Abstract
Ca2+ plays a complex role in the differentiation of committed pre-adipocytes into mature, fat laden adipocytes. Stim1 is a single pass transmembrane protein that has an essential role in regulating the influx of Ca2+ ions through specific plasma membrane store-operated Ca2+ channels. Stim1 is a sensor of endoplasmic reticulum Ca2+ store content and when these stores are depleted ER-localized Stim1 interacts with molecular components of store-operated Ca2+ channels in the plasma membrane to activate these channels and induce Ca2+ influx. To investigate the potential role of Stim1 in Ca2+-mediated adipogenesis, we investigated the expression of Stim1 during adipocyte differentiation and the effects of altering Stim1 expression on the differentiation process. Western blotting revealed that Stim1 was expressed at low levels in 3T3-L1 pre-adipocytes and was upregulated 4 days following induction of differentiation. However, overexpression of Stim1 potently inhibited their ability to differentiate and accumulate lipid, and reduced the expression of C/EBP alpha and adiponectin. Stim1-mediated differentiation was shown to be dependent on store-operated Ca2+ entry, which was increased upon overexpression of Stim1. Overexpression of Stim1 did not disrupt cell proliferation, mitotic clonal expansion or subsequent growth arrest. siRNA-mediated knockdown of endogenous Stim1 had the opposite effect, with increased 3T3-L1 differentiation and increased expression of C/EBP alpha and adiponectin. We thus demonstrate for the first time the presence of store-operated Ca2+ entry in 3T3-L1 adipocytes, and that Stim1-mediated Ca2+ entry negatively regulates adipocyte differentiation. We suggest that increased expression of Stim1 during 3T3-L1 differentiation may act, through its ability to modify the level of Ca2+ influx through store-operated channels, to balance the level of differentiation in these cells in vitro.
Keywords: Stim1, 3T3-L1, Adipocyte, Store-operated Ca2+ entry, BTP2, Differentiation
1. Introduction
Adipocytes play a fundamental role in the maintenance of energy balance in mammals and, via the secretion of a diverse variety of hormones and peptides, also play a critical role in such diverse processes as hematopoiesis, vascular remodeling, insulin sensitivity and the immune response (Morrison and Farmer, 1999). Excess accumulation of triglycerides in obesity hampers normal adipocyte function and results in a significant risk in the development of metabolic disorders (Pi-Sunyer, 1993). In many models of obesity, de novo adipocyte differentiation contributes to an increased total number of adipocytes and increased adipose stores (Hirsch and Batchelor, 1976). Understanding the molecular mechanisms regulating adipocyte differentiation has thus been the subject of intense investigation. An increase in intracellular Ca2+ concentration ([Ca2+]i) in adipocytes often accompanies the development of obesity (Draznin et al., 1988). In vitro, increasing [Ca2+]i by overexpressing neuronatin (Suh et al., 2005), a putative Ca2+-ATPase regulator, acts to increase the differentiation of the 3T3-L1 pre-adipocyte cell line, a well-characterized in vitro model of adipocyte differentiation. Paradoxically, directly raising [Ca2+]i in 3T3-L1 pre-adipocytes by calcium mobilizing agents efficiently inhibits differentiation, diminishes adipocyte-specific gene expression and reduces lipid accumulation (Ntambi and Takova, 1996). These inhibitory effects can be mimicked either by enhancing the activity of the Ca2+/calmodulin-dependent serine/ threonine phosphatase calcineurin (Neal and Clipstone, 2002) or by constitutive activation of calcineurin effectors such as members of the nuclear factor of activated T cell (NFAT) family (Neal and Clipstone, 2003). Conversely, inhibition of calcineurin activity by cyclosporin A (CsA) increases adipocyte differentiation and lipid accumulation (Neal and Clipstone, 2002), mimicking the obesogenic effects of CsA treatment in humans (Mathieu et al., 1994). These apparently contradictory effects suggest that elevating [Ca2+]i in discrete cellular microdomains likely has diverse effects on adipocyte differentiation, similar to that seen in other cell types (Berridge, 2006).
Ca2+ influx through plasma membrane store-operated Ca2+ channels (SOCs) provides for localized sub-plasma membrane increase in [Ca2+]i critical for sustained activity of several intracellular enzymes (Cooper et al., 1998), including calcineurin (Gwack et al., 2007). SOCs are uniquely activated by a mechanism critically dependent on the depletion of endoplasmic reticulum (ER) Ca2+ stores (Venkatachalam et al., 2002). The predominantly ER membrane protein Stromal interaction molecule-1 (Stim1) (Williams et al., 2002; Liou et al., 2005) plays a critical role in sensing ER [Ca2+] via its ER luminal N-terminal unpaired EF hand domain and activating SOCs (Liou et al., 2005). When ER [Ca2+] drops following receptor-mediated IP3 signaling, Ca2+ is no longer bound to the low affinity EF hand domain of Stim1, inducing a conformational change and aggregation of Stim1 within the ER membrane (Liou et al., 2005). These aggregates, forming within 10–25nm of the plasma membrane (Wu et al., 2006), initiate clustering of the plasma membrane store-operated Ca2+ channel component, Orai1 (Xu et al., 2006). Through an, as yet, unidentified mechanism, Stim1 and Orai1 together induce localized Ca2+ influx from the extracellular space (Soboloff et al., 2006b). In mice, targeted inactivation of Stim1 in T-cells severely impairs Ca2+ entry through SOCs, abolishes NFAT-mediated gene expression and impairs regulatory T-cell development and function (Oh-hora et al., 2008).
It is now clear that Stim1 mediates Ca2+ entry through SOCs in a varied selection of cell types, and that the expression level of Stim1 profoundly affects the level of Ca2+ influx (Roos et al., 2005; Soboloff et al., 2006a). The ubiquitous tissue expression pattern of Stim1 (Dziadek and Johnstone, 2007) and the perinatal lethal phenotype of Stim1−/− mice (Oh-hora et al., 2008) also suggest that Stim1 is critical for correct development and/or function of multiple cell types. However, it is not clear how Stim1 regulates developmental processes at a cellular level. Given that the activity of calcineurin in modifying 3T3-L1 adipocyte differentiation suggests the presence of functional SOCs in these cells, we investigated the role of Stim1 in 3T3-L1 pre-adipocyte differentiation. By modulating the expression level of Stim1 we demonstrate that increasing Ca2+ entry through SOCs via upregulation of Stim1 acts to inhibit 3T3-L1 differentiation without affecting proliferation, and downregulation of Stim1 promotes 3T3-L1 differentiation. These results suggest that Stim1 is a negative modulator of 3T3-L1 differentiation, and that its expression may act to balance the level of differentiation in 3T3-L1 cells in vitro.
2. Materials and methods
2.1. Cell culture and adipocyte differentiation
Mouse 3T3-L1 pre-adipocytes (ATCC) were maintained in Dulbecco’s modified Eagle’s medium with high glucose supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin G, 100 µg/ml streptomycin and 2 mM l-glutamine (all from Invitrogen). To induce adipocyte differentiation, cells were grown until 2 days postconfluence (day 0) and then treated for 2 days with growth medium plus MDI (0.5 mM methylisobutylxanthine, 1 µM dexamethasone and 10 µg/ml insulin; all from Sigma). Subsequently, cells were incubated in growth medium containing 10 µg/ml insulin for 2 days and then refed every 2 days in growth medium for the remainder of the culture period. Where indicated, differentiation was induced with 0.25 mM methylisobutylxanthine and 0.5 µM dexamethasone (0.5MD) for 2 days, and thereafter cells were refed every 2 days in growth medium. At the end of the culture period, cells were fixed in 4% paraformaldehyde and stained with 2.1 mg/ml Oil Red O (Sigma) in 60% isopropanol. Cells were photographed and Oil Red O eluted in 100% isopropanol for spectrophotometric quantification at OD520. Where indicated, cells were treated with 3 or 10 µM N-(4-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl]phenyl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (BTP2) (Merck), 1 µg/ml cyclosporin A (Sigma), 5 ng/ml FK-506 (Sigma) or vehicle control (DMSO, ethanol).
2.2. Generation of L1-control and L1-STIM1 cells
To create 3T3-L1 lines stably overexpressing STIM1 (L1-STIM1 lines), cells were transfected with a human pIRES::STIM1 expression plasmid (Williams et al., 2001) or empty pIRES plasmid (L1-control lines) using Lipofectamine 2000 according to the manufacturers instructions (Invitrogen). Stably overexpressing cell lines were selected in G418 (Sigma) and single clonal lines obtained by limiting dilution in 96 well plates.
2.3. Ca2+ measurements
Cells grown on coverslips were placed in “cation-safe” medium free of sulfate and phosphate anions (107 mM NaCl/7.2 mM KCl/1.2 mM MgCl2/11.5 mM glucose/20 mM Hepes-NaOH, pH 7.2) and loaded with 4 µM fura-2/acetoxymethyl ester (Molecular Probes) for 30 min at 20 °C in the presence of 0.01% Pluronic F-127 (Invitrogen). Cells were washed and dye was allowed to deesterify for a minimum of 30 min at 20 °C before measurement of Ca2+ using an InCyt dual-wavelength fluorescence imaging system (Intracellular Imaging, Cincinnati). Fluorescence emission at 505 nm was monitored with excitation at 340 and 380 nm; intracellular Ca2+ measurements are shown as 340- to 380-nm ratios. Store-operated Ca2+ entry was induced by incubation with 1 µM Tg (EMD Biosciences) or 100 µUTP (Sigma) in zero Ca2+ conditions. All traces are averages from multiple (20–50) cells and are representative of at least three separate experiments.
2.4. Western blot analysis
Total 3T3-L1 protein extracts were prepared and quantified as previously described (Williams et al., 2001). Protein samples (10–20 µg) were separated on 12% SDS-PAGE or 4–16% gradient (Invitrogen) gels under reducing conditions and Western blotting performed as previously described (Williams et al., 2001). Primary antibodies were affinity purified rabbit anti-STIM1-CT at 1:1000 (Williams et al., 2001), mouse anti-β-actin (Sigma) at 1:2000, rabbit anti-C/EBPα (14AA), C/EBPβ (C-19) and PPARγ (H-100) (all Santa Cruz Biotechnology) all at 1:500, and rabbit anti-Adpn (Sigma) at 1:1000. Secondary antibodies (anti-mouse-AP, anti-rabbit-AP, anti-rabbit-BIO) were from Jackson Immunochemicals used at 1:2000 and ExtrAvidin-AP (Sigma) at 1:300,000. Alkaline phosphatase was detected using NBT-BCIP substrate (Invitrogen) or by CDP-Star chemiluminescent substrate solution (Sigma). Where indicated, image capture and quantification of Western blots were performed with the LAS-3000 imaging system and MultiGuage software (Fujifilm).
2.5. siRNA design and transfection
Pre-designed Stealth™ siRNA duplexes to mouse Stim1 (MSS209660, MSS209661) and control medium GC siRNA duplexes (12935-300) were purchased from Invitrogen. 3T3-L1 cells at 30–50% confluency were transfected with Lipofectamine 2000 (Invitrogen) in 6-well plates according to the protocol described by Fox et al. (2006). Following transfection and recovery, cells were harvested in 0.25% trypsin (Invitrogen) and re-seeded at 2 × 104/well in 24 well plates for differentiation.
2.6. Real-time RT-PCR
Total RNA was prepared using TRIzol Reagent (Invitrogen) and genomic DNA-free RNA converted to cDNA using Superscript Vilo cDNA synthesis kit (Invitrogen). Samples prepared without reverse transcriptase were used as negative controls. Analysis of Stim1 and β-actin mRNA levels in pentuplicate wells were performed using Express Two-Step SYBR GreenER (Invitrogen) on a ABI-7700HT (Applied Biosystems) using fast cycling conditions and the exon spanning PCR primers 5′-ttgccaagcaggaagctc-3′,5′-ctccttctctgctttcctcaag-3′ (Stim1) and 5′-aaggccaaccgtgaaaagat-3′,5′-gtggtacgaccagaggcatac-3′ (β-actin). The presence of single products were confirmed by melting point analysis of every sample. Stim1 gene expression was analyzed using the 2−ddCT method (Livak and Schmittgen, 2001) relative to β-actin levels.
2.7. Data analysis
Statistical analysis was performed using a Student’s t-test with a p-value of 0.05 considered significant. All data shown are representative of at least 3 individual experiments.
3. Results
3.1. Stim1 is expressed in pre-adipocytes and is upregulated following adipocyte differentiation
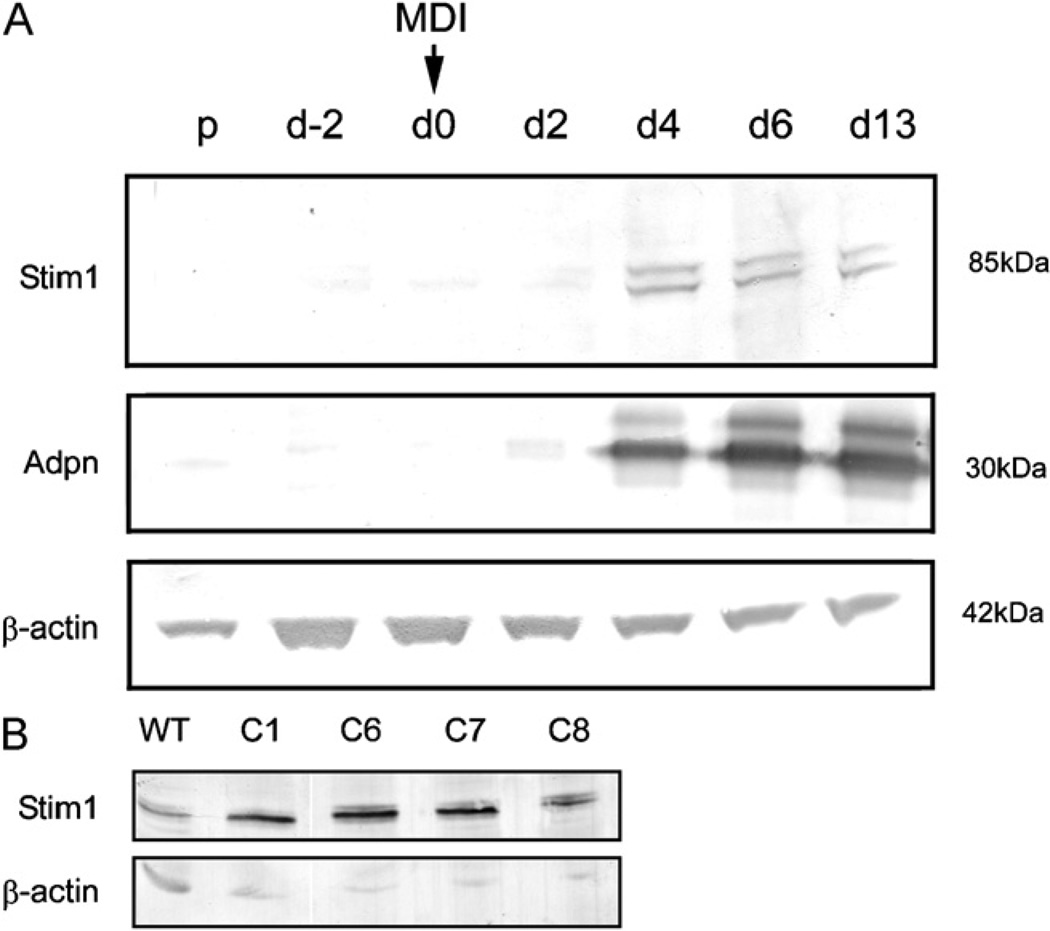
The 3T3-L1 pre-adipocyte cell line efficiently differentiates into morphologically distinct, triglyceride laden adipocytes over a 10-day differentiation period when confluent cells are treated with a differentiation cocktail comprising methylisobutylxanthine (IBMX), dexamethasone and insulin (MDI) (Cowherd et al., 1999). Western blotting showed cell lysates prepared from cells prior to differentiation contained very low levels of Stim1 (Fig. 1A, top panel). Stim1 levels increased markedly 4 days following stimulation with MDI, and remained stable throughout the remaining differentiation period. Adiponectin (Adpn), a known marker of terminal 3T3-L1 differentiation, was robustly induced at day 4 post MDI treatment (Fig. 1A, middle panel) and continued to increase throughout the remaining differentiation period as expected (Hu et al., 1996). This analysis indicated that Stim1 is upregulated during adipogenic differentiation of 3T3-L1 cells.
Fig. 1.
Stim1 expression is upregulated during differentiation of 3T3-L1 cells. (A) Western blotting for Stim1, Adpn and β-actin in cell lysates from 3T3-L1 cells collected at the proliferative phase (p), day-2 of differentiation (d-2), at day 0 = MDI addition (d0) and at days 2, 4, 6 and 13 post MDI treatment (d2, d4, d6, d13). (B) Western blotting for Stim1 and β-actin in lysates from proliferating wild-type 3T3-L1 cells and L1-STIM1 clones.
3.2. Overexpression of STIM1 in 3T3-L1 cells increases Ca2+ entry through SOCs
To determine the role of Stim1 in the differentiation process, 3T3-L1 pre-adipocytes were stably transfected with a human pIRES::STIM1 expression construct (Williams et al., 2001). Ten clonally derived STIM1-overexpressing cell lines (L1-STIM1) and 10 control empty vector pIRES-transfected lines (L1-control) were generated. Western blot analysis revealed a moderate upregulation of STIM1 expression levels in the C8 clonal line, and higher expression in the C1, C6 and C7 clonal lines compared to wild-type 3T3-L1 cells and the β-actin loading control (Fig. 1B).
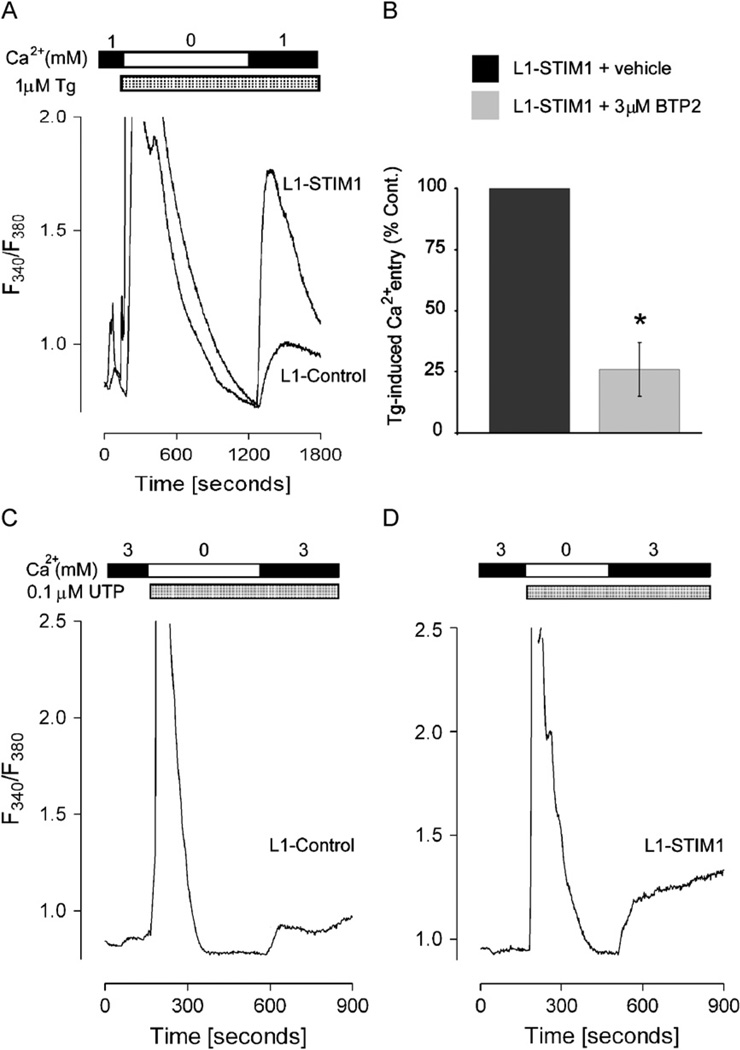
To determine whether STIM1 overexpression in 3T3-L1 pre-adipocytes increased the level of Ca2+ entry in response to store-depletion, the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase Ca2+ pump blocker thapsigargin (Tg) was used to deplete ER Ca2+ stores, and fura-2 was used to monitor cytosolic Ca2+ levels (Soboloff et al., 2006a). In the absence of external Ca2+, Tg induced a large and transient increase in intracellular Ca2+ as stores were initially emptied, followed by a return to baseline values as plasma membrane Ca2+ pumps facilitated extrusion into the extracellular medium (Fig. 2A). The level of Tg-induced ER Ca2+ release was similar in both L1-control and L1-STIM1 pre-adipocytes. Addition of extracellular Ca2+ caused a rapid entry of Ca2+ through SOCs similar to that described in other cells (Venkatachalam et al., 2002), the extent of which was almost 2-fold greater in L1-STIM1 cells compared to L1-controls (p < 0.0001); these increases correlated with the gain in Tg-induced Ca2+ entry in single HEK293 cells overexpressing STIM1 (Soboloff et al., 2006a). Pre-incubation of L1-STIM1 cells with the store-operated Ca2+ channel blocker BTP2 for 10 min prior to Tg treatment reduced Ca2+ entry to approximately 25% of that seen in vehicle control-treated cells (p < 0.0001) (Fig. 2B), correlating with the BTP2-mediated decrease in Tg-induced Ca2+ entry in B-lymphocytes (He et al., 2005). This analysis indicated that the increased Ca2+ entry seen in L1-STIM1 cells could be attributed to activity of store-operated Ca2+ channels.
Fig. 2.
Overexpression of STIM1 leads to increased store-operated Ca2+ entry. (A) Representative traces of Tg-induced Ca2+ release and Ca2+ entry in L1-control and L1-STIM1 C7 cell lines. Tg and Ca2+ were introduced at the times indicated by the bars above the graph. (B) L1-STIM1 C7 cells were preincubated for 10 min with 3 µM BTP2 prior to stimulation with 1 µM Tg in Ca2+-free media followed by reintroduction of 1 mM Ca2+. Data represents the maximum level of store-operated Ca2+ entry in L1-STIM1 C7 cells treated with BTP2 or vehicle, expressed as a percentage of the vehicle control. (C, D) Representative traces of UTP-induced Ca2+ release and Ca2+ entry in L1-control (C) and L1-STIM1 C7 (D) cells. UTP and Ca2+ were introduced at the times indicated by the bars above the graphs, *p < 0.0001.
To demonstrate that STIM1-mediated SOC activation could contribute to a physiological signal, we tested the responses of L1-control and L1-STIM1 cells to UTP. In white adipocytes this nucleotide likely activates the P2Y purinergic receptor subclass (Lee et al., 2005), a typical phospholipase C-linked G-protein coupled receptor. In both L1-control (Fig. 2C) and L1-STIM1 (Fig. 2D) pre-adipocytes, UTP rapidly induced InsP3-mediated Ca2+ release from stores in the absence of extracellular Ca2+. Addition of external Ca2+ resulted in rapid entry of Ca2+ in L1-control cells, which was greatly enhanced in L1-STIM1 pre-adipocytes. Thus, enhanced activation of SOCs by STIM1 was not limited to pharmacological ER Ca2+ depletion; Ca2+ entry in L1-STIM1 cells was also enhanced in response to physiological signals.
3.3. STIM1 overexpression inhibits 3T3-L1 pre-adipocyte differentiation
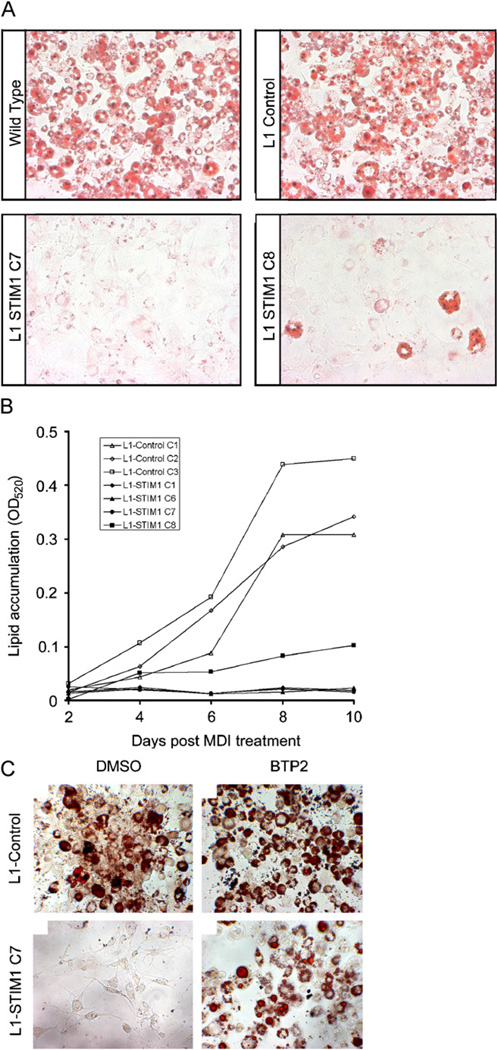
To investigate the effects of increasing Ca2+ entry through SOCs on the differentiation of 3T3-L1 cells, confluent L1-STIM1 or L1-control pre-adipocytes were induced to differentiate by MDI and the extent of differentiation was quantified by staining lipid with Oil Red O (Kutt and Tsaltas, 1959). At the end of the 8-day differentiation period, both wild-type 3T3-L1 and L1-control cells had accumulated a comparable amount of lipid (Fig. 3A, top panels). In the higher expressing L1-STIM1 C7 line, lipid accumulation was inhibited by 90% (Fig. 3A, bottom left panel; p = 0.0002) whereas in the lower expressing L1-STIM1 C8 line, lipid accumulation was reduced by 67% (Fig. 3A, bottom right panel; p = 0.0005). This experiment was repeated with four individually created L1-STIM1 and three L1-control cell lines over a 10-day differentiation period (Fig. 3B). Poor differentiation of L1-STIM1 lines (closed symbols) was observed in every experiment compared to the L1-control lines (open symbols).
Fig. 3.
STIM1 overexpression inhibits 3T3-L1 adipogenesis. (A) Oil Red O staining of fixed wild-type 3T3-L1, L1-control, L1-STIM1 C7 and C8 cells at day 10 post MDI induction. (B) Spectrophometric quantitation of Oil Red O in cell extracts at various timepoints post MDI treatment of individual L1-STIM1 cell lines (closed symbols) and individual L1-control lines (open symbols). (C) Oil Red O staining of fixed L1-control and L1-STIM1 C7 cells at day 10 following incubation with either DMSO or 10 µM BTP2 between days 0 and 6 post MDI treatment.
BTP2 was used to determine whether inhibition of Ca2+ entry through SOCs in L1-STIM1 cells would reverse the inhibitory effects of STIM1 overexpression on the differentiation process. BTP2 concentrations of up to 10 µM have been successfully used in extended culture conditions to inhibit hypertrophy-associated gene expression in cardiomyocytes (Bush et al., 2006). Confluent L1-STIM1 C7 or L1-control cells were incubated with 3 µM or 10 µM BTP2 or an equivalent final dilution of the DMSO vehicle control, and following the standard differentiation protocol, cells were stained with Oil Red O. Incubation with BTP2 had no measurable effect on lipid accumulation in L1-control cells (Fig. 3C, top right panel) when compared to cells treated with vehicle control (Fig. 3C, top left panel), whereas L1-STIM1 C7 cells treated with vehicle control did not accumulate lipid (Fig. 3C, bottom left panel). In contrast, 10 µM BTP2 partially reversed the inability of the L1-STIM1 C7 cells to differentiate, with an increase in both the size and number of adipogenic clusters (Fig. 3C, bottom right panel), whereas 3 µM BTP2 had little effect (not shown). 10 µM BTP2 also reversed the differentiation block in several other individually created L1-STIM1 lines (not shown). Together, these results indicated that overexpression of STIM1 inhibits adipocyte differentiation, that the level of inhibition correlates with the expression level of STIM1 and that this inhibition of differentiation could be partially reversed by inhibition of store-operated Ca2+ channel activity with BTP2.
3.4. Overexpression of STIM1 does not affect 3T3-L1 proliferation, MCE or growth arrest
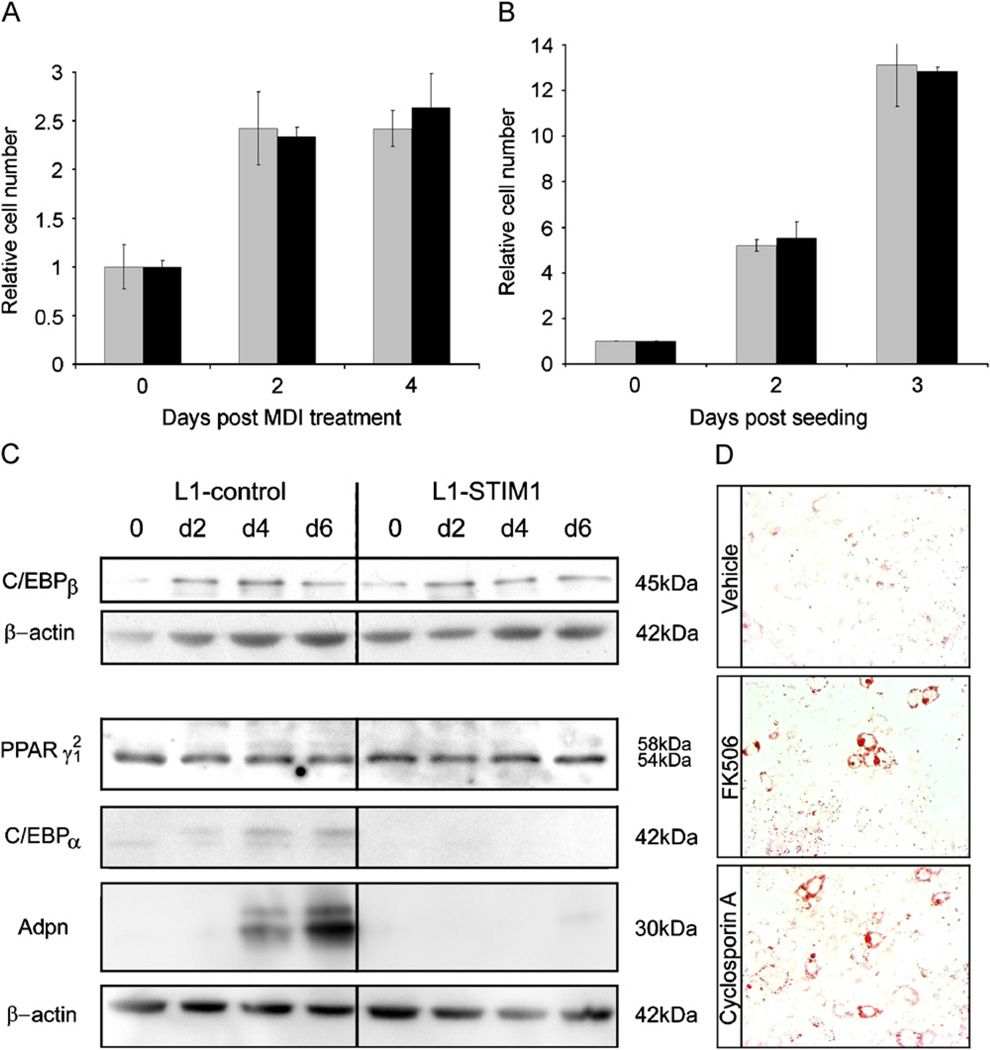
To investigate the cellular and molecular mechanisms by which STIM1 overexpression inhibited differentiation, several critical phases of the differentiation process were examined. Confluent 3T3-L1 cells undergo one to two rounds of cell division (Cowherd et al., 1999), known as mitotic clonal expansion (MCE) over the 4 days following MDI treatment followed by growth arrest, and this process is critical for robust differentiation (Tang et al., 2003). To test the effects of STIM1 overexpression on MCE, confluent L1-STIM1 and control pre-adipocytes were induced to differentiate and total cell numbers quantified at day 0 (MDI addition), day 2 and day 4 (Fig. 4A). An identical 2.5-fold increase in cell number was observed at day 2 in both L1-STIM1 and control cells, indicating that each cell line had undergone at least one round of cell division. No further increase in cell number in either cell line between days 2 and 4 indicated that both lines had once again growth arrested. Therefore STIM1 overexpression did not appear to inhibit differentiation by blocking post-confluent MCE or subsequent growth arrest. The intrinsic proliferative capacity of L1-STIM1 cells was also examined since aberrant proliferation reduces 3T3-L1 differentiation (Neal and Clipstone, 2003). L1-STIM1 or control pre-adipocytes were seeded at low density and cell numbers counted 2 and 3 days post seeding (Fig. 4B). No difference in proliferation rates between the two cell lines could be discerned, with cell numbers increasing 6-fold at day 2 and 13-fold at day 3 in both cell lines.
Fig. 4.
STIM1 overexpression leads to downregulation of C/EBPα and adiponectin expression. (A) 3T3-L1-control (gray bars) and L1-STIM1 C7 (black bars) cell numbers at day 0 (MDI addition), days 2 and 4 post MDI treatment as determined by cell counting with a haemocytometer. For each cell line, data is expressed as the cell number relative to that of 3T3-L1-control and L1-STIM1 C7 cell numbers at day 0, each of which were normalized to 1. Error bars represent the standard error of triplicate wells. (B) 3T3-L1-control (gray bars) and L1-STIM1 C7 (black bars) cell numbers at 0 (day of seeding), 2 and 3 days of proliferation as determined by cell counting with a haemocytometer. Data is expressed as for (A). Error bars represent the standard error of triplicate wells. (C) Western blotting for C/EBPβ, C/EBPα, PPARγ1 and 2, Adpn and β-actin in cell lysates collected at days 0, 2, 4 and 6 post MDI treatment in 3T3-L1-control and L1-STIM1 C7 cell lines. (D) Oil-red-O staining of fixed L1-STIM1 C7 cells at day 8 post MDI induction. Cells were treated with vehicle (ethanol), FK506 or cyclosporin A from day 0.
3.5. STIM1 overexpression results in downregulation of C/EBPα and adiponectin
To determine the effects of STIM1 overexpression on adipogenic gene regulation, the expression of transcription factors essential for 3T3-L1 differentiation were determined. The transcription factors CCAAT/enhancer binding protein (C/EBP) β and C/EBPδ which activate transcription of peroxisome proliferators-activated receptor gamma (PPARγ), are induced by differentiation (Salma et al., 2006). C/EBPβ and PPARγ induce expression of C/EBPα, which reinforces expression of PPARγ in a positive feedback loop, and also induces expression of other proteins such as adiponectin (Adpn) involved in terminal differentiation (Park et al., 2004). The expression of C/EBPβ, C/EBPα and PPARγ 1 and 2 isoforms were examined by Western blot during differentiation of confluent L1-STIM1 and control cells (Fig. 4C). Levels of C/EBPβ and PPARγ1 and 2 isoforms were indistinguishable in control and L1-STIM1 cells over the 6-day differentiation period while both C/EBPα and Adpn expression were visibly reduced in L1-STIM1 cells compared to controls at all time-points examined, by 99% and 91%, respectively at day 6. Together, these data, and the data presented in Fig. 4A and B, suggested that STIM1 overexpression results in the downregulation of C/EBPα and Adpn expression.
3.6. The effects of STIM1 overexpression can be reversed by calcineurin inhibitors
STIM1 overexpression activates calcineurin/NFAT signaling in lymphocytes (Huang et al., 2006), and overexpression of activated calcineurin potently inhibits 3T3-L1 differentiation (Neal and Clipstone, 2002). To determine whether the effects of STIM1 overexpression on adipocyte differentiation could be mediated by calcineurin, L1-STIM1 cells were induced to differentiate at day 0 with MDI, or with MDI and the specific calcineurin inhibitors cyclosporin A or FK506 (Neal and Clipstone, 2002). Compared with L1-STIM1 cells treated with vehicle control (Fig. 4D, top panel), lipid accumulation in FK506 or cyclosporin A-treated L1-STIM1 cells (Fig. 4D, middle, bottom panels) increased 5.5-fold (p = 0.001) and 6.5-fold (p = 0.0008), respectively. These data indicate that calcineurin activity is required to mediate the inhibitory effects of STIM1 overexpression on adipocyte differentiation.
3.7. Reducing endogenous STIM1 expression enhances 3T3-L1 differentiation, resulting in upregulation of C/EBPα and adiponectin
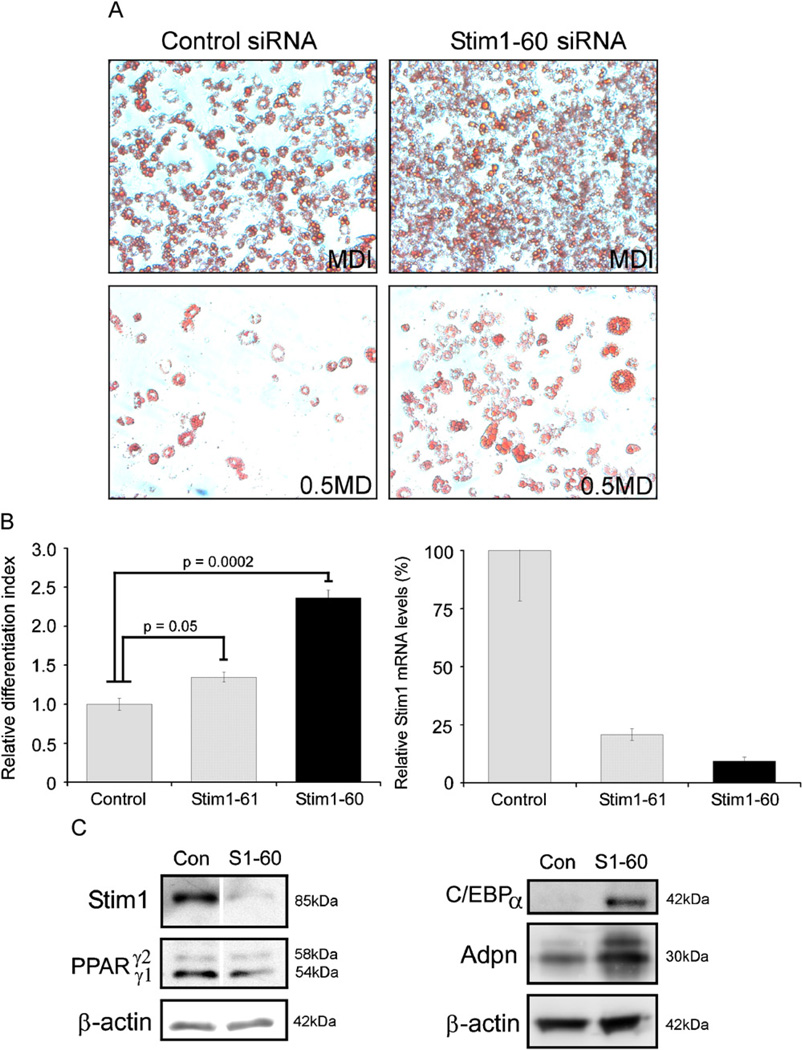
To determine the effects of reducing endogenous Stim1 expression on 3T3-L1 differentiation, 3T3-L1 cells were transfected with Stim1-specific Stealth™ siRNA (Stim1–60) or control siRNA and induced to differentiate under sub-optimal differentiation conditions by modifying the concentration of the MDI adipogenic cocktail (Neal and Clipstone, 2002). This modification resulted in the expected progressive reduction in both size and number of adipogenic colonies detected by Oil Red O staining (Fig. 5A). Control siRNA-transfected 3T3-L1 cells differentiated fully under optimal MDI conditions (Fig. 5A, top left) and accumulated 3.5-fold less lipid in sub-optimal 0.5MD conditions (Fig. 5A, bottom left). An increased number of Stim1–60-transfected cells appeared to stain Oil Red O positive than control siRNA-transfected cells in MDI (Fig. 5A, top right), but quantification of extracted lipid showed that this difference was not significant. In contrast, in 0.5MD, a greater number of Stim1–60-transfected cells stained Oil Red O positive (Fig. 5A, bottom right) and accumulated 2.5-fold more lipid than control siRNA-transfected cells (p = 0.0002). To confirm specificity, a second siRNA was tested. The Stim1–61 siRNA also increased lipid accumulation in 0.5MD conditions (Fig. 5B), by 1.4-fold (p = 0.05). Real-time RT-PCR confirmed that the Stim1–61 siRNA was less efficient at reducing endogenous Stim1 mRNA levels compared to the Stim1–60 siRNA (Fig. 5C), indicating that the enhancing effects of reduced Stim1 levels on adipocyte differentiation were dose dependent.
Fig. 5.
Stim1 downregulation enhances adipogenesis in 3T3-L1 cells and leads to upregulation of C/EBPα and Adpn expression. (A) Oil Red O staining of 3T3-L1 cells at day 8 following transfection with standard control Stealth™ siRNA duplexes or Stim1–60 Stealth™ siRNA duplexes and induced to differentiate with either MDI, or 0.5MD. (B) Quantification at OD520 of lipid extracted from triplicate wells of 3T3-L1 cells at day 8. Cells were transfected with standard control, Stim1–60 or Stim1–61 Stealth™ siRNA duplexes and induced to differentiate with 0.5MD. (C) Relative Stim1 mRNA levels in Stim1 or control siRNA transfected 3T3-L1 cells harvested 48 h post transfection. Western blotting for (D) Stim1, PPARγ1 and γ2, β-actin and (E) C/EBPα, Adpn, β-actin in cell lysates collected from Stealth™ Stim1-60 (S1–60) or control (Con) siRNA duplex-transfected cells at 4 days post 0.5MD treatment.
We confirmed by Western blotting that the Stim1–60 siRNA virtually abolished endogenous Stim1 expression at day 4 post 0.5MD treatment compared to control siRNA-transfected cells (Fig. 5D, top panel). Expression of PPARγ isoforms appeared relatively unaffected in Stim1-siRNA-treated cells (Fig. 5D, middle panel) whereas C/EBPα levels were increased by 5.7-fold and Adpn by 2.3-fold (Fig. 5E) in Stim1 siRNA-transfected cells at day 4. These data indicated that reducing endogenous Stim1 expression increases adipocyte differentiation, accompanied by an increase in expression of C/EBPα and Adpn.
4. Discussion
In the current study, we provide several lines of evidence indicating that Ca2+ entry via store-operated channels is functional in 3T3-L1 pre-adipocytes and, through a Stim1-mediated pathway, acts to negatively regulate adipocyte differentiation. We demonstrate that overexpression of Stim1 in 3T3-L1 pre-adipocytes specifically increases Ca2+ influx through SOCs and consequently inhibits differentiation associated with reduced expression of C/EBPα and Adpn. In contrast, we find that reducing Stim1 levels with siRNA markedly increases differentiation in response to sub-optimal adipogenic stimuli, associated with increased expression of C/EBPα and Adpn. However, in apparent contradiction, we find that endogenous Stim1 is itself upregulated during adipocyte differentiation. Altogether, our data suggests that the level of Ca2+ influx though SOCs may be intrinsically regulated during differentiation by the expression level of Stim1, and that this in turn influences adipocyte gene expression, and ultimately may set the level of commitment of pre-adipocytes to the terminal differentiation phase.
We have found that the inhibitory effects of STIM1 over-expression on adipocyte differentiation requires calcineurin activity. The calcineurin/NFAT signaling pathway is an important downstream effector of Ca2+ entry via SOCs in lymphocytes (Gwack et al., 2007). Ca2+-activated calcineurin is best known for it’s ability to dephosphorylate cytosolic NFAT and trigger its nuclear translocation where it acts both as a positive and negative regulator of target gene transcription (Crabtree and Olson, 2002). Activation of the calcineurin/NFAT pathway in 3T3-L1 cells by prostaglandin F2a (Liu and Clipstone, 2007) or by the introduction of constitutively active calcineurin or NFATC1 (Neal and Clipstone, 2002, 2003) potently inhibits their differentiation, similar to the effects of Stim1 overexpression. In addition, cyclosporin A markedly increases adipocyte differentiation in response to suboptimal differentiation stimuli (Neal and Clipstone, 2002), similar to the effects of siRNA-mediated knockdown of Stim1. Modulation of Stim1 levels directly affects the activity of the calcineurin/NFAT pathway in epithelial and hematopoietic cells (Wang et al., 2006; Huang et al., 2006; Liou et al., 2005), and this study suggests that Stim1 is able to exert a similar influence in 3T3-L1 adipocytes. However, this does not preclude the involvement of additional signaling pathways in Stim1-mediated inhibition of differentiation since cyclosporin A and FK506, at the concentrations used here, did not completely rescue the effects of STIM1 overexpression. In some cells, Ca2+ is known to enhance the activation of transcription factors such as NFκB (Crabtree, 2001), also an inhibitor of adipocyte differentiation (Chae and Kwak, 2003). It remains to be tested whether modulation of Stim1 levels directly affects additional signaling pathways in 3T3-L1 cells.
Overexpression of Stim1 virtually abolished C/EBPα and Adpn expression. Antisense knockdown of C/EBPα alone in 3T3-L1 pre-adipocytes is sufficient to inhibit triglyceride accumulation and terminal gene expression (Lin and Lane, 1992) and C/EBPα is a critical regulator of Adpn expression (Park et al., 2004) therefore it is likely that reduced C/EBPα expression is the major contributor to the reduced Adpn expression levels and inhibition of adipocyte differentiation observed in this study. While expression and activity of C/EBPβ and PPARγ are required for efficient induction of C/EBPα expression (Park et al., 2004; Zuo et al., 2006b) we show that Stim1 overexpression does not affect MDI-induced C/EBPβ or PPARγ expression, nor does it affect C/EBPβ-dependent mitotic clonal expansion or cell cycle arrest. However, Stim1-mediated Ca2+ entry may interfere with C/EBPβ phosphorylation, which is critical for induction of C/EBPα expression but not required for induction of PPARγ expression (Park et al., 2004). Alternatively, Stim1-mediated effects may be distal to C/EBPβ activity but instead may inhibit PPARγ activity, since selective antagonists of PPARγ activity reduce C/EBPα expression and inhibit 3T3-L1 adipogenesis (Zuo et al., 2006a, b). Another possible mechanism underlying Stim1-mediated effects is direct repression of C/EBPα expression after Ca2+ entry independently of C/EBPβ and PPARγ activity. Prostaglandin F2a-mediated inhibition of 3T3-L1 adipo-genesis via Ca2+/calcineurin can be attenuated by a histone deacetylase inhibitor, which rescues PPARγ and C/EBPα expression, suggesting that activation of Ca2+/calcineurin signaling in 3T3-L1 cells likely promotes transcriptional repression (Liu and Clipstone, 2007). However, Stim1 overexpression does not result in complete abolition of C/EBPα and PPARγ expression as is seen with sustained Prostaglandin F2a treatment, or expression of constitutively active calcineurin. Whilst the reason for this discrepancy is unclear, we and others have found that Stim1-mediated SOC entry in cells stably overexpressing STIM1 is still regulated by ER Ca2+ store content (Soboloff et al., 2006a), whereas constitutively active calcineurin and NFAT are likely not subject to normal regulatory processes and may have additional effects on cell behavior and gene expression (Neal and Clipstone, 2003). This study has shown that reducing Stim1 levels increased expression of Adpn and C/EBPα. Both C/EBPα and Adpn are capable of increasing triglyceride accumulation in 3T3-L1 cells when overexpressed (Lin and Lane, 1994; Fu et al., 2005). Interestingly, 2-fold overexpression of Adpn increases triglyceride accumulation almost 3-fold at day 9 of differentiation which correlates well with the level of Adpn upregulation and increased triglyceride accumulation observed in this study.
It is becoming increasingly understood that Stim1 can act as a multifunctional regulator of Ca2+ influx through several classes of SOCs, including Orai1, Orai2 and transient receptor potential C1 (TRPC1) (Huang et al., 2006) and that its presence can dictate the level of expression of some channels, such as TRPC1 in HEK293 cells (Huang et al., 2006). The expression of Orai or TRP channels in 3T3-L1 pre-adipocytes has not been reported, however, transcript profiling of adipocytes in vivo indicate expression of Orai and TRPC1 genes (Su et al., 2004). It will now be important to characterize Orai and TRP channel expression in 3T3-L1 pre-adipocytes and determine through which of these Stim1 exerts its anti-adipogenic effects. Recent evidence also suggests that Stim1 situated in the plasma membrane may regulate non-store-operated Ca2+ channels such as the arachidonic acid regulated channels (ARC) (Mignen et al., 2007). AA is a potent inhibitor of 3T3-L1 pre-adipocyte differentiation, acting through the cyclooxygenase-dependent generation of AA eicosanoid metabolites (Petersen et al., 2003). However, activation of Ca2+ influx through ARC channels is likely a direct action of AA on the channel itself, independently of AA metabolites and cyclooxygenase activity (Shuttleworth and Thompson, 1998), arguing against a role for ARC channels in Stim1-mediated adipocyte differentiation.
Our study has, for the first time, provided evidence that adipocytes utilize a store-operated Ca2+ entry mechanism that may control commitment to differentiation via upregulation of the endoplasmic reticulum Ca2+ sensor Stim1. That Stim1 exerts an inhibitory effect on differentiation is perhaps surprising given its upregulation during the differentiation process. However, the functional attributes of mature adipocytes are one of lipid storage and lipid release and, by necessity, adipocytes obtain the proteins required to perform these functions during the differentiation process. Indeed, expression of another negative regulator of adipocyte differentiation, Sirtuin 1, is also upregulated during the differentiation process and appears to play a major role in promoting fat mobilization in mature adipocytes (Picard et al., 2004). Interestingly, NFAT proteins have recently been found to facilitate fat mobilization and metabolic gene expression in mature adipocytes (Holowachuk, 2007). Whether Stim1 plays a similar role remains to be determined. In conclusion, as in other cells, the subcellular source of Ca2+ is likely an important regulator of adipocyte differentiation. This could be especially important in human disease such as obesity where deregulation of calcium handling is evident. It will now be important to examine whether SOC entry play a role in the increased adipocyte Ca2+ levels and development of obesity in humans.
Acknowledgments
We thank Iain MacDonald and Vivian Ward for photography and graphics assistance, Annette Lasham for technical advice and Lance Xu for the kind gift of the anti-Adpn antibody. This work was supported by grants from the Health Research Council, New Zealand, the Auckland Medical Research Foundation, New Zealand and the School of Biological Sciences, The University of Auckland.
References
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- Chae GN, Kwak SJ. NF-kappaB is involved in the TNF-alpha induced inhibition of the differentiation of 3T3-L1 cells by reducing PPARgamma expression. Exp. Mol. Med. 2003;35:431–437. doi: 10.1038/emm.2003.56. [DOI] [PubMed] [Google Scholar]
- Cooper DM, Karpen JW, Fagan KA, Mons NE. Ca(2+)-sensitive adenylyl cyclases. Adv. Second Messenger Phosphoprotein Res. 1998;32:23–51. [PubMed] [Google Scholar]
- Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin. Cell Dev. Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Calcium, calcineurin, and the control of transcription. J. Biol. Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Draznin B, Sussman KE, Eckel RH, Kao M, Yost T, Sherman NA. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J. Clin. Investigat. 1988;82:1848–1852. doi: 10.1172/JCI113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadek MA, Johnstone LS. Biochemical properties and cellular localisation of STIM proteins. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Fox KE, Fankell DM, Erickson PF, Majka SM, Crossno JT, Jr, Klemm DJ. Depletion of cAMP-response element-binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer-binding protein (C/EBP) alpha, C/EBP beta, or PPAR gamma 2. J. Biol. Chem. 2006;281:40341–40353. doi: 10.1074/jbc.M605077200. [DOI] [PubMed] [Google Scholar]
- Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,5- bis(trifluoromethyl)pyrazole derivative, BTP2. J. Biol. Chem. 2005;280:10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin. Endocrinol. Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Holowachuk EW. Nuclear factor of activated T cell (NFAT) transcription proteins regulate genes involved in adipocyte metabolism and lipolysis. Biochem. Biophys. Res. Commun. 2007;361:427–432. doi: 10.1016/j.bbrc.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat. Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Kutt H, Tsaltas TT. Staining properties of Oil Red O and a method of partial purification of the commercial product. Clin. Chem. 1959;5:149–160. [PubMed] [Google Scholar]
- Lee H, Jun DJ, Suh BC, Choi BH, Lee JH, Do MS, Suh BS, Ha H, Kim KT. Dual roles of P2 purinergic receptors in insulin-stimulated leptin production and lipolysis in differentiated rat white adipocytes. J. Biol. Chem. 2005;280:28556–28563. doi: 10.1074/jbc.M411253200. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lane MD. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Clipstone NA. Prostaglandin F2alpha inhibits adipocyte differentiation via a G alpha q-calcium-calcineurin-dependent signaling pathway. J. Cell Biochem. 2007;100:161–173. doi: 10.1002/jcb.21044. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, CA) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mathieu RL, Casez JP, Jaeger P, Montandon A, Peheim E, Horber FF. Altered body composition and fuel metabolism in stable kidney transplant patients on immuno-suppressive monotherapy with cyclosporine A. Eur. J. Clin. Invest. 1994;24:195–200. doi: 10.1111/j.1365-2362.1994.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J. Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RF, Farmer SR. Insights into the transcriptional control of adipocyte differentiation. J. Cell Biochem. Suppl. 1999;32-33:59–67. doi: 10.1002/(sici)1097-4644(1999)75:32+<59::aid-jcb8>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J. Biol. Chem. 2002;277:49776–49781. doi: 10.1074/jbc.M207913200. [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J. Biol. Chem. 2003;278:17246–17254. doi: 10.1074/jbc.M300528200. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Takova T. Role of Ca2+ in the early stages of murine adipocyte differentiation as evidenced by calcium mobilizing agents. Differentiation. 1996;60:151–158. doi: 10.1046/j.1432-0436.1996.6030151.x. [DOI] [PubMed] [Google Scholar]
- Oh-hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B-H, Qiang L, Farmer SR. Phosphorylation of C/EBP{beta} at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol. Cell. Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RK, Jorgensen C, Rustan AC, Froyland L, Muller-Decker K, Furstenberger G, Berge RK, Kristiansen K, Madsen L. Arachidonic acid-dependent inhibition of adipocyte differentiation requires PKA activity and is associated with sustained expression of cyclooxygenases. J. Lipid Res. 2003;44:2320–2330. doi: 10.1194/jlr.M300192-JLR200. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer FX. Medical hazards of obesity. Ann. Intern. Med. 1993;119:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salma N, Xiao H, Imbalzano AN. Temporal recruitment of CCAAT/ enhancer-binding proteins to early and late adipogenic promoters in vivo. J. Mol. Endocrinol. 2006;36:139–151. doi: 10.1677/jme.1.01918. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Muscarinic receptor activation of arachidonate-mediated Ca2+ entry in HEK293 cells is independent of phospholipase C. J. Biol. Chem. 1998;273:32636–32643. doi: 10.1074/jbc.273.49.32636. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated storeoperated Ca2+ entry. Curr. Biol. 2006a;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 2006b;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Kim WH, Moon C, Hong YH, Eun SY, Lim JH, Choi JS, Song J, Jung MH. Ectopic expression of Neuronatin potentiates adipogenesis through enhanced phosphorylation of cAMP-response element-binding protein in 3T3- L1 cells. Biochem. Biophys. Res. Commun. 2005;337:481–489. doi: 10.1016/j.bbrc.2005.09.078. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. U S A. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang N, Xie J, Walton SC, McKown RL, Raab RW, Ma P, Beck SL, Coffman GL, Hussaini IM, Laurie GW. Restricted epithelial proliferation by lacritin via PKCalpha-dependent NFAT and mTOR pathways. J. Cell Biol. 2006;174:689–700. doi: 10.1083/jcb.200605140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem. J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Senior PV, Van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim. Biophys. Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem. Biophys. Res. Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Qiang L, Farmer SR. Activation of CCAAT/enhancer-binding protein (C/EBP) {alpha} expression by C/EBPbeta during adipogenesis requires a peroxisome proliferator-activated receptor-{gamma}-associated repression of HDAC1 at the C/ebp{alpha} gene promoter. J. Biol. Chem. 2006a;281:7960–7967. doi: 10.1074/jbc.M510682200. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Qiang L, Farmer SR. Activation of CCAAT/enhancer-binding protein (C/EBP) alpha expression by C/EBP beta during adipogenesis requires a peroxisome proliferator-activated receptor-gamma-associated repression of HDAC1 at the C/ebp alpha gene promoter. J. Biol. Chem. 2006b;281:7960–7967. doi: 10.1074/jbc.M510682200. [DOI] [PubMed] [Google Scholar]