Abstract
The association of inflammation with modern human diseases (e.g. obesity, cardiovascular disease, type 2 diabetes mellitus, cancer) remains an unsolved mystery of current biology and medicine. Inflammation is a protective response to noxious stimuli that unavoidably occurs at a cost to normal tissue function. This fundamental tradeoff between the cost and benefit of the inflammatory response has been optimized over evolutionary time for specific environmental conditions. Rapid change of the human environment due to niche construction outpaces genetic adaptation through natural selection, leading increasingly to a mismatch between the modern environment and selected traits. Consequently, multiple tradeoffs that affect human physiology are not optimized to the modern environment, leading to increased disease susceptibility. Here we examine the inflammatory response from an evolutionary perspective. We discuss unique aspects of the inflammatory response and its evolutionary history that can help explain the association between inflammation and modern human diseases.
Introduction
Inflammation is classically described as a response to infection or injury. It is now increasingly appreciated that chronic inflammation is universally associated with diseases of affluence and extended lifespan such as obesity [1], cardiovascular [2] and neurodegenerative diseases [3], and cancer [4]. The prevalence of these diseases has increased rapidly over the past decades [5], raising two important questions — what aspects of human biology make us susceptible to these diseases and why are diseases of disparate etiology all associated with chronic inflammation?
The underlying reasons for the intimate association of inflammation and disease are currently understood mainly in the extreme cases when diseases are caused by a dysregulated inflammatory response, such as inflammatory tissue damage and sepsis [6,7]. Under these conditions, the normally protective role of inflammation becomes detrimental when the response becomes excessive in magnitude or duration. Anaphylaxis and septic shock are the two notorious examples of excessive and potentially lethal inflammatory responses. However, although excessive inflammation can clearly be pathogenic, the near-universal association of inflammation with human diseases cannot be explained by dysregulation alone. The fundamental features of the inflammatory process that contribute to disease susceptibility are still poorly understood, as are the characteristics of the target tissues and organs that make them vulnerable to inflammatory pathologies.
Here we discuss inflammation and inflammatory diseases from the evolutionary perspective. We focus on cost–benefit trade-offs of the inflammatory response and discuss the impact of our changing environment on human disease susceptibility. Finally, we discuss inflammation in the context of life history evolution.
Inflammation and Disease
An inflammatory response can be triggered by a variety of noxious stimuli, including infection and injury. Accordingly, the inflammatory responses are highly heterogeneous in terms of the cell types and molecular mediators involved. Inflammation also comes in different modalities that can be classified as acute versus chronic and local versus systemic [8]. Despite this complexity, all inflammatory responses can be broken down into four common components that align in a universal configuration of the inflammatory pathway: inflammatory inducers, sensors, mediators, and target tissues [9]. Inflammatory inducers can be exogenous signals (e.g. pathogens and toxins [10]) or endogenous signals (e.g. ATP or urate crystals [11]) that report on tissue stress, injury, or malfunction [12]. Sensor cells, such as tissue-resident macrophages and mast cells, detect inducers with specific receptors and respond by producing inflammatory mediators. Depending on the nature of the inducers, sensor cells produce different combinations and amounts of mediators, creating a unique mediator signature for the inducer. Inflammatory mediators, in turn, act on target tissues and alter their functional states, promoting elimination of the inducers, adaptation to the noxious state, and restoration of tissue homeostasis [9].
The alteration of target tissue functional states generally occurs at the expense of the normal tissue function [12–16], thus accounting for the fifth cardinal sign of inflammation — functio laesa (‘disturbance of function’) [17]. This fundamental property of inflammatory mediators underlies the pathological potential of the inflammatory response. Examples of inflammation-induced alteration in target tissue functional states include: increased endothelial adhesiveness and permeability in response to the inflammatory cytokines tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β), to allow exudate formation [6]; increased mucus production by goblet cells in response to IL-13, to enhance epithelial barrier defenses [18]; and synthesis and secretion by hepatocytes of acute phase proteins (APPs) in response to IL-6, to promote host defense from infection [19]. Each of these effects provides an adaptive benefit in response to infection; however, they come with a cost imposed by a decline in the normal function of target tissues. In the above examples, increased endothelial permeability and adhesiveness can impair hemostasis [20], increased mucus production leads to mucus plug formation and reduced gas exchange at respiratory epithelia [21,22], and increased synthesis of APPs leads to a reduction in synthesis of albumin and other serum proteins [19,23–25]. It is the alteration of tissue and organ functional states that underlies the pathological potential of the inflammatory response. Importantly, because alteration of tissue functional states is a fundamental consequence of any inflammatory response, the cost and pathological potential of inflammation is present regardless of the magnitude and duration of the response. Thus, while inflammatory tissue damage caused by an excessive response is the most noticeable negative outcome, it is not the only, and not even the most common, cost of the inflammatory response.
Functional states of tissues and organs are maintained by a variety of homeostatic control mechanisms operated by the endocrine and the nervous systems [21,26]. Hormones and neurotransmitters regulate tissue and organ functional activities to maintain key physiological variables, such as blood pressure, blood glucose level, and core body temperature, within a desired range around a characteristic set point. Homeostatic control mechanisms also enable adaptation to a changing environment or physiological needs [27]. For example, the autonomic nervous system controls thermogenesis and heat dissipation to adapt to ambient temperature and blood flow to the skeletal muscle and the intestine depending on current priorities (hunting or escape from predators versus food digestion) [28]. The pancreatic hormones insulin and glucagon reciprocally control gluconeogenesis and glycogenolysis to regulate blood glucose level in response to fasting and feeding [29]. Homeostatic control mechanisms thus maintain and adjust the functional states of tissues and organs according to stereotypical changes in environmental conditions and predefined sets of physiological priorities that are characteristic for a given species in a given ecological niche.
However, when homeostasis is disrupted by noxious conditions, including infection or tissue damage, homeostasis control mechanisms are insufficient to maintain or restore homeostasis. It is under these conditions that inflammatory control mechanisms are engaged in order to eliminate the noxious stimulus and to restore homeostasis. In order to perform these functions, inflammatory control mechanisms alter the functional states of tissues and organs in a way that is usually antagonistic to the homeostatic control mechanisms. The reason for this is the difference in the proximal objectives of homeostatic and inflammatory controls: the former aims to maintain the normal functional state, while the latter aims to temporarily alter the functional state in order to deal with the noxious stimulus or condition. In addition to being antagonistic, inflammatory control mechanisms are dominant over homeostatic controls [30]. The reason inflammatory control mechanisms can be dominant over homeostatic controls is the higher priority of the objectives of the former: the inflammatory response is engaged by conditions that threaten an organism's fitness or even survival. For example, inflammatory mediators can induce changes in core body temperature, appetite, sleep patterns, nociceptive thresholds, vascular and airway smooth muscle tone, and a variety of metabolic processes [21]. These effects of inflammatory mediators are antagonistic to the homeostatic controls mediated by the hypothalamus, autonomic nervous system, and endocrine hormones. The changes in homeostatic parameters are beneficial, since they aid the defense against noxious insults, and costly, as they interfere with normal functions regardless of the intensity or the duration of the inflammatory response.
These characteristics account for the pathological potential of the inflammatory response and underlie a fundamental cost–benefit trade-off inherent to inflammation — a point we discuss in more detail next.
Cost–Benefit Trade-Offs and the Inflammatory Response
The trade-off between beneficial and detrimental aspects of the inflammatory response has several characteristics that may account for the rapid increase in inflammatory disease prevalence in modern human populations. As these characteristics are not unique to inflammation, we will discuss them from a more general perspective.
The cost–benefit trade-off of a given trait is optimized by maximizing the benefit while minimizing the cost. This optimization usually is specific for a particular environment: the trait can be optimal in one environment and suboptimal or even detrimental in other environments. Thus, changes to the relevant aspects of the environment can alter the cost–benefit balance, making it suboptimal and, at the extreme, maladaptive if the cost outweighs the benefit. If the environmental change is stable, the trait can be either optimized to the new environment through natural selection or lost altogether if it negatively impacts reproductive fitness. The degree of sensitivity to environmental factors can also vary for different traits. Some traits, including many core metabolic and developmental processes, are relatively independent of the environment. Other traits, and their associated trade-offs, can be highly sensitive to environmental change. These traits tend to be ones that directly interface with the environment, including immune and inflammatory responses, and would be expected to be particularly sensitive to the relevant environmental changes. Indeed, the cost–benefit trade-off of the inflammatory response can be affected by many environmental factors, including altered exposure to commensal and pathogenic microorganisms caused by high population density, changes in diet and availability of hygiene products, antibiotics, and vaccines. Because many of these environmental factors changed dramatically in the past century, one can expect that the cost–benefit trade-off of the inflammatory response in modern human populations is not optimized to the current environment.
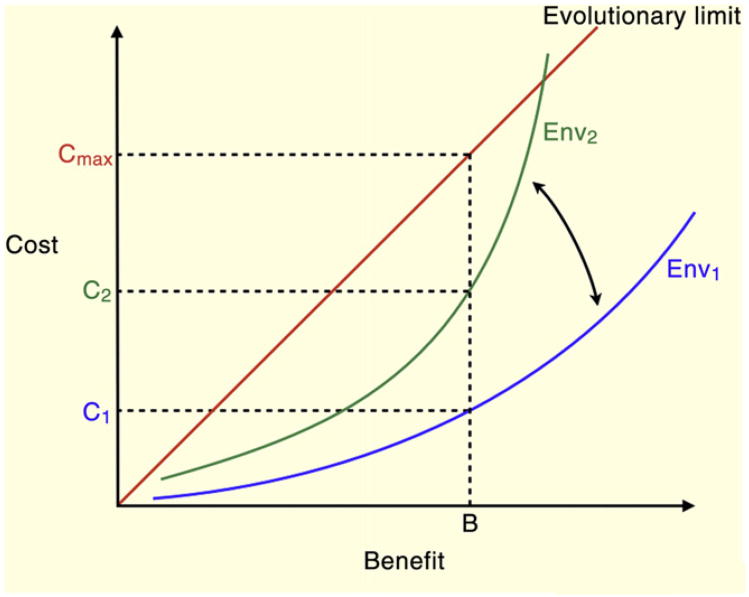
Generally, the benefit of a trait defines the acceptable cost: as long as the benefit outweighs the cost the trait can be selected and maintained by evolution. This is true up to a point: the cost can reach a physiological or evolutionary limit (that is, affect survival or reproduction) when it becomes unacceptable regardless of the associated benefit (Figure 1). Within these limits, however, high-benefit traits (for example, the traits that directly afford survival or reproductive success) can be associated with high costs. Although in a given environment the benefits of a trait are higher than the costs, the traits differ in terms of the absolute values of the costs and benefits: there are high-cost, high-benefit traits and low-cost, low-benefit traits. Because a change of the environment can alter the optimal cost–benefit balance, the high-cost, high-benefit traits should generally be more vulnerable to environmental change. Conversely, the traits that have very low cost can be maintained as atavisms even when the benefits once afforded by the trait are no longer there. Non-adaptive traits can also be maintained through sexual selection or through their association with different beneficial traits. Therefore, all traits and their associated trade-offs form a spectrum defined by their sensitivity to changes in any given environmental factor (Figure 2). Accordingly, a given change in the environment would affect different trade-offs to different extents.
Figure 1.
Cost–benefit trade-offs are optimized to specific environments and can be affected by environmental change.
A particular trait can be selected and maintained by evolution as long as the benefit of the trait outweighs the cost. Here the trait exists in Environment 1 (Env1) with benefit B and cost C1. As the environment changes, the cost of maintaining benefit B increases to C2. In all conditions, the boundary of adaptive traits is defined by the line of cost ≤ benefit, where traits below and to the right of the line are considered adaptive and traits above and to the left of the line are maladaptive. However, there is an upper limit to the acceptable cost where the trait becomes detrimental regardless of the benefit. These traits are defined by a high absolute cost.
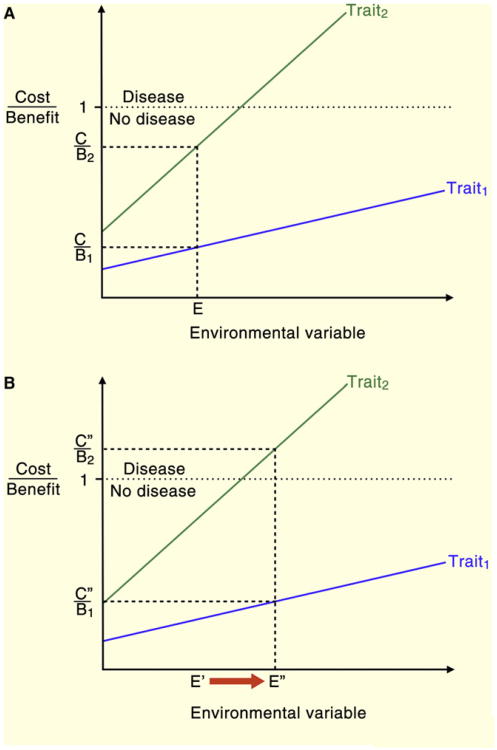
Figure 2.
Individual traits are characterized by cost–benefit ratios (C/B).
C/B < 1 is required for a trait to be adaptive. However, cost–benefit ratio is a function of the environment, and a given change in the environmental factor can affect C/B of different traits to different degrees. Here Trait1 is less sensitive than Trait2 to a given environmental change. (A) In environment E, Trait1 has C/B1 and Trait2 has C/B2, both of which are less than 1. (B) Environmental change, E′ → E″, causes a shift in the C/B ratio for both traits. Trait1 remains adaptive, with C″/B1 < 1, but Trait2 becomes maladaptive, with C″/B2 > 1.
The inflammatory response is a high-cost, high-benefit trait: high benefit because it can be life-saving in the face of noxious challenges and high cost because inflammatory defenses interfere with normal functions and, at the extreme, can cause tissue damage and death. Therefore, the inflammatory response is particularly vulnerable to changes in relevant environmental factors, a few examples of which are changes in microbial exposure, diet, stress, toxins and physical activity. This, in turn, likely contributes to the dramatic increase in the prevalence of inflammatory diseases that took place over the last few decades.
Direct Costs and Vulnerabilities
In considering the impact of cost–benefit balance on human disease, it may be useful to distinguish between two types of cost: direct costs and vulnerabilities. Direct costs are unavoidable, recurring costs that are continuously accrued as a result of deployment or maintenance of a trait. Vulnerabilities are rare but catastrophic consequences of a trait that can be incurred by a fraction of a population. In terms of cost–benefit balance, direct costs are optimized by reducing their absolute value while vulnerabilities are optimized by reducing their frequency or probability. Conversely, environmental changes can increase either the value of direct costs or increase the chances of experiencing the consequences of vulnerabilities. However, the difference between direct costs and vulnerabilities is not absolute: if vulnerabilities become very common they can turn into direct costs.
To illustrate the differences between direct costs and vulnerabilities, consider the analogy of car ownership. Some direct costs associated with the benefit of owning a car are the payments for the gasoline, insurance, and parking. A vulnerability of having a car is the possibility of getting into a fatal car crash. Both types of costs can increase or decrease depending on both internal factors (engine efficiency and car safety) and external factors (amount of traffic and bad drivers). Furthermore, direct costs (high gasoline and parking costs) or vulnerabilities (too many bad drivers) can reach a point where they outweigh the benefit of owning a car. Similarly, both direct costs and vulnerabilities of biological traits can contribute to increased susceptibility to human diseases in the face of suboptimal trade-offs caused by changes in the environment. In the next section we discuss several examples of human traits and their associated disease susceptibilities.
Anatomical and Physiological Trade-Offs
A spectrum of common human diseases reflects, in part, the direct costs and vulnerabilities of various traits of human anatomy and physiology. Here we discuss four examples that illustrate how trait-specific direct costs and vulnerabilities impact the susceptibility to inflammatory diseases. Additional examples of direct costs and vulnerabilities are given in Table 1.
Table 1.
Trait-specific adaptive benefits, direct costs, and vulnerabilities.
| Trait | Adaptive benefit | Direct cost | Vulnerability |
|---|---|---|---|
| Epithelial regeneration | Constantly replaces tissue, ensures new and functional tissue available at all times; tissue repair [21] | Metabolic costs of continuous regeneration [21] | Slow accumulation of mutations leading to development of cancer [69] |
| Oxygen metabolism | Dramatic increase in energy extraction [35] | Generation of reactive oxygen species (ROS) [21] | Neurodegenerative disease, cellular senescence [5,21,70] |
| Antioxidants (e.g. uric acid) | Protect against ROS-mediated damage [71] | Maintain serum urate through production and renal resorption [72] | Antioxidant mechanisms predispose humans to development of gout or acute renal failure [37,38,41,42] |
| Endoskeleton | Strong support system; removes thickness to volume constraint on size; increases types of movement [31] | Absence of exoskeleton-mediated protection [31] | Fat embolism, osteoporosis, osteopetrosis, fracture [5,21] |
| Synovial joints | Increased freedom of motion [31] | Wear and tear can lead to osteoarthritis [33] | Development of inflammatory arthritis [73] |
| Hemostasis | Protects against trauma-induced exsanguination [21] | Increased plasma viscosity places increased demand on the heart [46,47,74] | Inappropriate activation can lead to sudden tissue ischemia; certain tissues (e.g. brain and heart) are highly susceptible to ischemia |
| Inflammation | Restores homeostasis; clears infectious pathogens [12] | Generation of ROS leads to immunopathology and localized host tissue damage [43] | Overwhelming, hyperactivation can cause systemic inflammatory response and lead to septic shock [45] |
| Adaptive immunity | Allows for removal of pathogens in an antigen-specific manner; allows for memory of previous infections [8] | Maintenance of naïve and memory lymphocyte populations [8] | Inappropriate activation against self antigen can lead to autoimmunity [8] |
| Microbiota | Increased energy extraction from environment; generation of specific micronutrients humans are incapable of producing [75] | Defense systems such as mucus barrier and leukocyte presence are energetically costly [76] and can impede systemic nutrient absorption [77] | Inflammatory colitis [78] and recurrent infections |
| Mucosal epithelia | Protection from particulate matter and infection [5,21] | Decreased diffusion and decreased function [77] | Asthma and allergy [79] |
| Energy storage | Ability to withstand short-term food and nutrient insecurity [80] | Become an easy prey due to movement limitation [80] | Loss of predation can lead to diabetes or obesity [80] |
Exoskeleton and Endoskeleton
The exoskeleton and endoskeleton represent two different solutions in the animal kingdom for mechanical support and motility. The choice of a particular type of skeleton determines the general body plan and functions as a constraint on other evolutionary changes [31]. Animals with exoskeletons are better protected from traumatic injury, at the direct cost of constrained mobility, whereas animals with endoskeletons enjoy increased mobility of body parts but are more susceptible to injury (with the exception of the brain where the skull effectively functions as an exoskeleton). Additionally, exoskeletons impose other constraints on growth, heat dissipation, secretion, and gas exchange that are not present in species with endoskeletons [32]. The increased freedom of movement in animals with an endoskeleton is most pronounced at synovial joints [21]. These joints are distinguished by the presence of a synovial cavity that contains a lubricating fluid as well as cartilage plates on each articulating bone that function to absorb shock and reduce friction.
Although synovial joints have numerous mechanical benefits, they also present both direct costs and vulnerabilities. A direct cost of this system is that persistent stress on the joint leads to gradual erosion of articular cartilage, promoting bone-on-bone friction and bone damage. Eventually the persistent damage leads to both movement limitation and movement-induced pain [21,33]. Thus, direct costs associated with the design of synovial joints make us susceptible to osteoarthritis. The spatially confined structure of the synovial joint makes it vulnerable to inflammatory exudate formation. Unlike soft tissues that can withstand swelling caused by an exudate, synovial joints are a confined space where swelling in the joint is limited by the synovial membrane. In addition, recruitment of leukocytes to the joint, specifically macrophages and neutrophils, can create a self-sustaining inflammatory cycle that leads to progressive joint dysfunction and destruction [6,7]. This condition — rheumatoid arthritis — represents a vulnerability of the synovial joints.
Oxidative Metabolism
Increased concentration of atmospheric oxygen drove the evolution of oxidative metabolism, increasing energy extraction efficiency and allowing for the evolution of larger body size [34–36]. One corollary (direct cost) of oxidative metabolism is the elevated generation of reactive oxygen species (ROS) that can cause tissue damage [21]. Potential ROS-induced tissue damage drove the evolution of intracellular and extracellular antioxidants that help mitigate the elevated levels of ROS and maintain tissue homeostasis. Primates are unique in their extracellular antioxidant mechanisms, utilizing urate and bilirubin as the two main plasma antioxidants [37,38]. Elevated urate level in primates is the result of an inactive form of the enzyme uricase that converts urate to allantoin. Consequently, the plasma urate levels are 10-fold higher in primates than in other animals, reaching the limit of solubility at body temperature [39]. Interestingly, the presence of inactive uricase is unique to primates and parallels the loss in ability to synthesize another potent antioxidant, ascorbic acid [40], suggesting a potential compensatory mechanism.
The presence of high plasma urate levels exposes primates to unique vulnerabilities. Serum urate levels that border on saturation create the potential for urate crystal precipitation, particularly at places where temperature is slightly lower than the core body temperature (e.g. joints of toes). Precipitated urate crystals can activate the NALP3 inflammasome complex, resulting in IL-1β production and the development of gout [41]. In addition, urate can precipitate in the kidney, leading to acute renal failure and potentially death [42]. Taken together, high levels of urate function as potent antioxidants but create vulnerabilities to diseases. The propensity for urate crystallization is strongly influenced by diet [5] and therefore this vulnerability should be highly sensitive to environmental change (e.g. nutrient composition).
Immune Response
Because microbial infections can be life threatening, immune defenses provide very high benefit and therefore the acceptable costs can be very high as well (as discussed earlier). The direct costs of the immune response include energetic costs as well as collateral tissue damage. For example, neutrophil-mediated anti-bacterial defense involves the generation of very potent bactericidal ROS, such as hypochlorite, superoxide, and hydrogen peroxide [43]. The generation of ROS is associated with host tissue damage [6]. This is an unavoidable consequence of bacterial clearance, which is partially mitigated by tissue-protective and wound-repair mechanisms [44]. In addition to direct costs, the presence of a rapid, overwhelming immune response creates a vulnerability manifested as systemic immune hyperactivation. This vulnerability, known as septic shock, develops upon systemic activation of the inflammatory response, leading to a cascade of tissue and organ failures due to improper tissue perfusion, disseminated intravascular coagulation, and ultimately vascular collapse [45]. Autoimmune disease is an example of vulnerability of the adaptive immune system, and occurs when an immune response is directed against an organism's own antigens [8].
Blood Clotting
The coagulation cascade is another trait associated with both direct costs and vulnerabilities. A closed circulatory system has the benefit of highly efficient nutrient and oxygen distribution. However, it creates a secondary problem of maintaining hemostasis to ensure optimal vascular function and to promote repair upon vascular damage. The components of the clotting system are constitutively present in the blood to ensure a rapid hemostatic response to damage. Consequently, a direct cost of the clotting system is an increase in plasma viscosity, which, in turn, increases strain on the heart and necessitates increased resource investment in heart function and size [46,47]. Fibrinogen is a key component of the coagulation cascade and its concentration has been linked to plasma viscosity and disease [48]. Vulnerabilities of the clotting system are thrombosis, embolism, ischemia and stroke, which can be caused by inappropriate activation of the coagulation cascade [21].
Life History Trade-Offs
Life history traits are characteristics that directly affect reproductive success and include age and size at maturity, reproductive lifespan and aging, and number and size of offspring [49]. Allocation of resources to any of these functions is governed by a fitness trade-off and the allocation strategy is specific to each organism–environment interaction. Life history theory has shown that the balance of resource investment in these traits has been shaped by natural selection to maximize an organism's reproductive success [49].
Resource investment in different life history traits is dependent on the status of the environment. In general, a favorable environment (e.g., abundant food, lack of predators, optimal temperature) promotes investment in growth and/or reproduction while an unfavorable environment promotes investment in maintenance programs (Figure 3A). At the extremes, the maintenance programs can take forms of suspended animation, such as hibernation, estivation, diapause and dauer.
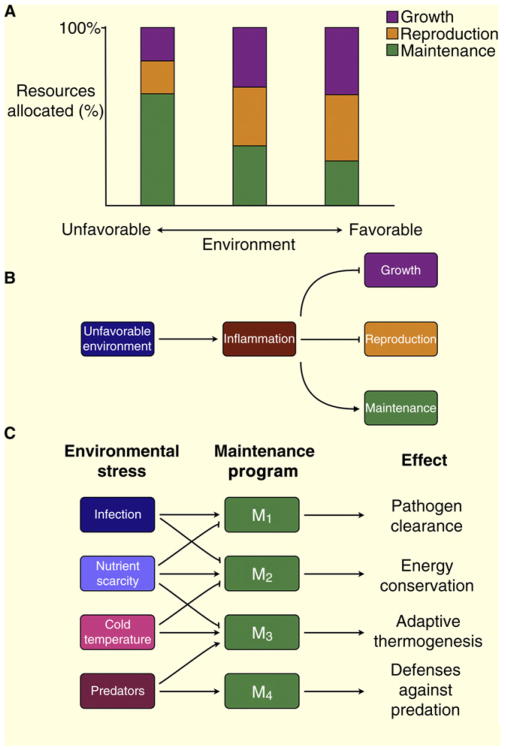
Figure 3.
Trade-off between life history characteristics is a function of the environment.
(A) Favorable environment promotes investment of resources in growth and reproduction while unfavorable environment promotes somatic maintenance at the expense of growth and reproduction. (B) Some aspects of unfavorable environment, such as infection or injury, lead to an inflammatory response that in turn promotes somatic maintenance and inhibits pathways controlling growth and reproduction. (C) Different environmental stresses activate different maintenance programs to deal with that stress and inhibit maintenance programs that are incompatible or linked by a functional or energetic trade-off.
Human life history traits include relatively long lifespan, large body size, delayed reproductive maturation, and low fecundity with large parental investment in each offspring [50]. One consequence of these life history traits is the potential for environmental signals to influence resource investment both in utero as well as during maturation. Given that inflammation is a broad response to noxious conditions, persistent inflammation may signal the presence of an unfavorable environment and should lead an organism to favor somatic maintenance over growth and reproduction (Figure 3B) [51]. Thus, the presence of a chronic inflammatory response would be expected to affect life history traits that can be interpreted under the rubric of direct costs versus vulnerabilities.
Chronic colitis provides a good example of the effects chronic inflammation can have on growth and maturation given its onset during the time of maturation [21]. It has been shown that colitis, including Crohn's disease, is associated with growth retardation [52,53] and delayed pubertal maturation [54,55] that is independent of nutrient absorption. The reduced growth and pubertal maturation are correlated with the elevated level of serum IL-6 and have been suggested to be the consequence of IL-6-mediated suppression of circulating insulin like growth factor-I (IGF-1) [56]. Further evaluation of the effects of chronically elevated cytokines was performed in mice that transgenically overexpress IL-6. These mice were substantially smaller than genetically identical non-transgenic animals [57] and females were either infertile or had very small litters [58], further demonstrating that inflammatory cytokines can alter life history traits. In addition, inflammation can affect human reproduction during pregnancy, being a major contributor to pre-term delivery, intrauterine growth restriction, pre-eclampsia and spontaneous abortion [59,60]. Finally, in humans, pro-inflammatory cytokine production in response to infection has been correlated with resistance to infection early in life but inversely correlated with overall reproductive fitness [61].
Taken together, these findings suggest that inflammatory mediators can ‘report’ on certain aspects of unfavorable environment (such as abundance of pathogens) and directly impact the life history traits of body size, age of reproduction, and overall reproductive capacity. A prediction of this model is that inflammation may promote somatic maintenance. It should be noted, however, that, while all types of environmental stress can promote somatic maintenance programs at the expense of growth and reproduction, different stresses induce different types of maintenance programs. Furthermore, different maintenance programs can be either compatible with or antagonistic to each other (Figure 3C). For example, if two maintenance programs rely on the same limited resource, they would be negatively correlated or connected by a trade-off of their own. Therefore, inflammation as a signal of environmental stress not only suppresses growth and reproduction during sensitive periods but also can inhibit antagonistic maintenance programs, such as those that control energy conservation. One simple example of this concept is infection-induced sickness behavior, which includes anorexia, fatigue and altered sleep patterns [21]. While sickness behavior may represent a maintenance program that promotes survival after infection [62], it also compromises defenses against predation, including the ‘fight or flight’ response.
Thus, from the life history perspective, inflammation may compromise human health in two ways: first, by inhibiting growth and reproduction programs, and second, by interfering with incompatible maintenance programs.
Perspectives: Evolutionary Adaptations, Inflammation and Diseases
The evolutionary perspective discussed above suggests that several factors may contribute to the increased incidence of inflammatory diseases.
First, the cost–benefit trade-off of inflammatory defenses has been optimized to an environment that no longer exists for most human populations, particularly in industrialized countries. Adaptation to the environment can occur by several mechanisms, including genetic adaptation through natural selection and non-genetic mechanisms of physiological adaptation and phenotypic plasticity [63]. In addition, some animals can alter the environment to various extents to match their needs, a process known as niche construction [64]. Examples of human niche construction include agriculture, Western diet, urban dwelling, and the use of clothing, hygiene products, antibiotics and other medicines. Humans rely on niche construction more than any other organism, and this is responsible for the rapid, unprecedented change in our modern environment [65,66]. This rapid change of multiple environmental factors caused by niche construction in turn alters the optimal cost–benefit balance of many traits, including the inflammatory response (Figure 1). Consequently, the inflammatory processes can be inappropriately engaged or dysregulated, causing the disruption of homeostasis and promoting pathology, including atopic and cardiovascular diseases, obesity and type 2 diabetes.
Second, inflammation is a high-cost, high-benefit trait, making it very vulnerable to the environmental changes that can shift cost–benefit balances (Figure 2). Given the high-cost nature of the inflammatory response, a suboptimal cost–benefit balance has a high potential to negatively impact fitness and cause disease. In addition, the benefits of the inflammatory response tend to be manifested early in life, when they have the highest impact on reproductive fitness, while the ‘payment’ of the costs is often delayed until the time when the organism is either going to die anyway (due to high extrinsic mortality) or when the death or disease will have little consequence for reproductive fitness. This phenomenon of antagonistic pleiotropy [67,68] may be particularly relevant to chronic inflammatory diseases, which are often manifested at an advanced age. The increase in human lifespan in industrialized countries is caused by a reduction of extrinsic mortality and improved health care, which in turn increased the fraction of the population that experiences the negative impact of the traits that exhibit antagonistic pleiotropy.
Third, direct costs and vulnerabilities of most traits of human anatomy and physiology can negatively affect homeostasis, particularly when these traits are not optimized to a changing environment or exhibit antagonistic pleiotropy. In the latter case, the value of direct costs and frequency of vulnerabilities would increase with age. As these costs increase, the disruption of homeostasis increases to the point that it can no longer be controlled by homeostatic control mechanisms, leading to the activation of inflammatory control mechanisms. Thus, activation of inflammatory control mechanisms leads to chronic inflammation, further altering tissue functional states and creating a vicious circle that converges on common inflammatory disease phenotypes, including cardiovascular, metabolic, and neurodegenerative diseases. It should be noted in this context that common inflammatory diseases are indeed diseases of disrupted homeostasis. Thus, the costs of human anatomical and physiological traits not only directly impact human fitness, but also, by engaging inflammatory pathways, create chronic inflammatory states that trigger, perpetuate or exacerbate diseases of homeostasis.
Fourth, inflammation can promote diseases through its effect on life history traits. Inflammation is induced by noxious environmental stimuli and therefore can signal an unfavorable environment, a situation known to promote an organism's maintenance programs at the expense of growth and reproduction. Thus, inflammation can cause certain pathologies (growth stunting, spontaneous abortions) because of the fundamental trade-off between the core life history traits. In addition, inflammation can promote a different class of diseases, such as metabolic diseases and other diseases of homeostasis, due to the interference with maintenance programs incompatible with inflammation.
In conclusion, an evolutionary perspective can provide several insights into human susceptibility to inflammatory diseases as well as the increased incidence of these diseases in modern human populations. Ironically, improvements in health care and reduced extrinsic mortality from infections, starvation, and predation have created a new set of vulnerabilities to diseases of affluence and old age. This may be the biggest trade-off of them all.
Acknowledgments
D.O. is supported by a training grant 2T32GM7205-36 of the MD/PhD program, Yale University Medical School, and an NIDDK fellowship 1F30DK094480. R.M. is supported by the HHMI and the NIH.
References
- 1.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nut. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 3.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease–a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 5.Cecil RL, Goldman L, Ausiello DA. Medicine. 23rd. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 6.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Murphy K, Travers P, Walport M, Janeway C. Immunobiology. 7th. New York: Garland Science; 2008. [Google Scholar]
- 9.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. TLR signaling. Cell Death Diff. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 11.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 13.Rocha e Silva M. A brief survey of the history of inflammation. Agents Actions. 1978;8:45–49. doi: 10.1007/BF01972401. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majno G. The Healing Hand: Man and Wound in the Ancient World. Cambridge, Mass: Harvard University Press; 1975. [Google Scholar]
- 18.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 19.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 20.Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22(Suppl 4):S1–14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Pathologic Basis of Disease. 7th. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- 22.Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Resp Cell Mol Biol. 1996;14:425–438. doi: 10.1165/ajrcmb.14.5.8624247. [DOI] [PubMed] [Google Scholar]
- 23.Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979;64:1118–1129. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon AH, Koj A. The Acute-Phase Response to Injury and Infection: The Roles of Interleukin I and Other Mediators. New York: Elsevier; 1985. [Google Scholar]
- 25.Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36:611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- 26.Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 27.Cannon WB. The Wisdom of the Body. New York: W.W. Norton & Company; 1939. [Google Scholar]
- 28.Pick J. The evolution of homeostasis: The phylogenetic development of the regulation of bodily and mental activities by the autonomic nervous system. Proc Am Philos Soc. 1954;98:298–303. [Google Scholar]
- 29.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 31.Primrose WB. The evolution of the vertebrate endoskeleton: An essay on the significance and meaning of segmentation in coelomate animals. J Anat. 1921;55:119–137. [PMC free article] [PubMed] [Google Scholar]
- 32.Losos JB, Mason KA, Singer SR, Raven PH, Johnson GB. Biology. 8th. Boston: McGraw-Hill Higher Education; 2008. [Google Scholar]
- 33.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Falkowski PG, Katz ME, Milligan AJ, Fennel K, Cramer BS, Aubry MP, Berner RA, Novacek MJ, Zapol WM. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science. 2005;309:2202–2204. doi: 10.1126/science.1116047. [DOI] [PubMed] [Google Scholar]
- 35.Falkowski PG. Tracing oxygen's imprint on earth's metabolic evolution. Science. 2006;311:1724–1725. doi: 10.1126/science.1125937. [DOI] [PubMed] [Google Scholar]
- 36.Hedges SB, Blair JE, Venturi ML, Shoe JL. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol Biol. 2004;4:2. doi: 10.1186/1471-2148-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker BF. Towards the physiological-function of uric-acid. Free Radical Biol Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 38.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15:189–192. doi: 10.1002/art.1780150209. [DOI] [PubMed] [Google Scholar]
- 40.Proctor P. Similar functions of uric acid and ascorbate in man? Nature. 1970;228:868. doi: 10.1038/228868a0. [DOI] [PubMed] [Google Scholar]
- 41.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 42.Conger JD. Acute uric acid nephropathy. Med Clin North Am. 1990;74:859–871. doi: 10.1016/s0025-7125(16)30522-3. [DOI] [PubMed] [Google Scholar]
- 43.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 44.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 45.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 46.Dormandy JA. Clinical significance of blood viscosity. Ann R Coll Surg Eng. 1970;47:211–228. [PMC free article] [PubMed] [Google Scholar]
- 47.Dormandy J, Hoare E. Significance of raised blood viscosity in circulatory disease. Surg Forum. 1972;23:251–253. [PubMed] [Google Scholar]
- 48.Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM-Mon J Assoc Phys. 2003;96:711–729. doi: 10.1093/qjmed/hcg129. [DOI] [PubMed] [Google Scholar]
- 49.Stearns SC. The Evolution of Life Histories. Oxford, New York: Oxford University Press; 1992. [Google Scholar]
- 50.Hill K, Kaplan H. Life history traits in humans: theory and empiricial studies. Annu Rev Anthropol. 1999;28:397–430. doi: 10.1146/annurev.anthro.28.1.397. [DOI] [PubMed] [Google Scholar]
- 51.Kopp EB, Medzhitov R. Infection and inflammation in somatic maintenance, growth and longevity. Evol Appl. 2009;2:132–141. doi: 10.1111/j.1752-4571.2008.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballinger AB, Camacho-Hubner C, Croft NM. Growth failure and intestinal inflammation. QJM-Mon J Assoc Phys. 2001;94:121–125. doi: 10.1093/qjmed/94.3.121. [DOI] [PubMed] [Google Scholar]
- 53.Sawczenko A, Azooz O, Paraszczuk J, Idestrom M, Croft NM, Savage MO, Ballinger AB, Sanderson IR. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the-174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci USA. 2005;102:13260–13265. doi: 10.1073/pnas.0503589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azooz OG, Farthing MJG, Savage MO, Ballinger AB. Delayed puberty and response to testosterone in a rat model of colitis. Am J Physiol Reg I. 2001;281:R1483–R1491. doi: 10.1152/ajpregu.2001.281.5.R1483. [DOI] [PubMed] [Google Scholar]
- 55.Ballinger AB, Savage MO, Sanderson IR. Delayed puberty associated with inflammatory bowel disease. Pediatr Res. 2003;53:205–210. doi: 10.1203/01.PDR.0000047510.65483.C9. [DOI] [PubMed] [Google Scholar]
- 56.Ballinger AB, Azooz O, El-Haj T, Poole S, Farthing MJ. Growth failure occurs through a decrease in insulin-like growth factor 1 which is independent of undernutrition in a rat model of colitis. Gut. 2000;46:694–700. doi: 10.1136/gut.46.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, Martini A, Ciliberto G, Fattori E. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–650. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Benedetti F, Pignatti P, Vivarelli M, Meazza C, Ciliberto G, Savino R, Martini A. In vivo neutralization of human IL-6 (hIL-6) achieved by immunization of hIL-6-transgenic mice with a hIL-6 receptor antagonist. J Immunol. 2001;166:4334–4340. doi: 10.4049/jimmunol.166.7.4334. [DOI] [PubMed] [Google Scholar]
- 59.Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138:903–919. doi: 10.1530/REP-09-0247. [DOI] [PubMed] [Google Scholar]
- 60.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 61.Van Den Biggelaar AH, De Craen AJ, Gussekloo J, Huizinga TW, Heijmans BT, Frolich M, Kirkwood TB, Westendorp RG. Inflammation underlying cardiovascular mortality is a late consequence of evolutionary programming. FASEB J. 2004;18:1022–1024. doi: 10.1096/fj.03-1162fje. [DOI] [PubMed] [Google Scholar]
- 62.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stearns SC, Hoekstra RF. Evolution: An Introduction. 2nd. Oxford, New York: Oxford University Press; 2005. [Google Scholar]
- 64.Laland KN, Odling-Smee FJ, Feldman MW. Evolutionary consequences of niche construction and their implications for ecology. Proc Natl Acad Sci USA. 1999;96:10242–10247. doi: 10.1073/pnas.96.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laland KN, Brown G. Niche construction, human behavior, and the adaptive-lag hypothesis. Evol Anthropol. 2006;15:95–104. [Google Scholar]
- 66.Rendell L, Fogarty L, Laland KN. Runaway cultural niche construction. Phil Trans Roy Soc B Biol Sci. 2011;366:823–835. doi: 10.1098/rstb.2010.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medawar PB. An Unsolved Problem of Biology. London: Published for the college by H. K. Lewis; 1952. [Google Scholar]
- 68.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 69.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 71.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 72.Levinson DJ, Sorensen LB. Renal handling of uric acid in normal and gouty subject: evidence for a 4-component system. Ann Rheum Dis. 1980;39:173–179. doi: 10.1136/ard.39.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 74.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Throm Hem. 2003;29:435–450. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 75.Hord NG. Eukaryotic-microbiota crosstalk: potential mechanisms for health benefits of prebiotics and probiotics. Annu Rev Nutr. 2008;28:215–231. doi: 10.1146/annurev.nutr.28.061807.155402. [DOI] [PubMed] [Google Scholar]
- 76.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smithson KW, Millar DB, Jacobs LR, Gray GM. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981;214:1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- 78.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 79.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Speakman JR. A nonadaptive scenario explaining the genetic predisposition to obesity: the “predation release” hypothesis. Cell Metab. 2007;6:5–12. doi: 10.1016/j.cmet.2007.06.004. [DOI] [PubMed] [Google Scholar]