol. 2012;88:1373–1377. doi: 10.1111/j.1751-1097.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kellner U. Kellner S. Weber B.H. Fiebig B. Weinitz S. Ruether K. Lipofuscin- and melanin-related fundus autofluorescence visualize different retinal pigment epithelial alterations in patients with retinitis pigmentosa. Eye. 2009;23:1349–1359. doi: 10.1038/eye.2008.280. [DOI] [PubMed] [Google Scholar]
- 72.Herrera W. Aleman T.S. Cideciyan A.V. Roman A.J. Banin E. Ben-Yosef T. Gardner L.M. Sumaroka A. Windsor E.A. Schwartz S.B. Stone E.M. Liu X.Z. Kimberling W.J. Jacobson S.G. Retinal disease in Usher syndrome III caused by mutations in the clarin-1 gene. Invest. Ophthalmol. Vis. Sci. 2008;49:2651–2660. doi: 10.1167/iovs.07-1505. [DOI] [PubMed] [Google Scholar]
- 73.Keilhauer C.N. and Delori, F.C. Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest. Ophthalmol. Vis. Sci. 2006;47:3556–3564. doi: 10.1167/iovs.06-0122. [DOI] [PubMed] [Google Scholar]
- 74.Marmorstein A.D. Marmorstein L.Y. Sakaguchi H. Hollyfield J.G. Spectral profiling of autofluorescence associated with lipofuscin, Bruch's Membrane, and sub-RPE deposits in normal and AMD eyes. Invest. Ophthalmol. Vis. Sci. 2002;43:2435–2441. [PubMed] [Google Scholar]
- 75.Roehlecke C. Schaller A. Knels L. Funk R.H. The influence of sublethal blue light exposure on human RPE cells. Mol. Vis. 2009;15:1929–1938. [PMC free article] [PubMed] [Google Scholar]
- 76.King A. Gottlieb E. Brooks D.G. Murphy M.P. Dunaief J.L. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem. Photobiol. 2004;79:470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- 77.Williams M.A. Craig D. Passmore P. Silvestri G. Retinal drusen: harbingers of age, safe havens for trouble. Age Ageing. 2009;38:648–654. doi: 10.1093/ageing/afp136. [DOI] [PubMed] [Google Scholar]
- 78.Hageman G.S. Luthert P.J. Victor Chong N.H. Johnson L.V. Anderson D.H. Mullins R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 79.Abdelsalam A. Del Priore L. Zarbin M.A. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 1999;44:1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 80.Farkas T.G. Sylvester V. Archer D. The ultrastructure of drusen. Am. J. Ophthalmol. 1971;71:1196–1205. doi: 10.1016/0002-9394(71)90963-9. [DOI] [PubMed] [Google Scholar]
- 81.Farkas T.G. Sylvester V. Archer D. Altona M. The histochemistry of drusen. Am. J. Ophthalmol. 1971;71:1206–1215. doi: 10.1016/0002-9394(71)90964-0. [DOI] [PubMed] [Google Scholar]
- 82.Kaarniranta K. Hyttinen J. Ryhanen T. Viiri J. Paimela T. Toropainen E. Sorri I. Salminen A. Mechanisms of protein aggregation in the retinal pigment epithelial cells. Front. Biosci. 2010;2:1374–1384. doi: 10.2741/e198. [DOI] [PubMed] [Google Scholar]
- 83.Wang A.L. and Neufeld, A.H. Smoking mice: a potential model for studying accumulation of drusen-like material on Bruch's membrane. Vis. Res. 2010;50:638–642. doi: 10.1016/j.visres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 84.Nittala M.G. Ruiz-Garcia H. Sadda S.R. Accuracy and reproducibility of automated drusen segmentation in eyes with non-neovascular age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2012;53:8319–8324. doi: 10.1167/iovs.12-10582. [DOI] [PubMed] [Google Scholar]
- 85.Bharadwaj A.S. Appukuttan B. Wilmarth P.A. Pan Y. Stempel A.J. Chipps T.J. Benedetti E.E. Zamora D.O. Choi D. David L.L. Smith J.R. Role of the retinal vascular endothelial cell in ocular disease. Prog. Retin. Eye Res. 2012;32:102–180. doi: 10.1016/j.preteyeres.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Regatieri C.V. Branchini L. Fujimoto J.G. Duker J.S. Choroidal imaging using spectral-domain optical coherence tomography. Retina. 2012;32:865–876. doi: 10.1097/IAE.0b013e318251a3a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lopez-Lopez F. and Gomez-Ulla, F. Update on the imaging techniques in the diagnosis of diabetic retinopathy. Curr. Diabetes. Rev. 2012;8:200–208. doi: 10.2174/157339912800564025. [DOI] [PubMed] [Google Scholar]
- 88.Takeda A. Baffi J.Z. Kleinman M.E. Cho W.G. Nozaki M. Yamada K. Kaneko H. Albuquerque R.J. Dridi S. Saito K. Raisler B.J. Budd S.J. Geisen P. Munitz A. Ambati B.K. Green M.G. Ishibashi T. Wright J.D. Humbles A.A. Gerard C.J. Ogura Y. Pan Y. Smith J.R. Grisanti S. Hartnett M.E. Rothenberg M.E. Ambati J. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H. Wittchen E.S. Jiang Y. Ambati B. Grossniklaus H.E. Hartnett M.E. Upregulation of CCR3 by age-related stresses promotes choroidal endothelial cell migration via VEGF-dependent and -independent signaling. Invest. Ophthalmol. Vis. Sci. 2011;52:8271–8277. doi: 10.1167/iovs.11-8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y. Yang Y. Hong H. Cai W. Multimodality molecular imaging of CD105 (Endoglin) expression. Int. J. Clin. Exp. Med. 2011;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- 91.Fonsatti E. Nicolay H.J. Altomonte M. Covre A. Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc. Res. 2010;86:12–19. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- 92.Fonsatti E. and Maio, M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J. Transl. Med. 2004;2:18. doi: 10.1186/1479-5876-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seon B.K. Haba A. Matsuno F. Takahashi N. Tsujie M. She X. Harada N. Uneda S. Tsujie T. Toi H. Tsai H. Haruta Y. Endoglin-targeted cancer therapy. Curr. Drug Deliv. 2011;8:135–143. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grisanti S. Canbek S. Kaiserling E. Adam A. Lafaut B. Gelisken F. Szurman P. Henke-Fahle S. Oficjalska-Mlynczak J. Bartz-Schmidt K.U. Expression of endoglin in choroidal neovascularization. Exp. Eye Res. 2004;78:207–213. doi: 10.1016/j.exer.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 95.Yasukawa T. Kimura H. Tabata Y. Miyamoto H. Honda Y. Ikada Y. Ogura Y. Active drug targeting with immunoconjugates to choroidal neovascularization. Curr. Eye Res. 2000;21:952–961. doi: 10.1076/ceyr.21.6.952.6992. [DOI] [PubMed] [Google Scholar]
- 96.Winter P.M. Morawski A.M. Caruthers S.D. Fuhrhop R.W. Zhang H. Williams T.A. Allen J.S. Lacy E.K. Robertson J.D. Lanza G.M. Wickline S.A. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 97.Peiris P.M. Toy R. Doolittle E. Pansky J. Abramowski A. Tam M. Vicente P. Tran E. Hayden E. Camann A. Mayer A. Erokwu B.O. Berman Z. Wilson D. Baskaran H. Flask C.A. Keri R.A. Karathanasis E. Imaging metastasis using an integrin-targeting chain-shaped nanoparticle. ACS Nano. 2012;6:8783–8795. doi: 10.1021/nn303833p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng Z. Wu Y. Xiong Z. Gambhir S.S. Chen X. Near-infrared fluorescent RGD peptides for optical imaging of integrin alphavbeta3 expression in living mice. Bioconjug. Chem. 2005;16:1433–1441. doi: 10.1021/bc0501698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F. Liu J. Jas G.S. Zhang J. Qin G. Xing J. Cotes C. Zhao H. Wang X. Diaz L.A. Shi Z.Z. Lee D.Y. Li K.C. Li Z. Synthesis and evaluation of a near-infrared fluorescent non-peptidic bivalent integrin alpha(v)beta(3) antagonist for cancer imaging. Bioconjug. Chem. 2010;21:270–278. doi: 10.1021/bc900313d. [DOI] [PubMed] [Google Scholar]
- 100.Curnis F. Gasparri A. Sacchi A. Longhi R. Corti A. Coupling tumor necrosis factor-alpha with alphaV integrin ligands improves its antineoplastic activity. Cancer Res. 2004;64:565–571. doi: 10.1158/0008-5472.can-03-1753. [DOI] [PubMed] [Google Scholar]
- 101.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. 2012;24:3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Friedlander M. Theesfeld C.L. Sugita M. Fruttiger M. Thomas M.A. Chang S. Cheresh D.A. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nahrendorf M. Keliher E. Panizzi P. Zhang H. Hembrador S. Figueiredo J.L. Aikawa E. Kelly K. Libby P. Weissleder R. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc. Imaging. 1996;2009;2:1213–1222. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsourkas A. Shinde-Patil V.R. Kelly K.A. Patel P. Wolley A. Allport J.R. Weissleder R. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug. Chem. 2005;16:576–581. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 105.Zhang H. Molecular Imaging and Contrast Agent Database (MICAD) Bethesda, MD: National Center for Biotechnology Information (NCBI); 2004. Anti-ICAM-1 antibody-conjugated paramagnetic liposomes. [PubMed] [Google Scholar]
- 106.Sipkins D.A. Gijbels K. Tropper F.D. Bednarski M. Li K.C. Steinman L. ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J. Neuroimmunol. 2000;104:1–9. doi: 10.1016/s0165-5728(99)00248-9. [DOI] [PubMed] [Google Scholar]
- 107.Jayagopal A. Linton M.F. Fazio S. Haselton F.R. Insights into atherosclerosis using nanotechnology. Curr. Atheroscler. Rep. 2010;12:209–215. doi: 10.1007/s11883-010-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jayagopal A. Russ P.K. Haselton F.R. Surface engineering of quantum dots for in vivo vascular imaging. Bioconjug. Chem. 2007;18:1424–1433. doi: 10.1021/bc070020r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang S. Le-Ruppert K.C. Gabel V.P. Expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on proliferating vascular endothelial cells in diabetic epiretinal membranes. Br. J. Ophthalmol. 1994;78:370–376. doi: 10.1136/bjo.78.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caicedo A. Espinosa-Heidmann D.G. Pina Y. Hernandez E.P. Cousins S.W. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp. Eye Res. 2005;81:38–47. doi: 10.1016/j.exer.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 111.Jayagopal A. Halfpenny K.C. Perez J.W. Wright D.W. Hairpin DNA-functionalized gold colloids for the imaging of mRNA in live cells. J. Am. Chem. Soc. 2010;132:9789–9796. doi: 10.1021/ja102585v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dubois L. Lieuwes N.G. Maresca A. Thiry A. Supuran C.T. Scozzafava A. Wouters B.G. Lambin P. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother. Oncol. 2009;92:423–428. doi: 10.1016/j.radonc.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 113.Vullo D. Scozzafava A. Pastorekova S. Pastorek J. Supuran C.T. Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with fluorine-containing sulfonamides. The first subnanomolar CA IX inhibitor discovered. Bioorg. Med. Chem. Lett. 2004;14:2351–2356. doi: 10.1016/j.bmcl.2004.01.095. [DOI] [PubMed] [Google Scholar]
- 114.Shahidi M. Wanek J. Blair N.P. Mori M. Three-dimensional mapping of chorioretinal vascular oxygen tension in the rat. Invest. Ophthalmol. Vis. Sci. 2009;50:820–825. doi: 10.1167/iovs.08-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shahidi M. Wanek J. Blair N.P. Little D.M. Wu T. Retinal tissue oxygen tension imaging in the rat. Invest. Ophthalmol. Vis. Sci. 2010;51:4766–4770. doi: 10.1167/iovs.09-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shahidi M. Shakoor A. Blair N.P. Mori M. Shonat R.D. A method for chorioretinal oxygen tension measurement. Curr. Eye Res. 2006;31:357–366. doi: 10.1080/02713680600599446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shonat R.D. Wilson D.F. Riva C.E. Cranstoun S.D. Effect of acute increases in intraocular pressure on intravascular optic nerve head oxygen tension in cats. Invest. Ophthalmol. Vis. Sci. 1992;33:3174–3180. [PubMed] [Google Scholar]
- 118.Khoobehi B. Beach J.M. Kawano H. Hyperspectral imaging for measurement of oxygen saturation in the optic nerve head. Invest. Ophthalmol. Vis. Sci. 2004;45:1464–1472. doi: 10.1167/iovs.03-1069. [DOI] [PubMed] [Google Scholar]
- 119.Okuda K. Okabe Y. Kadonosono T. Ueno T. Youssif B.G. Kizaka-Kondoh S. Nagasawa H. 2-Nitroimidazole-tricarbocyanine conjugate as a near-infrared fluorescent probe for in vivo imaging of tumor hypoxia. Bioconjug. Chem. 2012;23:324–329. doi: 10.1021/bc2004704. [DOI] [PubMed] [Google Scholar]
- 120.McIntyre J.O. and Matrisian, L.M. Optical proteolytic beacons for in vivo detection of matrix metalloproteinase activity. Methods Mol. Biol. 2009;539:155–174. doi: 10.1007/978-1-60327-003-8_9. [DOI] [PubMed] [Google Scholar]
- 121.McIntyre J.O. Scherer R.L. Matrisian L.M. Near-infrared optical proteolytic beacons for in vivo imaging of matrix metalloproteinase activity. Methods Mol. Biol. 2010;622:279–304. doi: 10.1007/978-1-60327-299-5_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee S. Xie J. Chen X. Peptide-based probes for targeted molecular imaging. Biochemistry. 2010;49:1364–1376. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Egeblad M. and Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 124.Barnett J.M. McCollum G.W. Fowler J.A. Duan J.J. Kay J.D. Liu R.Q. Bingaman D.P. Penn J.S. Pharmacologic and genetic manipulation of MMP-2 and -9 affects retinal neovascularization in rodent models of OIR. Invest. Ophthalmol. Vis. Sci. 2007;48:907–915. doi: 10.1167/iovs.06-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cunha-Vaz J. Faria de Abreu J.R. Campos A.J. Early breakdown of the blood-retinal barrier in diabetes. Br. J. Ophthalmol. 1975;59:649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Do carmo A. Ramos P. Reis A. Proenca R. Cunha-vaz J.G. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp. Eye Res. 1998;67:569–575. doi: 10.1006/exer.1998.0546. [DOI] [PubMed] [Google Scholar]
- 127.Cunha-Vaz J.G. and Shakib, M. Ultrastructural mechanisms of breakdown of the blood-retina barrier. J. Pathol. Bacteriol. 1967;93:645–652. doi: 10.1002/path.1700930225. [DOI] [PubMed] [Google Scholar]
- 128.Russ P.K. Gaylord G.M. Haselton F.R. Retinal vascular permeability determined by dual-tracer fluorescence angiography. Ann. Biomed. Eng. 2001;29:638–647. doi: 10.1114/1.1385809. [DOI] [PubMed] [Google Scholar]
- 129.Cunha-Vaz J.G. Vitreous fluorophotometry studies in diabetes. Dev. Ophthalmol. 1981;2:214–221. doi: 10.1159/000395326. [DOI] [PubMed] [Google Scholar]
- 130.Cunha-Vaz J.G. Vitreous fluorophotometry recordings in posterior segment disease. Graefe's Arch. Clin. Exp. Ophthalmol. 1985;222:241–247. doi: 10.1007/BF02133688. [DOI] [PubMed] [Google Scholar]
- 131.Cunha-Vaz J.G. Fonseca J.R. Abreu J.F. Vitreous fluorophotometry and retinal blood flow studies in proliferative retinopathy. Albrecht von Graefe's Arch. Clin. Exp. Ophthalmol. 1978;207:71–76. doi: 10.1007/BF00414303. [DOI] [PubMed] [Google Scholar]
- 132.Cunha-Vaz J.G. Goldberg M.F. Vygantas C. Noth J. Early detection of retinal involvement in diabetes by vitreous fluorophotometry. Ophthalmology. 1979;86:264–275. doi: 10.1016/s0161-6420(79)35516-6. [DOI] [PubMed] [Google Scholar]
- 133.Tolentino M.J. Husain D. Theodosiadis P. Gragoudas E.S. Connolly E. Kahn J. Cleland J. Adamis A.P. Cuthbertson A. Miller J.W. Angiography of fluoresceinated anti-vascular endothelial growth factor antibody and dextrans in experimental choroidal neovascularization. Arch. Ophthalmol. 2000;118:78–84. doi: 10.1001/archopht.118.1.78. [DOI] [PubMed] [Google Scholar]
- 134.Xu Q. Qaum T. Adamis A.P. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest. Ophthalmol. Vis. Sci. 2001;42:789–794. [PubMed] [Google Scholar]
- 135.Wilson C.A. Berkowitz B.A. Funatsu H. Metrikin D.C. Harrison D.W. Lam M.K. Sonkin P.L. Blood-retinal barrier breakdown following experimental retinal ischemia and reperfusion. Exp. Eye Res. 1995;61:547–557. doi: 10.1016/s0014-4835(05)80048-x. [DOI] [PubMed] [Google Scholar]
- 136.Berkowitz B.A. Sato Y. Wilson C.A. de Juan E. Blood-retinal barrier breakdown investigated by real-time magnetic resonance imaging after gadolinium-diethylenetriaminepentaacetic acid injection. Invest. Ophthalmol. Vis. Sci. 1991;32:2854–2860. [PubMed] [Google Scholar]
- 137.Tang J. and Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miyamoto K. Khosrof S. Bursell S.E. Rohan R. Murata T. Clermont A.C. Aiello L.P. Ogura Y. Adamis A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miyamoto K. Ogura Y. Hamada M. Nishiwaki H. Hiroshiba N. Honda Y. In vivo quantification of leukocyte behavior in the retina during endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 1996;37:2708–2715. [PubMed] [Google Scholar]
- 140.Nishiwaki H. Ogura Y. Kimura H. Kiryu J. Miyamoto K. Matsuda N. Visualization and quantitative analysis of leukocyte dynamics in retinal microcirculation of rats. Invest. Ophthalmol. Vis. Sci. 1996;37:1341–1347. [PubMed] [Google Scholar]
- 141.Kimura H. Kiryu J. Nishiwaki H. Ogura Y. A new fluorescent imaging procedure in vivo for evaluation of the retinal microcirculation in rats. Curr. Eye Res. 1995;14:223–228. doi: 10.3109/02713689509033518. [DOI] [PubMed] [Google Scholar]
- 142.Saetzler R.K. Jallo J. Lehr H.A. Philips C.M. Vasthare U. Arfors K.E. Tuma R.F. Intravital fluorescence microscopy: impact of light-induced phototoxicity on adhesion of fluorescently labeled leukocytes. J. Histochem. Cytochem. 1997;45:505–513. doi: 10.1177/002215549704500403. [DOI] [PubMed] [Google Scholar]
- 143.Chen W. Vucic E. Leupold E. Mulder W.J. Cormode D.P. Briley-Saebo K.C. Barazza A. Fisher E.A. Dathe M. Fayad Z.A. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol. Imaging. 2008;3:233–242. doi: 10.1002/cmmi.257. [DOI] [PubMed] [Google Scholar]
- 144.Mulder W.J. Strijkers G.J. Briley-Saboe K.C. Frias J.C. Aguinaldo J.G. Vucic E. Amirbekian V. Tang C. Chin P.T. Nicolay K. Fayad Z.A. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn. Reson. Med. 2007;58:1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 145.Hyafil F. Cornily J.C. Feig J.E. Gordon R. Vucic E. Amirbekian V. Fisher E.A. Fuster V. Feldman L.J. Fayad Z.A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007;13:636–641. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 146.Amirbekian V. Lipinski M.J. Briley-Saebo K.C. Amirbekian S. Aguinaldo J.G. Weinreb D.B. Vucic E. Frias J.C. Hyafil F. Mani V. Fisher E.A. Fayad Z.A. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl. Acad. Sci. U. S. A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jayagopal A. Su Y.R. Blakemore J.L. Linton M.F. Fazio S. Haselton F.R. Quantum dot mediated imaging of atherosclerosis. Nanotechnology. 2009;20:165102. doi: 10.1088/0957-4484/20/16/165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Asahara T. Kawamoto A. Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 149.Kalka C. Masuda H. Takahashi T. Kalka-Moll W.M. Silver M. Kearney M. Li T. Isner J.M. Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takahashi T. Kalka C. Masuda H. Chen D. Silver M. Kearney M. Magner M. Isner J.M. Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 151.Li Calzi S. Neu M.B. Shaw L.C. Grant M.B. Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits. EPMA J. 2010;1:88–100. doi: 10.1007/s13167-010-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bhatwadekar A.D. Glenn J.V. Curtis T.M. Grant M.B. Stitt A.W. Gardiner T.A. Retinal endothelial cell apoptosis stimulates recruitment of endothelial progenitor cells. Invest. Ophthalmol. Vis. Sci. 2009;50:4967–4973. doi: 10.1167/iovs.09-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Caballero S. Sengupta N. Afzal A. Chang K.H. Li Calzi S. Guberski D.L. Kern T.S. Grant M.B. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–967. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]