Abstract
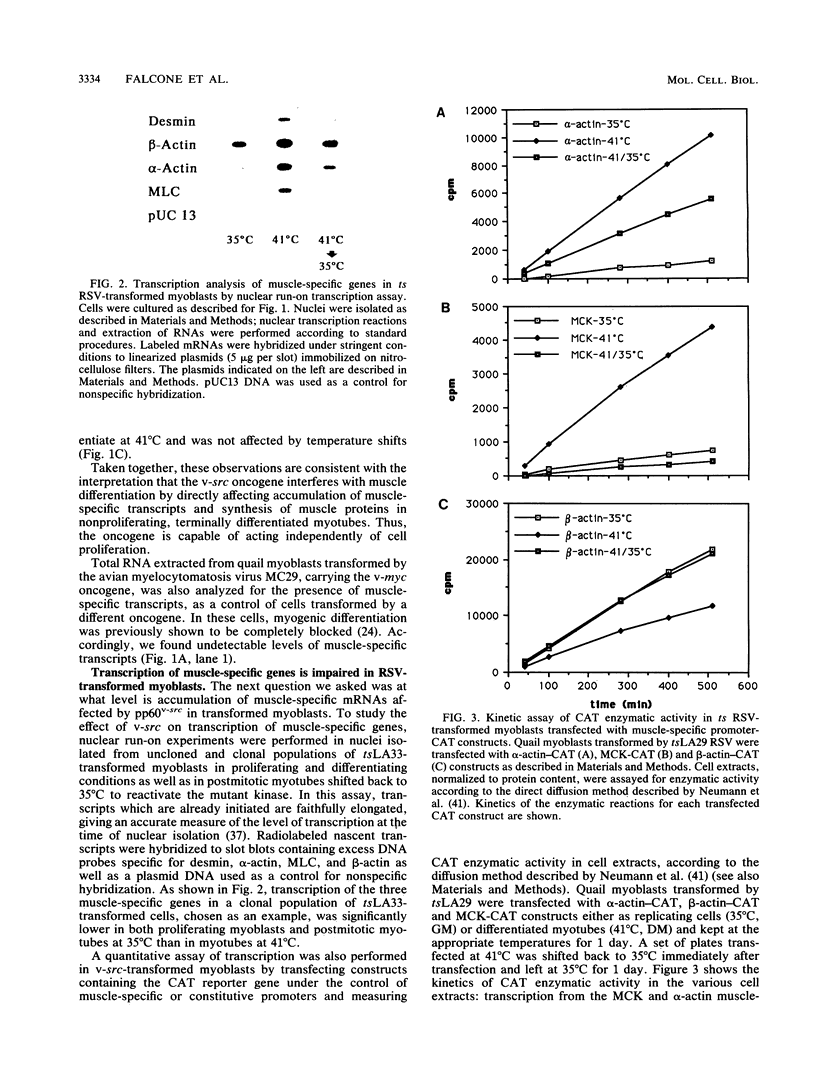
Quail myogenic cells infected with temperature sensitive (ts) mutants of Rous sarcoma virus (RSV) exhibit a temperature-dependent transformation and block of differentiation. When the cells are allowed to differentiate at the restrictive temperature (41 degrees C) and then shifted back to the permissive temperature (35 degrees C), a sharp reduction in the accumulation of muscle-specific mRNAs is observed, following reactivation of the transforming protein pp60v-src. A kinetic analysis of this down-regulation reveals that the reduction in the accumulation of muscle-specific transcripts occurs fairly rapidly within 6 to 20 h after the shift back, depending on the mRNA analyzed. Studies on transcription of endogenous muscle-specific genes and a transfected chloramphenicol acetyltransferase reporter gene under the control of muscle-specific promoters, at the different temperatures, suggest that the oncogene exerts its control mainly at the transcriptional level. On the contrary, transcription of the CMD1 gene, the avian homolog of the mouse muscle regulatory MyoD gene, is not significantly affected by the oncogene both in proliferating myoblasts and in myotubes shifted back to 35 degrees C. These findings are consistent with the conclusion that v-src blocks myogenesis by controlling transcription of muscle-specific genes independently of cell proliferation. Furthermore, they suggest the existence of an alternative pathway, not requiring the silencing of CMD1 transcription, through which the oncogene exerts its effect.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alema S., Tato F., Boettiger D. myc and src oncogenes have complementary effects on cell proliferation and expression of specific extracellular matrix components in definitive chondroblasts. Mol Cell Biol. 1985 Mar;5(3):538–544. doi: 10.1128/mcb.5.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Alemá S., Tató F. Interaction of retroviral oncogenes with the differentiation program of myogenic cells. Adv Cancer Res. 1987;49:1–28. doi: 10.1016/s0065-230x(08)60792-7. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Braun T., Bober E., Winter B., Rosenthal N., Arnold H. H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO J. 1990 Mar;9(3):821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Lazarides E. Characterization and regulation in the expression of a gene coding for the intermediate filament protein desmin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6909–6913. doi: 10.1073/pnas.81.22.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalbore P., Agostini E., Alemà S., Falcone G., Tatò F. The v-myc oncogene is sufficient to induce growth transformation of chick neuroretina cells. Nature. 1987 Mar 12;326(6109):188–190. doi: 10.1038/326188a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisanti-Combes P., Lorinet A. M., Girard A., Pessac B., Wasseff M., Calothy G. Expression of neuronal markers in chick and quail embryo neuroretina cultures infected with Rous sarcoma virus. Cell Differ. 1982 Jan;11(1):45–54. doi: 10.1016/0045-6039(82)90016-1. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- DePonti-Zilli L., Seiler-Tuyns A., Paterson B. M. A 40-base-pair sequence in the 3' end of the beta-actin gene regulates beta-actin mRNA transcription during myogenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1389–1393. doi: 10.1073/pnas.85.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Stoeckle M. Y., Hanafusa H. Serum and v-src increase the level of a CCAAT-binding factor required for transcription from a retroviral long terminal repeat. Genes Dev. 1990 Feb;4(2):243–254. doi: 10.1101/gad.4.2.243. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Eldridge J., Zehner Z., Paterson B. M. Nucleotide sequence of the chicken cardiac alpha actin gene: absence of strong homologies in the promoter and 3'-untranslated regions with the skeletal alpha actin sequence. Gene. 1985;36(1-2):55–63. doi: 10.1016/0378-1119(85)90069-1. [DOI] [PubMed] [Google Scholar]
- Falcone G., Boettiger D., Alemà S., Tatò F. Role of cell division in differentiation of myoblasts infected with a temperature-sensitive mutant of Rous sarcoma virus. EMBO J. 1984 Jun;3(6):1327–1331. doi: 10.1002/j.1460-2075.1984.tb01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Guermah M., Gillet G., Michel D., Laugier D., Brun G., Calothy G. Down regulation by p60v-src of genes specifically expressed and developmentally regulated in postmitotic quail neuroretina cells. Mol Cell Biol. 1990 Jul;10(7):3584–3590. doi: 10.1128/mcb.10.7.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltmeier H., Rohrer H. Distinct and different effects of the oncogenes v-myc and v-src on avian sympathetic neurons: retroviral transfer of v-myc stimulates neuronal proliferation whereas v-src transfer enhances neuronal differentiation. J Cell Biol. 1990 Jun;110(6):2087–2098. doi: 10.1083/jcb.110.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Adkins B., Beug H., Graf T. src- and fps-containing avian sarcoma viruses transform chicken erythroid cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7122–7126. doi: 10.1073/pnas.81.22.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny S. F., Drobes B. L., Menke S. L., Taparowsky E. J. Inhibition of myogenic differentiation by the H-ras oncogene is associated with the down regulation of the MyoD1 gene. Oncogene. 1989 Apr;4(4):473–481. [PubMed] [Google Scholar]
- La Rocca S. A., Grossi M., Falcone G., Alemà S., Tatò F. Interaction with normal cells suppresses the transformed phenotype of v-myc-transformed quail muscle cells. Cell. 1989 Jul 14;58(1):123–131. doi: 10.1016/0092-8674(89)90409-1. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Thayer M. J., Overell R. W., Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell. 1989 Aug 25;58(4):659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Lin Z. Y., Dechesne C. A., Eldridge J., Paterson B. M. An avian muscle factor related to MyoD1 activates muscle-specific promoters in nonmuscle cells of different germ-layer origin and in BrdU-treated myoblasts. Genes Dev. 1989 Jul;3(7):986–996. doi: 10.1101/gad.3.7.986. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Holtzer H. Myogenesis: fusion, myosin synthesis, and the mitotic cycle. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1484–1490. doi: 10.1073/pnas.56.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Spizz G., Tainsky M. A. The oncogenic forms of N-ras or H-ras prevent skeletal myoblast differentiation. Mol Cell Biol. 1987 Jun;7(6):2104–2111. doi: 10.1128/mcb.7.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Eldridge J. D. alpha-Cardiac actin is the major sarcomeric isoform expressed in embryonic avian skeletal muscle. Science. 1984 Jun 29;224(4656):1436–1438. doi: 10.1126/science.6729461. [DOI] [PubMed] [Google Scholar]
- Payne P. A., Olson E. N., Hsiau P., Roberts R., Perryman M. B., Schneider M. D. An activated c-Ha-ras allele blocks the induction of muscle-specific genes whose expression is contingent on mitogen withdrawal. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8956–8960. doi: 10.1073/pnas.84.24.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Schwarz R. I., Farson D. A., Soo W. J., Bissell M. J. Primary avian tendon cells in culture. An improved system for understanding malignant transformation. J Cell Biol. 1978 Dec;79(3):672–679. doi: 10.1083/jcb.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakata M., Nabeshima Y., Konishi K., Fujii-Kuriyama Y. Upstream regulatory region for inducible expression of the chicken skeletal myosin alkali light-chain gene. Mol Cell Biol. 1988 Jun;8(6):2581–2588. doi: 10.1128/mcb.8.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. A., Spizz G., Perry W. M., Vizard D., Weil T., Olson E. N. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol Cell Biol. 1988 Jul;8(7):2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Tyagi J. S., Hirano H., Merlino G. T., Pastan I. Transcriptional control of the fibronectin gene in chick embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1983 May 10;258(9):5787–5793. [PubMed] [Google Scholar]
- Vaidya T. B., Rhodes S. J., Taparowsky E. J., Konieczny S. F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989 Aug;9(8):3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Lockshon D., Lassar A. MyoD binds cooperatively to two sites in a target enhancer sequence: occupancy of two sites is required for activation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5623–5627. doi: 10.1073/pnas.87.15.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Tapscott S. J., Davis R. L., Thayer M. J., Adam M. A., Lassar A. B., Miller A. D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham M. J., Wyke J. A., Lang A., Wyke A. W. Mitogenesis induced by pp60v-src is not accompanied by increased expression of immediate early response genes. Oncogene. 1990 Feb;5(2):161–169. [PubMed] [Google Scholar]
- West C. M., Boettiger D. Selective effect of Rous sarcoma virus src gene expression on contractile protein synthesis in chick embryo myotubes. Cancer Res. 1983 May;43(5):2042–2046. [PubMed] [Google Scholar]
- Wyke J. A., Stoker A. W. Genetic analysis of the form and function of the viral src oncogene product. Biochim Biophys Acta. 1987 Apr 20;907(1):47–69. doi: 10.1016/0304-419x(87)90018-7. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Brousse F. C., Emerson C. P., Jr Localized expression of a myogenic regulatory gene, qmf1, in the somite dermatome of avian embryos. Genes Dev. 1990 Apr;4(4):567–581. doi: 10.1101/gad.4.4.567. [DOI] [PubMed] [Google Scholar]