Abstract
Chloride channels represent a relatively under-explored target class for drug discovery as elucidation of their identity and physiological roles has lagged behind that of many other drug targets. Chloride channels are involved in a wide range of biological functions, including epithelial fluid secretion, cell-volume regulation, neuroexcitation, smooth-muscle contraction and acidification of intracellular organelles. Mutations in several chloride channels cause human diseases, including cystic fibrosis, macular degeneration, myotonia, kidney stones, renal salt wasting and hyperekplexia. Chloride-channel modulators have potential applications in the treatment of some of these disorders, as well as in secretory diarrhoeas, polycystic kidney disease, osteoporosis and hypertension. Modulators of GABAA (γ-aminobutyric acid A) receptor chloride channels are in clinical use and several small-molecule chloride-channel modulators are in preclinical development and clinical trials. Here, we discuss the broad opportunities that remain in chloride-channel-based drug discovery.
Although there has been much activity in drug development for cation (sodium, potassium and calcium) channel targets, considerably less attention has been given to chloride channels. This is partly because their identification and analysis has lagged behind those of other targets, and because of technical challenges in screening for chloride-channel modulators. Electrophysiologists have historically considered anion channel currents as ‘unimportant leaks’ associated with cation channels in excitable cells. Also, although there are toxins known to modulate sodium, potassium and calcium channels with high affinity and selectivity1,2 — thereby allowing their functional and molecular characterization — comparable ligands for anion channels have yet to be identified.
Chloride channels are involved in a diverse set of functions in normal physiology and acquired diseases. Additionally, there are genetic diseases caused by mutations in chloride channels (channelopathies) — the most common being cystic fibrosis, which has a prevalence of 1 in 2,000 Caucasians. Cystic fibrosis remains an incurable disease despite considerable knowledge of its genetic basis; there is a great need to develop drugs to restore normal chloride-channel function in cystic fibrosis cells.
Chloride transport across the cell plasma membrane is involved in key cellular events including cell-volume regulation, transepithelial fluid transport, muscle contraction and neuroexcitation. Within cells, chloride transport across organellar membranes is involved in endosomal, lysosomal and Golgi acidification. Chloride channels provide the major route for transmembrane chloride transport in these processes. In contrast to cation channels, which often show high selectivity for a specific ion, chloride channels are also permeable to other anions including other halides, the pseudohalide SCN− and bicarbonate.
Mammalian chloride channels broadly fall into five classes based on their regulation: cystic fibrosis transmembrane conductance regulator (CFTR), which is activated by cyclic AMP-dependent phosphorylation; calcium-activated chloride channels (CaCCs); voltage-gated chloride channels (ClCs); ligand-gated chloride channels (GABA (γ-aminobutyric acid)and glycine-activated); and volume-regulated chloride channels (FIG. 1). TABLE 1 summarizes these chloride-channel classes, their subtypes, molecular identities, functional properties, physiological roles, associated diseases and pharmacological modulators. Modulators of ligand-gated chloride channels, such as barbiturates and benzodiazepines for GABAA -gated chloride channels, are in clinical use. In addition, several compounds are in Phase II clinical trials for cystic fibrosis, including potentiators of mutant CFTRs and activators of CaCCs, which act by elevating cytoplasmic calcium. Candidate inhibitors of CFTR, CaCCs and ClCs have also been identified and are in various stages of preclinical development. Several attractive chloride-channel targets remain relatively unexplored. This Review considers methods to assay chloride-channel function, with a focus on screening applications, and discusses the opportunities emerging from basic research for drug development for each of the chloride-channel classes.
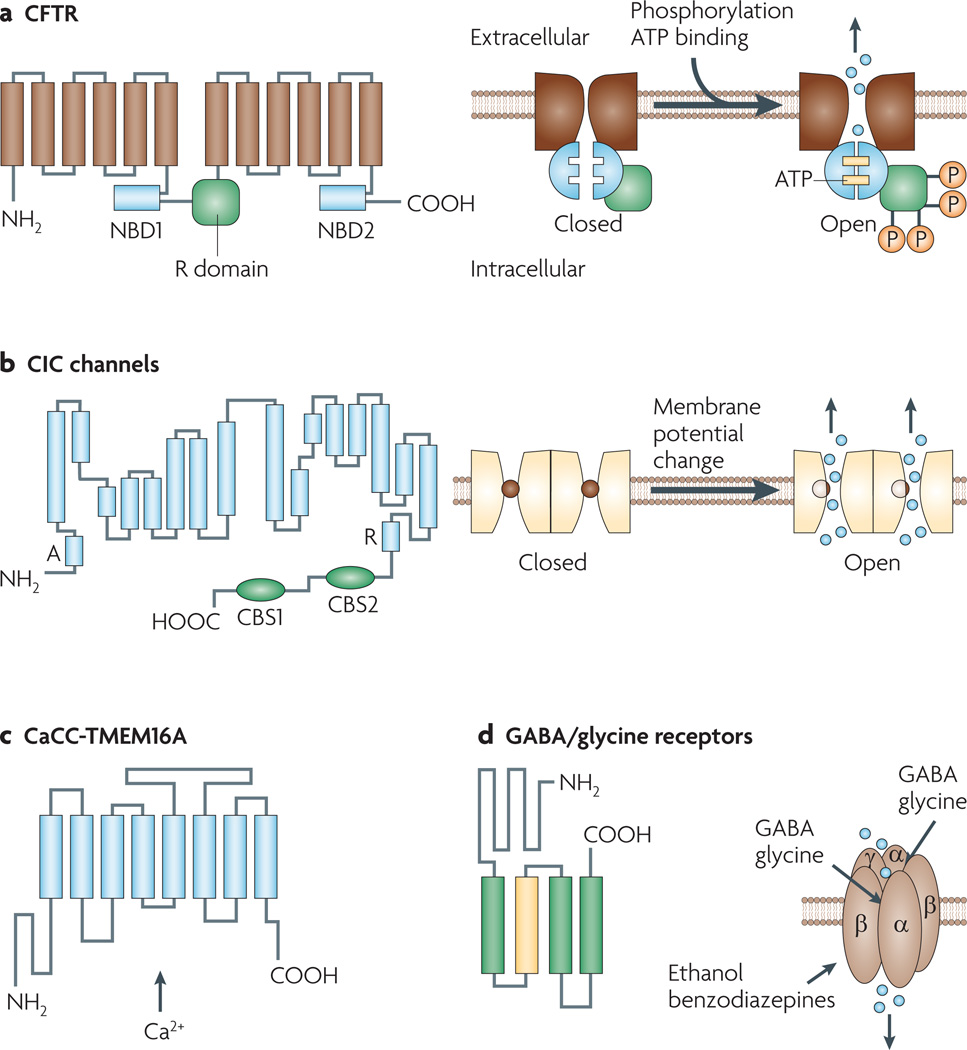
Figure 1. Structures and mechanisms of regulation of chloride channels.
a | Cystic fibrosis transmembrane conductance regulator (CFTR). Shown here are 12 membrane-spanning segments of CFTR plus two nucleotide binding domains (NBDs 1 and 2) and a regulatory R domain. CFTR activation involves cyclic AMP-dependent phosphorylation and binding of ATP molecules at the NBDs. b | The overall organization of voltage-gated chloride (ClC) channels is depicted, showing 18 segments (labelled A to R) most of which span the plasma membrane partially and in a strongly tilted configuration. Fast gating involves flipping of a pore-lining glutamate side chain into and out of the chloride pathway. Channels are arranged as dimers with a slow gate controlling the activity of both channels simultaneously. c | The calcium-activated chloride channel (CaCC) TMEM16A (anoctamin-1), with predicted topology showing eight transmembrane segments with cytosolic amino and carboxy termini. The mechanism of calcium activation is unknown. d | GABA (γ-aminobutyric acid) and glycine-gated chloride channels, showing pentameric channels formed by α, β and γ subunits. Each subunit has four transmembrane segments, with a large extracellular N terminus. The second transmembrane segment of each subunit contributes to the formation of the central pore. The N termini of the α and β subunits form the ligand binding site. Volume-sensitive chloride channels (not shown) have an unknown molecular structure. They activate upon cell swelling. CBS, cystathione β-synthase-related domain.
Table 1.
Examples of chloride-channel subtypes, functions and modulators
| Protein | Mechanism of regulation |
Channel properties | Physiological roles | Human diseases | Pharmacological modulators |
|---|---|---|---|---|---|
| CFTR | Activated by cyclic AMP-dependent phosphorylation | Linear I–V; Cl− > I− permeability | Cl− secretion by epithelial cells in airways, submucosal glands, pancreas, intestine and testis; Cl− absorption in sweat glands | Cystic fibrosis | Multiple, nanomolar-potency activators and inhibitors |
| ClC-1 | Activated by depolarization | Cl− > I− permeability; double-barrelled pore | Cl− conductance in skeletal muscle; repolarization after action potential | Myotonia | Weak inhibition by 9-AC, niflumic acid and DPC |
| ClC-2 | Slowly activated by hyperpolarization and cell swelling | Inward rectification of I–V; Cl− > I− permeability | Cl− homeostasis in neurons; cell-volume regulation | Epilepsy (controversial) | Weak inhibition by classical Cl− channel blockers, inhibited by Zn2+ |
| ClC-4 and ClC-5 | None identified | Electrogenic H+/Cl− exchange; strong outward rectification | Intracellular Cl− channels facilitating endosomal and synaptic vesicle acidification | ClC-5: Dent’s disease (proteinuria and kidney stones) | No known inhibitors |
| ClC-7 | Requires OSTM1 for membrane expression | Electrogenic H+/Cl− exchanger | Acidification of resorption lacuna in osteoblasts; lysosomal acidification | Osteopetrosis; lysosomal storage disease | No known inhibitors |
| ClC-Ka and ClC-Kb | Weak voltage-dependence; both require barttin for membrane targeting | Moderate outward rectification of I–V; Cl− > I− permeability | Transepithelial Cl− transport in kidney tubules and inner ear | ClC-Kb: Bartter’s syndrome (deafness when barttin affected) | Inhibited by phenylbenzofuran carboxylic acids |
| Bestrophins | Activated by elevated cytosolic Ca2+ | I− > Cl− permeability | Cl− transport in retinal pigment epithelium; Ca2+-stimulated Cl− secretion in epithelia | Best vitelliform macular dystrophy | Weakly inhibited by niflumic acid and stilbenes |
| TMEM16A (anoctamin-1) | Activated by elevated cytosolic Ca2+ | I− > Cl− permeability | Ca2+-stimulatd Cl− secretion in epithelia; smooth-muscle contraction | Not known | Strongly inhibited by niflumic acid and NPPB |
| GABA receptor | Activated by GABA | I− > Cl− permeability | Inhibitory synaptic transmission in the brain | Epilepsy | Potentiated by benzodiazepines and barbiturates |
| Glycine receptor | Activated by glycine, β-alanine and taurine | I− > Cl− permeability | Inhibitory synaptic transmission in the spinal cord | Hyperekplexia | Inhibited by strychnine and picrotoxin |
9-AC, 9-anthracene-carboxylic acid; CFTR, cystic fibrosis transmembrane conductance regulator; ClC, voltage-gated chloride channel; DPC, diphenylcarboxylate; GABA, γ-aminobutyric acid; I–V, current–voltage relationship; NPPB, 5-nitro-2-(3-phenylpropylamino)benzoic acid; OSTM1, osteopetrosis associated transmembrane protein 1.
Assaying chloride-channel function
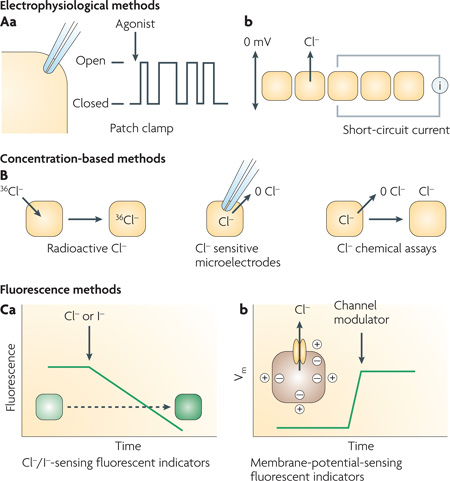
Functional assay of chloride-channel activity is required for screening of chloride-channel modulators and for elucidation of their mechanism of action. Until a few years ago, identification of chloride-channel modulators was hampered by the lack of suitable probes for high-throughput assays. BOX 1 summarizes the principles of electrophysiological, concentration-based and fluorescence methods that are now available to measure chloride transport, and their suitability for screening applications.
Box 1 | Methods to assay chloride-channel activity.
The gold standard in the study of ion-channel function is the patch-clamp technique, in which changes in single-channel activity are followed continuously, as shown in panel Aa for the cell-attached patch-clamp configuration. Transmembrane current is measured in response to applied potential differences and channel modulators. Other configurations that allow direct control of the composition of solutions bathing both sides of the channel-containing membrane patch include outside-out and inside-out configurations. Single-channel recordings provide a comprehensive description of channel parameters, including unitary conductance, open probability, channel gating kinetics (open and closed times) and gating mechanism.
Access of the patch pipette to the cell interior allows measurement of whole-cell macroscopic currents. The resultant macroscopic current–voltage relationship describes channel activity as a function of membrane potential. Voltage-dependence of membrane currents may be linear (ohmic response), inwardly-rectifying (larger currents at interior-negative potentials) or outwardly-rectifying (larger currents at exterior-negative potentials). For channels contained or expressed in epithelial cell monolayers having high electrical resistance, measurement of short-circuit current can provide quantitative information on channel activity and/or expression. As shown in panel Ab, short-circuit current is the transepithelial current applied to maintain zero transepithelial potential difference. Although short-circuit current depends in general on the activities of multiple transporters and on electrochemical driving forces, suitable experimental design can allow channel discrimination. While patch-clamp and short-circuit current measurements are of considerable use in secondary analysis of putative chloride-channel modulators, they are technically tedious for primary screening applications, even when using currently marketed high-throughput patch-clamp instruments. Electrophysiological measurements may be necessary to measure activity of certain ion channels, such as transiently gated sodium and potassium channels in excitable cells, but the relatively stable activation of most chloride channels allows the use of technically simple and relatively inexpensive methods for primary screening. Even for ligand-activated chloride channels, which show a transient response to GABA (γ-aminobutyric acid) and glycine, it is possible to apply screening methods based on anion flux.
Panel B shows concentration-based methods to assay chloride transport, which rely on changes in intracellular or extracellular chloride concentration in response to imposed chloride gradients. Concentration is measured using radioactive chloride (36Cl−), chloride-sensitive microelectrodes or chemical assay methods such as silver chloride gravimetry. In contrast to electrophysiological methods, concentration-based methods are sensitive to both conductive and electrically silent chloride transport. Although such methods have long been used to assay chloride transport, they are of limited use for screening applications because of their relatively poor reproducibility and the need to separate cellular and extracellular solutions or construct complex microelectrodes.
Fluorescence methods are best suited for high-throughput identification of chloride-channel modulators. Chemical-type chloride-sensitive fluorescent indicators of the quinolinium class and iodide-sensitive fluorescent indicators of the luminarine class can be loaded into cells to provide a direct readout of cytoplasmic halide concentration181,182. The fluorescence of these indicators is quenched by halides through a collisional mechanism. Panel Ca shows an assay in which indicator fluorescence is measured continuously following extracellular halide addition. The rate of fluorescence decline provides a quantitative measure of halide influx. As discussed in the main text, halide-sensing mutants of the green fluorescent protein are particularly useful for chloride-channel screening assays because they can be expressed stably in cells and so obviate the need to load and wash cells with chemical-type halide indicators. Also, fluorescent proteins do not leak out of cells. Membrane-potential-sensing fluorescent indicators provide an alternative method to assay chloride-channel function. As illustrated in panel Cb, changes in membrane potential (Vm) are measured in response to addition of channel modulators. Various membrane-potential-sensing fluorescent indicators are available with different sensitivities, response kinetics and sensing mechanisms, including genetically encoded fluorescent-protein-based sensors183,184. However, a limitation of membrane-potential-sensing methods to assay chloride channels is that cell membrane potential is determined by multiple factors, including cytoplasmic ionic composition and the activities of all ion channels; this can result in high false-positive rates.
Of particular utility are fluorescent-protein-based screening methods that use halide-sensing green fluorescent protein (GFP) mutants. As a genetically encoded, intrinsically fluorescent protein of ~30 kDa, GFP can be targeted to the cell cytoplasm or to specific intracellular sites. GFP fluorescence is sensitive to pH but not to halides. In the experiments depicted in FIG. 2Aa, halide sensitivity was conferred using a rational mutagenesis strategy based upon crystallographic data3, allowing halide penetration near the internal GFP chromophore. The emission maximum of the resultant yellow fluorescent protein (YFP) is red-shifted by ~20 nm (to 528 nm) compared with GFP, and is reduced by increasing halide concentration. The fluorescence of the original halide-sensing YFP, YFP-H148Q, is 50% quenched by 100 mM chloride or 21 mM iodide4.
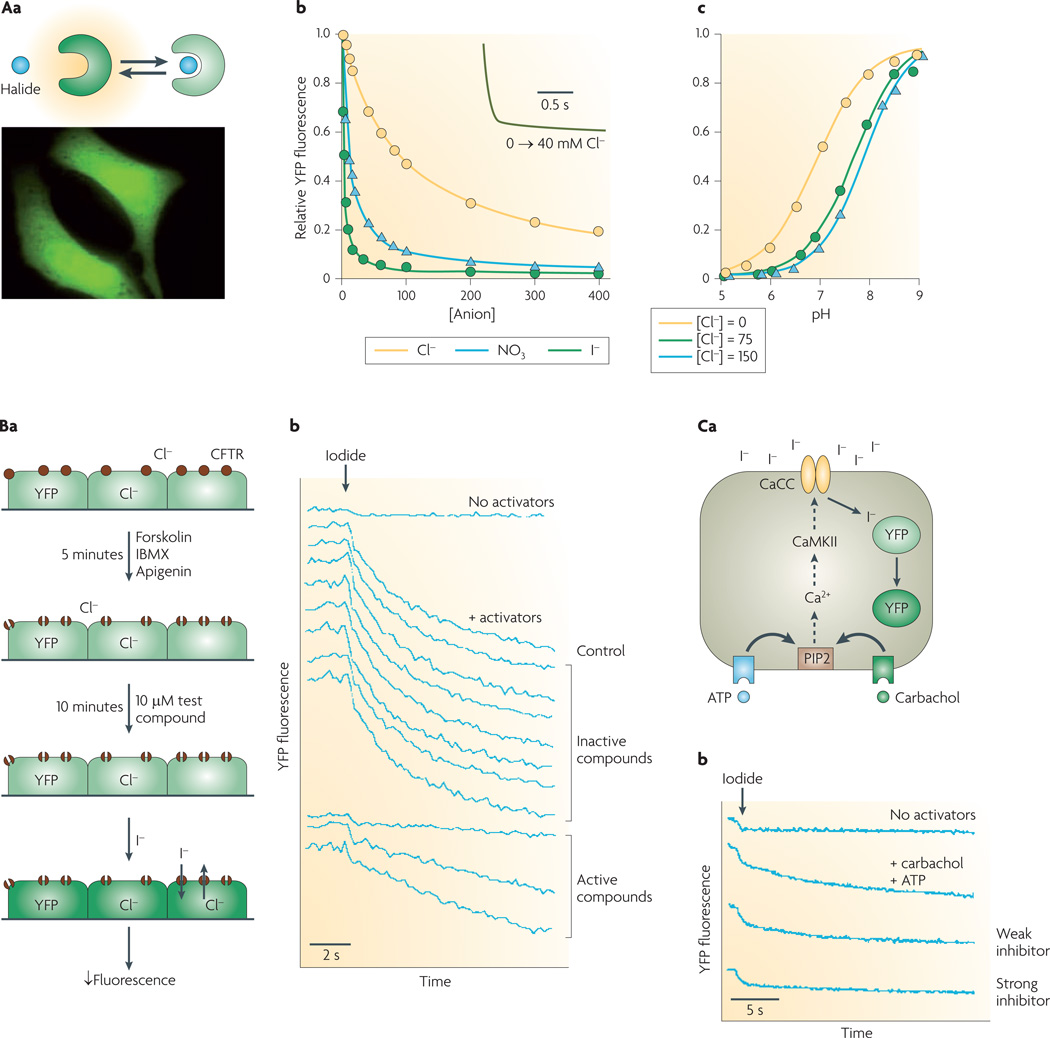
Figure 2. Cell-based screening assay of halide transport using a fluorescent protein mutant.
Aa | Reduced yellow fluorescence protein (YFP) fluorescence following halide binding (top). Cells expressing YFP in a cytoplasmic pattern (bottom). Ab | Titration of YFP-H148Q/I152L fluorescence with chloride, iodide and nitrate at pH 7.4. Inset shows rapid indicator response following an increase in Cl− concentration from 0 mM to 40 mM. Ac | pH titration at Cl− concentration of 0 mM, 75 mM and 150 mM. Ba | Screening protocol for cystic fibrosis transmembrane regulator (CFTR) inhibitors. CFTR halide conductance in cells co-expressing CFTR and YFP indicator stimulated by an agonist mixture (forskolin, 3-isobutyl-1-methylxanthine (IBMX), apigenin). After addition of test compound, iodide influx is measured by YFP fluorescence. Bb | Single-well fluorescence data showing controls (no activators, no test compound) and test wells. Ca | Screen for calcium-activated chloride channel (CaCC) inhibitors. CaCC halide conduction in human colonic cells expressing native CaCC and transfected with YFP indicator is measured following stimulation by an agonist mixture (ATP, carbachol). Iodide influx quenches YFP fluorescence. Cb | Fluorescence data showing controls (no activators, no test compounds) and examples of inhibitors. CaMKII, calcium/calmodulin kinase II; PIP2, phosphatidylinositol 4,5-bisphosphate. Panel A is modified, with permission, from REF. 5 © (2001) Elsevier Science. Panel B is modified, with permission, from REF. 6 © (2002) American Society for Clinical Investigation. Panel C is modified, with permission, from REF. 8 © (2008) American Society for Pharmacology and Experimental Therapeutics.
YFP-H148Q/I152L, discovered by mutation screening5, is highly sensitive to iodide compared with chloride (FIG. 2Ab). The halide-sensing mechanism of YFPs involves increases in their pKa with halide concentration: the pKa of YFP-H148Q/I152L is 6.95 in the absence of chloride, increasing to 7.89 in 150 mM chloride (FIG. 2Ac). YFP-H148Q/I152L fluorescence responds over tens of milliseconds to changes in chloride concentration (FIG. 2Ab, inset). YFPs can be stably expressed in cells where they are brightly fluorescent and strongly halide-sensitive, although their fluorescence is sensitive to both halide concentration and to pH.
FIGURE 2B,C show two examples of YFP/cell-based screening assays for chloride-channel modulators. Screening for small-molecule CFTR inhibitors6 (FIG. 2B) required selection of cell lines that stably expressed a YFP halide sensor together with wild-type human CFTR. Key requirements of cell lines for CFTR screening include rapid growth on relatively inexpensive media, good adherence to plastic multi-well plates during solution washing/exchange, low CFTR-independent halide permeability, and strong and stable expression of CFTR and the YFP sensor. In addition, an epithelial cell type is desirable because CFTR is normally expressed in these cells, and because they can form electrically tight monolayers that allow efficient secondary analysis of hits by measurement of short-circuit current. FIGURE 2Ba illustrates primary screening in a 96-well format with YFP/CFTR-transfected Fischer rat thyroid cells7. CFTR halide conductance is activated by an agonist mixture that targets different CFTR activation pathways, including CFTR itself; the reasoning behind this approach is that active compounds identified in the screen would be expected to target CFTR itself. Following incubation with test compounds, fluorescence is measured briefly before, and for 12 seconds after addition of iodide to the extracellular solution. Iodide rather than chloride is used because of strong YFP quenching by iodide, and because CFTR, like most chloride channels, is permeable to iodide. Fluorescence following iodide addition is stable in the absence of agonists, but declines rapidly with agonists, with hits identified from their reduced negative slope (FIG. 2Bb). As discussed below, several classes of small-molecule CFTR inhibitors have emerged from this screen.
FIGURE 2Ca illustrates a similar strategy used to identify small-molecule inhibitors of a human intestinal CaCC. Because the molecular identity of this channel was not known at the time of the screening, phenotype-based screening was performed on a human intestinal epithelial cell line (HT-29) following stable expression of YFP-H148Q/I152L using lentivirus8. The primary screen was done using a mixture of calcium-elevating agonists, with the reasoning that the target of active compounds should be downstream of calcium signalling. As for the CFTR screen, the readout was YFP fluorescence quenching by iodide influx.
Targeting CFTR
CFTR is a cAMP-activated chloride channel in the apical plasma membrane of epithelial cells in the airways, intestine, pancreas, sweat ducts, testis and other fluid-transporting tissues9,10. It is a member of the ATP-binding cassette (ABC) transporter superfamily. CFTR activation involves phosphorylation of multiple regulatory R-domain sites and ATP binding and hydrolysis at its nucleotide binding domains11,12.
The CFTR gene was identified in 1989 as the genetic basis of cystic fibrosis, which is an autosomal recessive disease13. The clinical features of cystic fibrosis include chronic lung infection with progressive deterioration of lung function, pancreatic exocrine insufficiency, male infertility, meconium ileus and various less common gastrointestinal complications14. Lung disease is the principal cause of morbidity and mortality in cystic fibrosis, with current median life expectancy of 37 years in the united States (see the Cystic Fibrosis Foundation Patient Registry Annual Data Report 2006). More than 1,500 loss-of-function mutations in CFTR have been identified that cause cystic fibrosis, with approximately 90% of patients with cystic fibrosis having the ΔF508 mutation (deletion of phenylalanine at 508 position) in one or both CFTR gene alleles. Although no gain-of-function CFTR mutations have been identified, inappropriately sustained activation of normal CFTR in intestinal epithelial cells caused by enterotoxins produces secretory diarrhoea15,16. CFTR activity in epithelial cells lining renal cysts is responsible for progressive fluid accumulation and cyst enlargement seen in autosomal dominant polycystic kidney disease (PKD)17,18. As discussed below, therapies aimed at restoring function to mutant CFTRs would be of benefit in cystic fibrosis, whereas inhibiting CFTR function is desirable in enterotoxin-mediated secretory diarrhoeas and PKD.
CFTR inhibitors
Before the development of small-molecule screening techniques, available inhibitors of CFTR chloride conductance included glibenclamide, diphenylamine-2-carboxylate, 5-nitro-2-(3-phenylpropylamino) benzoate and niflumic acid, which are nonspecific in their action and of low potency19. High-throughput screening of diverse small-molecule collections (FIG. 2) has yielded two classes of CFTR inhibitors.
The first class includes the thiazolidinone CFTRinh-172 (FIG. 3Aa), which acts on the cytoplasmic side of the plasma membrane to block CFTR chloride conductance with an IC50 value of ~300 nM6. Patch-clamp analysis indicated a voltage-independent channel blocking mechanism with prolongation of mean channel closed time20. This involves an interaction with arginine 347, which is located at the intracellular end of the CFTR pore21. CFTRinh -172 has low toxicity, undergoes renal excretion with minimal metabolism and accumulates in the intestine by entero-hepatic recirculation22. It has been used extensively to block CFTR chloride-channel function in various cell culture, tissue and in vivo systems. Recent structure–activity studies have identified thiazolidinone CFTR inhibitors that are more water soluble than CFTRinh-172, including an analogue that substitutes the 4-carboxyphenyl in CFTRinh-172 with 4-tetrazolophenyl23.
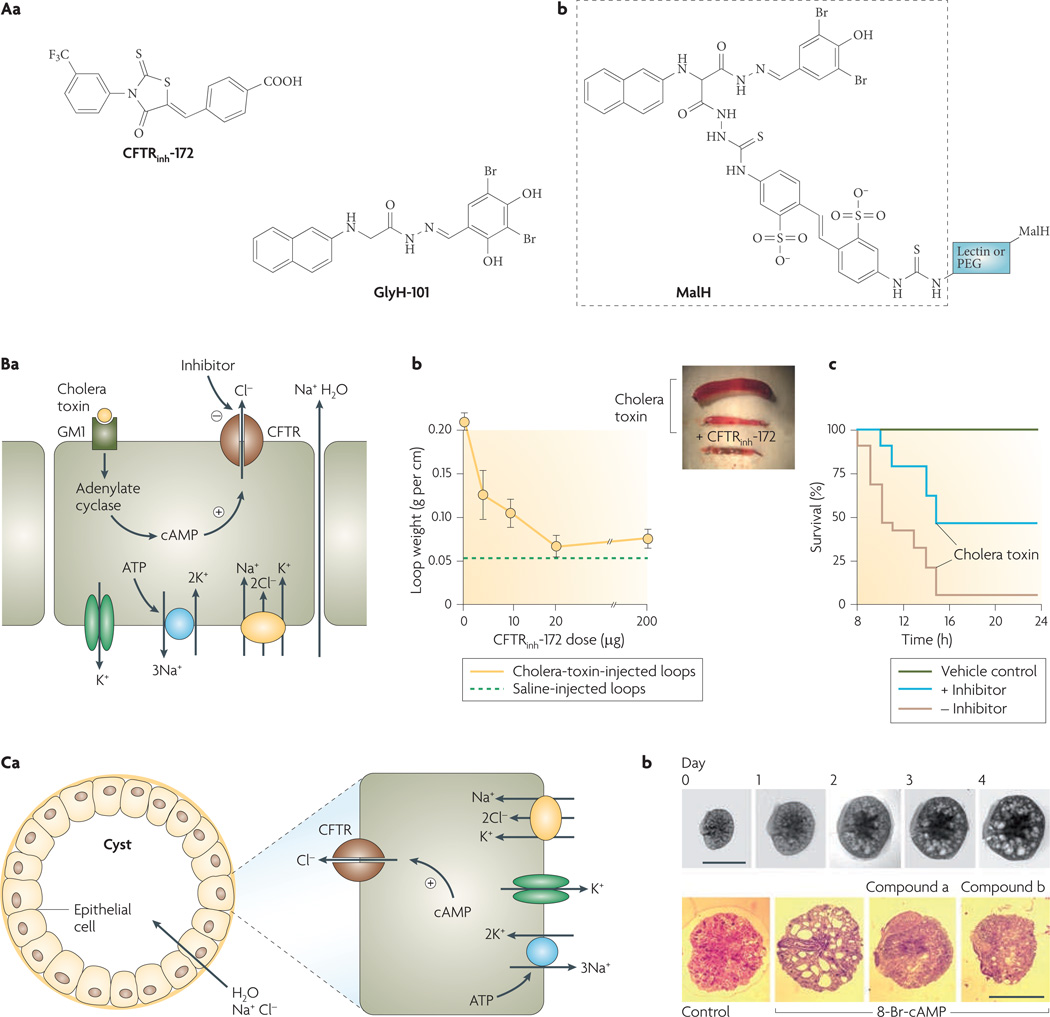
Figure 3. Cystic fibrosis transmembrane regulator (CFTR) inhibitors and their indications.
Aa | Structures of thiazolidinone CFTR inhibitor CFTRinh-172 and glycine hydrazide GlyH-101. Ab | Malonic acid hydrazide (MalH) conjugated to a macromolecular backbone (lectin or polyethylene glycol (PEG)). Ba | Intestinal fluid secretion in diarrhoea. Mechanism of enterotoxin-mediated diarrhoea showing CFTR chloride secretion following choleratoxin-induced cyclic AMP elevation. Sodium and water follow passively. Bb | CFTRinh-172 inhibits intestinal fluid accumulation in closed mouse ileal loops. Loops were injected with saline or cholera toxin and CFTRinh-172 was administered by intraperitoneal injection. Inset shows photograph of intestinal loops. Bc | Survival of suckling mice following oral administration of cholera toxin with or without CFTR inhibitor (MalH–lectin, 125 pmol). Vehicle control indicates no cholera toxin given. Ca | Cyst fluid secretion in polycystic kidney disease. Mechanism of fluid secretion into cysts, involving CFTR-dependent chloride secretion. Cb | CFTR inhibitors (compound a, a tetrazolo-derivatized thiazolidinone analogue; compound b, an absorbable, phenyl-derivatized glycine hydrazide analogue) cause slowing of cyst expansion in embryonic kidney organ culture. Upper panels show kidney growth and cyst formation in medium containing 8-Br-cAMP (scale bars, 1 mm). Bottom panels show day-4 kidneys in control and inhibitor-containing medium. Panel A is modified, with permission, from REF. 26 © (2007) W. B. Saunders. Panel B is modified, with permission, from REF. 28 © (2004) W. B. Saunders. Panel C is modified, with permission, from REF. 31 © (2008) American Society of Nephrology.
The second class of chemical CFTR inhibitors identified from screening was the glycine hydrazides (GlyH-101, FIG. 3Aa). Patch-clamp analysis showed that the CFTR current–voltage relationship changed from linear to inwardly rectifying in the presence of sub-maximal concentrations of GlyH-101 (REF. 24). Single-channel CFTR currents showed rapid channel ‘flicker’ (opening and closing) following GlyH-101 application, which, together with evidence that GlyH-101 inhibition potency is dependent on the extracellular chloride concentration, indicates that GlyH-101 acts by occluding the CFTR pore from its extracellular side. The external site of action of the glycine hydrazides provided a unique opportunity to develop non-absorbable compounds for antidiarrhoeal therapy. Based on structure–activity studies showing that substitutions on the glycyl methylene group of the glycine hydrazide scaffold could be tolerated, non-absorbable polyethylene glycol conjugates containing a malonic acid hydrazide (MalH) CFTR-inhibiting moiety were synthesized and were found to fully inhibit CFTR when added at the external surface of cells25. Various other MalH–macromolecular conjugates have been synthesized (generic structure shown in FIG. 3Ab) with the goal of improving CFTR inhibition potency and resisting possible dilutional washout during severe diarrhoea. Conjugates of MalH with various lectins inhibited CFTR at doses in the nanomolar range and resisted washout for many hours, probably because of entrapment in the dense enterocyte glycocalyx26. Divalent MalH–polyethylene glycol conjugates, which inhibit CFTR by an apparent cooperative mechanism, also showed potency in the nanomolar range27.
FIGURE 3Ba shows the mechanism of cholera-induced secretory diarrhoea. Cholera toxin from Vibrio cholerae increases cAMP levels, thereby activating CFTR. Consequent chloride secretion into the intestinal lumen drives sodium and water secretion. Various rodent models of intestinal fluid secretion, including the closed intestinal loop model, open intestinal loop model and suckling mouse survival model, have confirmed anti-diarrhoeal efficacy of the thiazolidinone and glycine hydrazide CFTR inhibitors. For example, FIG. 3Bb shows that a single intraperitoneal dose of CFTRinh-172 blocks cholera-toxin-induced fluid accumulation in closed ileal loops28. FIGURE 3Bc shows that oral administration of a MalH–lectin conjugate improves survival in suckling mice following oral administration of cholera toxin26.
Small-molecule CFTR inhibitors also reduce cyst expansion in models of PKD. Human autosomal dominant PKD, which results from mutations in the interacting PKD1 and PKD2 genes, is a major cause of chronic renal insufficiency29. Cyst growth in PKD is characterized by epithelial cell hyperplasia coupled with CFTR-dependent fluid secretion into the cyst lumen30 (FIG. 3Ca). In a proof-of-concept study, thiazolidinone and glycine hydrazide CFTR inhibitors slowed cyst expansion in both in vitro and in vivo models of PKD. Screening of CFTR inhibitor analogues in an MDCK (epithelial) cell cyst model indicated near complete suppression of cyst growth by CFTRinh-172 and by an absorbable, phenyl-derivatized glycine hydrazide31. These compounds also reduced cyst number and growth by more than 80% in an embryonic kidney cyst model involving 4-day organ culture of embryonic mouse kidneys in the presence of a cAMP agonist (FIG. 3Cb). Subcutaneous delivery of the CFTR inhibitors to neonatal, kidney-specific Pkd1 knockout mice, which normally develop large renal cysts in their first week of life, slowed their kidney enlargement and cyst expansion, and preserved renal function. Whether CFTR inhibition will be efficacious in human PKD, which has a much slower time course than that in PKD mouse models, will require clinical trials.
CFTR activators, potentiators and correctors
Inhibitors of wild-type CFTR are predicted to reduce excessive intestinal fluid secretion in infectious diarrhoeas in which CFTR is inappropriately activated by bacterial enterotoxins. By contrast, activators of wild-type CFTR are predicted to increase basal intestinal fluid secretion and are therefore of potential utility in the treatment of constipation. Another potential therapeutic indication of wild-type CFTR activators is keratoconjunctivitis sicca (dry eye), a common disorder in the elderly in which the tear film is defective because of reduced lacrimal gland fluid secretion and/or excessive tear-fluid evaporation. CFTR is strongly expressed in ocular surface epithelia where it functions, as in the intestine, in ion and fluid secretion32. CFTR can be activated by agents that elevate cAMP (such as receptor/G protein/adenylyl cyclase activators or phosphodiesterase inhibitors), stabilize phosphorylated CFTR (phosphatase inhibitors) or act directly on CFTR (for example, flavones). A screening study identified nanomolar-potency small-molecule CFTR activators with novel chemical structures that seemed to target CFTR directly33. Flavone analogues have also been synthesized with greatly increased CFTR-activating potency compared with genistein34, a natural compound found in tofu that has multiple biological activities including CFTR activation. With a more direct mode of action, one would expect fewer off-target effects of selective CFTR-interacting activators than of general modulators of cellular cAMP signalling.
Current cystic fibrosis therapies treat the symptoms rather than address the underlying CFTR defect, and include antibiotics, mucolytics (such as DNase), anti-inflammatory agents, nebulized hypertonic saline and lung transplantation. Compounds that restore apical membrane chloride permeability in epithelia lacking normal CFTR function hold considerable promise. The most common CFTR mutation that causes cystic fibrosis, ΔF508, produces relatively chloride-impermeable epithelial cells because of defective cellular processing of the channel protein, causing retention at the endoplasmic reticulum (ER), accelerated degradation and impaired chloride-channel gating35–38 (FIG. 4Aa). Some less common mutations, which individually occur in less than 5% of patients with cystic fibrosis, include mutations that cause defective channel gating, such as G551D39, and premature CFTR truncation, such as W1282X40. Of note, approximately one-third of all patients with cystic fibrosis harbour alleles other than ΔF508 (REF. 40), so therapies that are effective for these other mutations — such as gating or premature truncation mutations — might benefit more than 5% of the cystic fibrosis patient population. In developing chloride-channel enhancement therapy for cystic fibrosis, it is assumed that restoring chloride permeability would correct the underlying cellular defect that causes lung disease. However, the crucial link between CFTR dysfunction in cystic fibrosis and cystic fibrosis lung disease remains unresolved. As depicted in FIG. 4Ab, one theory suggests that the defect in the cystic fibrosis lung is reduced secretion of chloride, bicarbonate and fluid by airway submucosal glands, resulting in a viscous, acidic airway surface liquid (ASL) that promotes bacterial colonization by impairment of mucociliary clearance and bacterial killing. Another theory proposes that the major defect in cystic fibrosis is epithelial sodium channel (ENaC) hyperactivity involving a CFTR–ENaC interaction, in which excessive sodium absorption by the airway surface produces ASL dehydration. If correct, ENaC inhibitors — which are under development — rather than CFTR activators, would be of greatest benefit. Various other theories relating CFTR dysfunction to cystic fibrosis lung disease have been proposed, such as defective organellar function in airway epithelia, alveolar macrophage dysfunction, excessive ceramide accumulation and high ASL salt concentration41–43. As in other fields, the development and testing of therapies for cystic fibrosis are preceding full elucidation of disease pathogenesis.
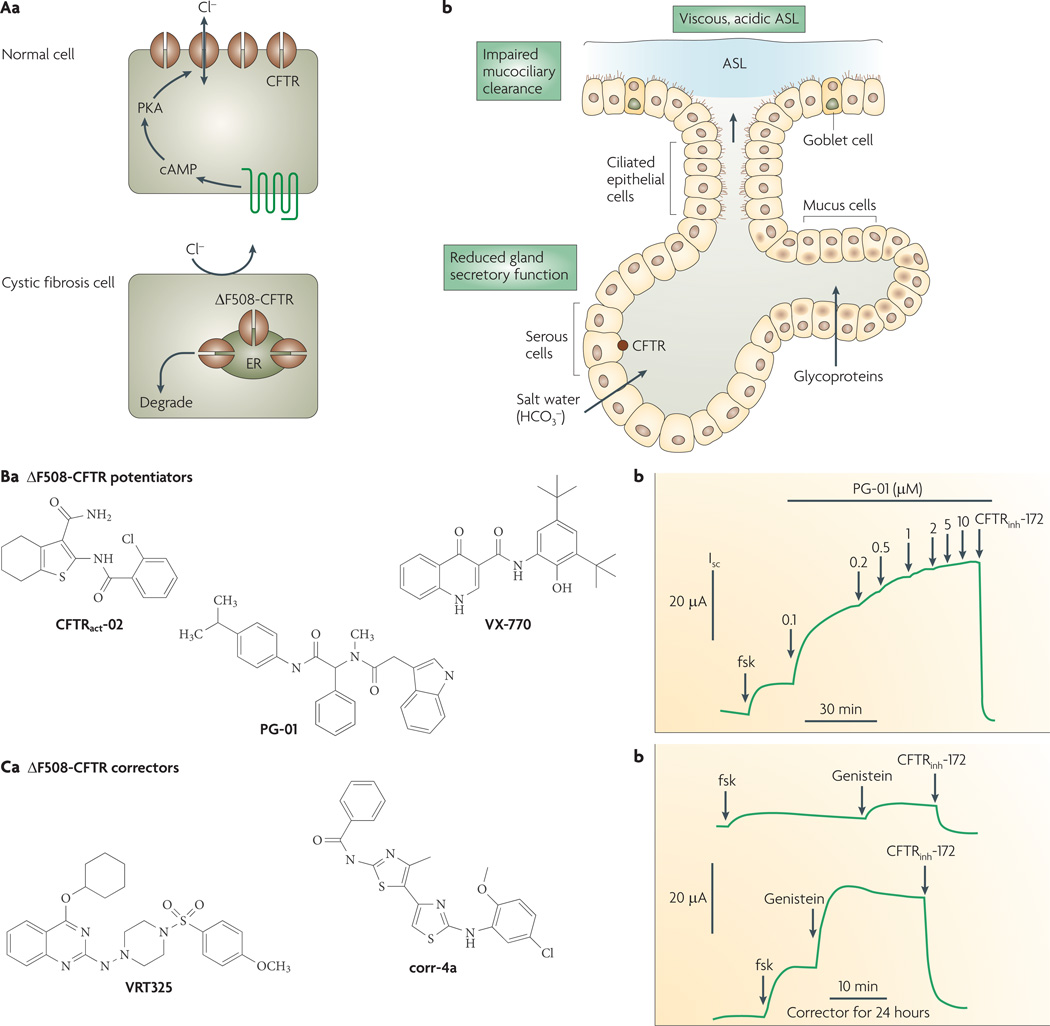
Figure 4. Lung pathophysiology in cystic fibrosis (CF) and activators of ΔF508-CFTR, the most common CF-causing mutation.
Aa | CF transmembrane regulator (CFTR) normally functions as a cyclic AMP-activated chloride channel at the apical plasma membrane of selected epithelial cells (top). ΔF508-CFTR is misfolded, retained at the endoplasmic reticulum (ER) and rapidly degraded (bottom). Ab | Lung pathophysiology in CF showing reduced chloride and bicarbonate secretion by submucosal glands, producing a viscous, acidic airway surface liquid (ASL) that promotes bacterial colonization. Ba | Structures of nanomolar-potency ΔF508-CFTR potentiators (correctors of defective channel gating). Bb | Short-circuit current analysis of ΔF508-CFTR-expressing epithelial cells (following low-temperature rescue to permit targeting of ΔF508-CFTR to the plasma membrane), showing small response to forskolin (fsk, 20 µM), followed by activation by PG-01 and inhibition by 10 µM CFTRinh-172. The small response to a high concentration of forskolin represents the ΔF508-CFTR gating defect, as forskolin alone fully activates wild-type CFTR. PG-01 strongly increases ΔF508-CFTR chloride conductance, with the increase inhibited by CFTRinh-172. Patch-clamp analysis indicated that PG-01 increases ΔF508-CFTR chloride current with open probability similar to that of activated wild-type CFTR. Ca | Structures of ΔF508-CFTR correctors (correctors of defective folding/cellular processing). Cb | Short-circuit analysis of cells as in (Bb), but cultured for 24 hours in the absence (top trace) or presence (bottom trace) of corr-4a. ΔF508-CFTR activated by 20 µM forskolin and 50 µM genistein. Isc, short-circuit current; PKA, protein kinase A. Panel Bb is modified, with permission, from REF. 45 © (2005) American Society for Pharmacology and Experimental Therapeutics. Panel Cb is modified, with permission, from REF. 57 © (2005) The American Society for Clinical Investigation.
Small-molecule therapies are under development for cystic fibrosis caused by the ΔF508 mutation. Efforts have focused on two distinct types of compounds: correctors, which rescue defective ΔF508-CFTR cellular processing and promote plasma membrane expression, and potentiators, which increase ΔF508-CFTR open channel probability. A single compound with both types of activity is preferable.
Several classes of compounds act as potentiators, including flavones, xanthines, benzimidazoles and dihydropyridines10,19. A general characteristic of potentiators is that they are ineffective on wild-type CFTR when the channel is already maximally activated by cAMP, whereas they increase open probability of mutant CFTR even under conditions of maximal phosphorylation. High-throughput screening efforts by two groups, each screening diverse collections of approximately 150,000 compounds, have identified additional small-molecule potentiators44–46. Three classes of potentiators of nanomolar potency are shown in FIG. 4B. In addition to correcting defective gating of ΔF508-CFTR, PG-01 and compound VX-770 (which has recently received orphan designation by the FDA) (FIG. 4Ba) correct the defective gating of other CFTR mutants, including G551D-CFTR. VX-770 is currently in Phase II clinical trials in patients with cystic fibrosis carrying the G551D mutation (see Vertex web site). It remains unclear whether a potentiator alone would benefit ΔF508 patients with cystic fibrosis because little, if any, ΔF508-CFTR protein is expressed at the plasma membrane.
Identification of correctors that rescue ΔF508-CFTR misprocessing presents a substantially greater challenge because of the complex, multi-step nature of protein processing and the ubiquitous expression of genes encoding protein processing machinery. Some natural products and drugs, including curcumin47, miglustat (Zavesca; Actelion)48 and sildenafil (Revatio/viagra; Pfizer)49,50, while approved for other indications, have also shown ΔF508-CFTR corrector activity. However, although curcumin has ΔF508-CFTR potentiator function51, several laboratories could not confirm curcumin corrector activity52–54, thereby contradicting the original report. The compound 4-phenylbutryate (Buphenyl; ucyclyd Pharma), a drug approved for an erythrocyte urea-cycle disorder, increases ΔF508-CFTR plasma membrane expression in cell culture models, possibly by nonspecific transcriptional enhancement and/or modulation of interactions with the chaperone protein HSC70 (REF. 55). In Phase III clinical trials, a large dose (20 g three times a day) of 4-phenylbutryate was tolerated, but produced only small increases in apparent ΔF508-CFTR conductance in nasal potential difference measurements56. Several ΔF508-CFTR correctors have been identified by high-throughput screening46,57,58; structures of two examples are shown in FIG. 4Ca. Epithelial cells expressing ΔF508-CFTR had increased short-circuit currents when incubated with bisaminomethylbithiazole corr-4a for 24 hours compared with the cells that had no compound exposure (FIG. 4Cb). The restoration of the chloride current is comparable to that conferred by low-temperature rescue. Mechanism-of-action studies suggest that corr-4a improves ΔF508-CFTR folding, probably by a direct interaction, resulting in ER-to-Golgi trafficking and increased ΔF508-CFTR stability57. Although the compounds shown in FIG. 4Ca correct the defective ΔF508-CFTR processing in primary cultures of human airway epithelial cells from ΔF508 homozygous subjects, the chloride current after stimulation by forskolin and a potentiator is less than 20% of that in airway cell cultures from subjects who do not have cystic fibrosis. The percentage correction necessary to confer clinical benefit in cystic fibrosis remains unknown59, as does how findings in cell culture models translate to efficacy in patients with cystic fibrosis. Clinical trials are needed to establish efficacy of the various correctors under development.
‘Read-through’ therapies aimed at bypassing premature termination codons caused by certain CFTR mutations are under investigation. Gentamicin, which like other aminoglycosides has read-through activity, was shown in human nasal potential difference measurements to partially restore CFTR function60, but its renal and oto toxicity preclude long-term use in patients with cystic fibrosis. A more recent candidate, PTC124, which is under development for Duchenne muscular dystrophy, produces ribosomal read-through of premature but not of normal stop codons61. Phase II clinical trials of PTC124 in cystic fibrosis caused by nonsense CFTR mutations show promise in nasal potential difference measurements62.
Targeting CaCCs
CaCCs are broadly expressed in mammalian cells where they are involved in a wide range of physiological functions, including transepithelial fluid secretion, oocyte fertilization, olfactory and sensory signal transduction, smooth-muscle contraction, and neuronal and cardiac excitation. Whole-cell current analysis indicates several common features between CaCC subfamilies, including slow activation following membrane depolarization, out-wardly rectifying steady-state currents and greater iodide than chloride permeability. Single-channel analysis has suggested four or more distinct CaCC subclasses, with a wide range of reported single-channel conductances from less than 2 pS in cardiac myocytes to 50 pS in airway epithelial cells.
FIGURE 5a summarizes the cellular mechanisms involved in the various physiological CaCC functions (reviewed in REFs 63–65). CaCCs are activated in some cell types by direct action of calcium, as in salivary gland epithelium, or through calcium action on calcium/calmodulin kinase II, as in intestinal epithelium. Various stimuli cause elevation in cytoplasmic calcium, such as cholinergic agonists in glandular secretory epithelium, odorants in olfactory epithelium and pain in dorsal root ganglia neurons. These stimuli elevate cytoplasmic calcium through the release of intracellular calcium stores via inositol 1,4,5-trisphosphate (IP3) signalling, and/or calcium influx through plasma membrane voltage-gated calcium channels. Increases in cytoplasmic calcium can produce both membrane depolarization, by CaCC activation, and membrane hyperpolarization, by activation of calcium-sensitive potassium channels. The consequences of CaCC activation are cell-type specific, for example, chloride secretion in epithelial cells, action-potential generation in olfactory receptor neurons, smooth-muscle contraction, and prevention of polyspermia in oocytes. In some cell types, such as smooth muscle cells, membrane depolarization activates voltage-gated calcium channels, increasing intracellular calcium concentration.
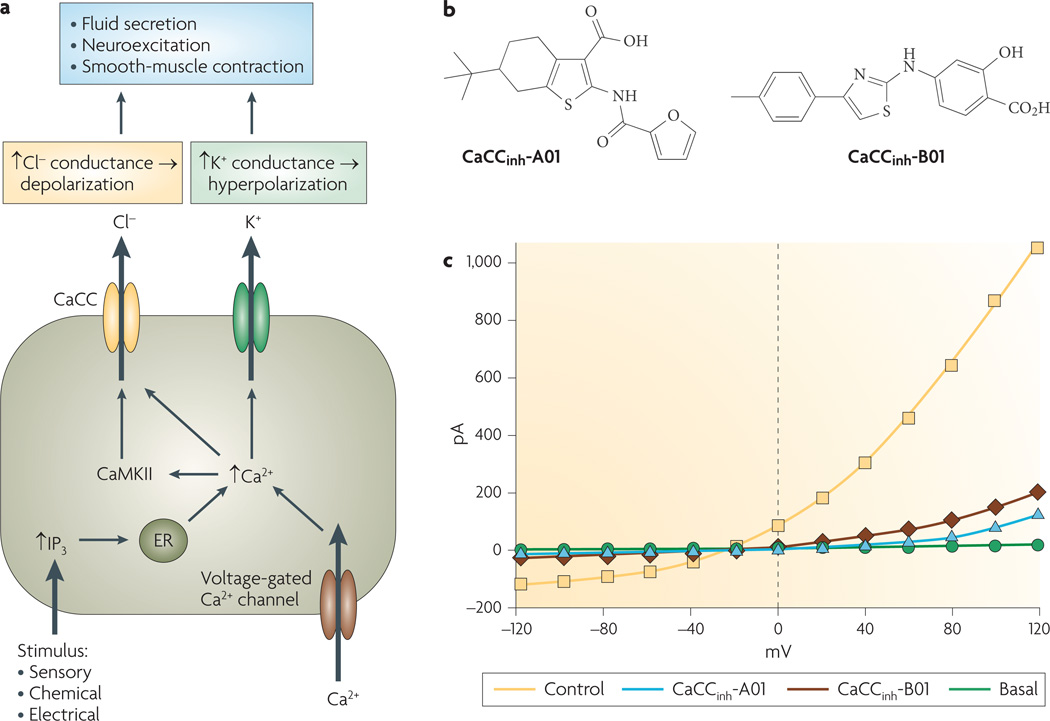
Figure 5. Cellular physiology of calcium-activated chloride channels (CaCCs) and small-molecule inhibitors.
a | Cellular roles of CaCCs. Cytoplasmic calcium elevation following various stimuli activates CaCCs directly or through calcium/calmodulin kinase II (CaMKII)-mediated phosphorylation. CaCC activation facilitates epithelial cell chloride secretion, and by depolarizing the plasma membrane it modulates neuroexcitation and smooth-muscle contraction. b | Inhibitors of human intestinal CaCC identified by high-throughput screening. c | Whole-cell currents in HT-29 cells following CaCC stimulation by ATP and carbachol, in the absence (control) and presence of indicated compounds. ‘Basal’ refers to absence of activators. ER, endoplasmic reticulum; IP3, inositol 1,4,5-trisphosphate. Panel b is modified, with permission, from REF. 8 © (2008) American Society for Pharmacology and Experimental Therapeutics.
Although CaCCs were functionally characterized nearly three decades ago, their molecular identity has remained unclear until recently, with potential candidates including bestrophins (BEST1–BEST4)66,67, the calcium-activated chloride channel ClCA family proteins68 and ClC-3 (REF. 69). It has been controversial whether these candidate gene products are chloride channels themselves, regulators of chloride channels or unrelated to chloride channels. The reader is referred to several recent reviews on this evolving subject63,70,71. Of the various candidates, the bestrophins seemed to be the strongest contenders for bona fide CaCCs. Heterologous expression, mutagenesis and RNAi knockdown studies have provided evidence for their involvement in calcium-activated chloride currents67,72,73. Mutations in one of the four bestrophin subclasses, BEST1, which is expressed in retinal pigment epithelial cells, causes Best vitelliform macular dystrophy. However, recent evidence suggests that defective bestrophin causes pathology not through impaired chloride conductance, but by its role as a regulator of calcium channels74
Three independent laboratories have identified TMEM16A, also called anoctamin-1, as a strong candidate for a CaCC75–77. Three different strategies were used: database searching for membrane proteins with multiple transmembrane segments and unknown function75, functional genomics following the observation that interleukin 4 (Il-4)-treated bronchial epithelial cells show increased CaCC activity76, and expression cloning using axolotl oocytes that do not have endogenous CaCC activity77. There is strong evidence to suggest TMEM16A is a key component of CaCC, including similarity to native CaCCs in its electrophysiological properties, appearance of CaCC currents in various transfected cell systems, reduction in CaCC currents following RNAi knockdown, and its tissue distribution. TMEM16A has eight putative transmembrane segments (FIG. 1c) without domains evidently involved in calcium regulation
A significant limitation in studying CaCCs has been the lack of potent or selective inhibitors. Available compounds — including fenamates, anthracene-9-carboxylic acid, indanyloxyacetic acid, ethacrynic acid and tamoxifen — have low potency, inhibit multiple types of chloride channels and transporters, and in some cases cause activation of large conductance calcium-dependent potassium channels (BKCa)9,19,63. Recent small-molecule screening to identify inhibitors of human intestinal CaCC(s), using the halide-influx assay described in FIG. 2C, identified several classes of CaCC inhibitors8. The most potent inhibitors identified were of the 3-acyl-2-aminothiophene and 5-aryl-2-aminothiazole classes (CaCCinh-A01 and CaCCinh-B01; FIG. 5b), which fully inhibited halide flux in intestinal cell lines and in response to different agonists, with IC50 values as low as 1 µM. Whole-cell patch-clamp in FIG. 5c shows inhibition of outwardly rectifying CaCCs in HT-29 cells after activation by the calcium ionophore ionomycin. Potent, small-molecule CaCC inhibitors have potential utility in the therapy of certain infectious and drug-induced secretory diarrhoeas78–80, in reducing mucus secretion in asthma and cystic fibrosis81, and in the treatment of hypertension by reducing vascular resistance.
By elevating cytoplasmic calcium, CaCC activators are thought to be of potential benefit in cystic fibrosis therapy as they might activate non-CFTR chloride channels in airway epithelial cells when CFTR is dysfunctional. This approach is based on the hypothesis that correction of reduced plasma membrane chloride permeability will be beneficial in cystic fibrosis, and in the case of CaCC activators, that activation of chloride channels by non-physiological mechanisms will be beneficial. Two CaCC activator therapies are in clinical trials. The purinergic P2y2 receptor antagonist denufosol (INS37217) is in Phase II clinical trials, with a 28-day treatment in patients with mild cystic fibrosis being well tolerated and showing preliminary evidence of potential benefit to lung function82. Moli1901 (duramycin) — a 19-amino-acid residue bacterial polycylic peptide that elevates calcium by interaction with membrane phospholipids — is also in Phase II clinical trials, with 5-day inhalation therapy showing improvement in lung function83. The long-term efficacy of these therapies for cystic fibrosis requires further study. CaCC activators that target CaCCs directly without cytoplasmic calcium elevation have not been developed, but they might offer more targeted therapy than general agonists of cellular calcium signalling. The recent identification of TMEM16A might facilitate the discovery of CaCC-targeted activators.
Targeting ClC-type chloride channels
ClCs were discovered by expression cloning using mRNA isolated from the electrical organ of Torpedo marmorata, which has a very large chloride conductance generating high voltages9,84. ClC family proteins are broadly expressed in prokaryotes and eukaryotes. In mammals, there are nine ClC genes that encode plasma membrane chloride channels (ClC-1 (CLCN1), ClC-2 (CLCN2), ClC-Ka (CLCNKA), and ClC-Kb (CLCNKB)) and intracellular proteins (ClC-3 (CLCN3), ClC-4 (CLCN4), ClC-5 (CLCN5), ClC-6 (CLCN6), and ClC-7 (CLCN7)). There is strong evidence that ClC-4, ClC-5 and ClC-7, like the bacterial ClCs85, function as electrogenic Cl−/H+ exchangers86–88. Recent data suggest that Cl− influx is coupled to H+ efflux with a stoichiometry of 2Cl−:1H+ (REFs 85,88). This transport mechanism would account for the shunt conductance needed to maintain electroneutrality during H+ pumping into the organelle lumen by vacuolar-type ATPases.
The crystal structures of two bacterial ClC proteins have been determined89. The structures of eukaryotic ClCs, although not yet crystallized, have been modelled by homology to prokaryotic ClCs. In contrast to early models that were based on hydropathy analysis, ClC proteins have a complex topology with 18 segments, eight of which form hairpin structures that do not completely span the membrane (FIG. 1b). The cytosolic carboxyl terminus of eukaryotic ClCs contains two copies of a cystathionine-β-synthase motif that are important for protein–protein interactions and channel activity. One of the transmembrane segments contains a highly conserved glutamate residue that is crucial for ClC channel and transporter functions90. For ClC channel function, movement of the glutamate carboxyl into and out of the pore seems to account for fast channel gating. For ClC transport function, the same carboxyl is involved in proton translocation across the membrane. It has been proposed that ClC channel function has evolved from its transporter function by loss of proton coupling90.
Early electrophysiological analysis of chloride conductance in the T. marmota electrical organ showed coordinated bursts of openings, suggesting the presence of a double-barrelled channel containing two pores for chloride transport91. Similar results were obtained for cloned ClC-0 (REF. 92) and ClC-1 (REF. 93). Evidence from expression of heterodimeric channels suggests that the ClC channels (at least ClC-0, ClC-1 and ClC-2) assemble as dimers, with each subunit forming a separate pore92–94. Analysis of ClC channel gating indicates a fast gate, occurring separately in each pore of the dimer, and a common gate that coordinates opening and closing of the two subunits at the same time. Whereas the fast gate involves the crucial glutamate residue mentioned above, the location of the common gate is less clear, but may involve cystathionine-β-synthase domains at the dimer interface95. Naturally occurring mutations in ClC genes produce human diseases including myotonia, Dent’s disease and osteopetrosis (TABLE 1), and there is a considerable body of phenotype data from mice lacking individual ClCs9,96.
ClC-1
ClC-1 is a voltage-dependent chloride channel with both fast and common gates opened by membrane depolarization. The fast gate in ClC-1 (and ClC-0) is strongly influenced by extracellular chloride concentration: a tenfold reduction in chloride shifts the current–voltage relationship by 40–60 mV to more positive (depolarizing) membrane potentials97,98. ClC-1 is expressed in skeletal muscle, where its expression in the sarcolemmal membrane is sensitive to innervation and muscle electrical activity99. ClC-1 is responsible for the large chloride conductance in skeletal muscle allowing for membrane repolarization after each action potential. Action potentials that originate at the neuromuscular junction reach the interior of muscle fibre by travelling along the membrane of T-tubules, where depolarization triggers muscle contraction by calcium release from internal stores (FIG. 6a). In other excitable cell types, termination of action potentials involves membrane repolarization by potassium channels. However, in the small spaces of the T-tubule system, a potassium repolarization current would increase extracellular potassium concentration and produce undesired membrane depolarization and muscle contraction. ClC-1 chloride conductance is an appropriate repolarizing mechanism in muscle because extracellular chloride concentration is more than tenfold higher than that of potassium. As expected, loss-of-function mutations in ClC-1 cause myotonia, which is characterized by exaggerated muscle contractions in response to normal stimuli. Depending on the mutation, myotonia congenita may be transmitted in a dominant (Thomsen-type) or a recessive (Becker-type) pattern100. Mutations causing dominant myotonia typically affect the common gating mechanism, causing a shift in voltage-dependence to more positive, non-physiological membrane potentials. Recessive mutations may have various effects such as channel loss of function and altered voltage dependence100. ClC-1 is also involved in dystrophic myotonia, where mutations occurring in a different gene, dystrophia myotonica protein kinase (DMPK), cause aberrant RNA splicing and ClC-1 loss of function101,102.
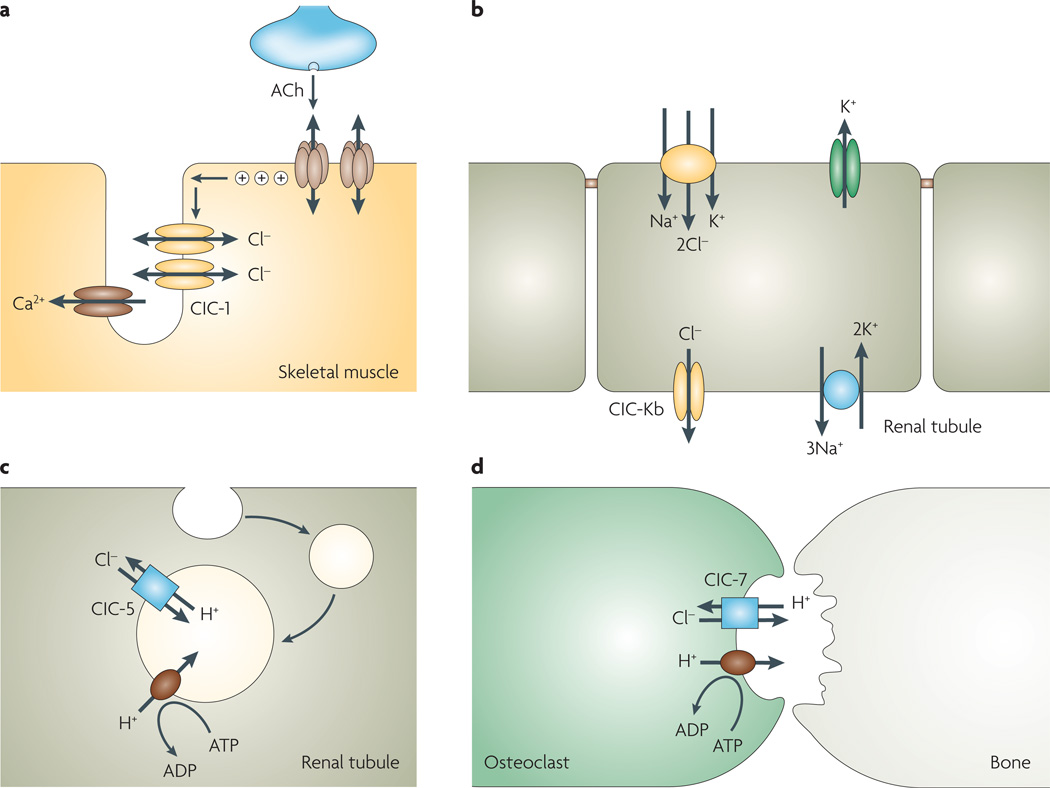
Figure 6. Physiology of selected voltage-gated (ClC)-type chloride channels.
a | ClC-1 in skeletal muscle stabilizes membrane potential. ClC-1 loss-of-function mutations causes myotonia. b | ClC-Kb in the basolateral membrane of kidney distal tubular cells facilitates transepithelial sodium chloride absorption, through coordinated activity with the apical bumetanide-sensitive Na+/K+/2Cl− cotransporter, apical potassium channel (to recycle potassium in the tubule lumen) and basolateral Na+/K+-ATPase. c | ClC-5 in kidney proximal tubule epithelial cells facilitates endocytosis and endosomal acidification. ClC-5 loss-of function mutations cause proteinuria and kidney stones (Dent’s disease). Organellar ClCs such as ClC-5 and ClC-7 function as electrogenic Cl−/H+ exchangers. d | ClC-7 chloride transport in bone osteoclasts facilitates net secretion of HCl into the lacuna for bone demineralization. ClC-7 loss-of-function mutations cause osteopetrosis. ACh, acetylcholine.
ClC-2
ClC-2 is a ubiquitously expressed, inwardly rectifying chloride channel that is activated by cell swelling103,104. ClC-2 was thought to be involved in cell-volume regulation, but it has different biophysical characteristics from the volume-sensitive chloride channels that have been characterized in many tissues. It was anticipated that Clcn2 knockout mice would have an epileptic phenotype because ClC-2 was predicted to maintain low cytoplasmic chloride in GABAergic neurons105. However, mice lacking ClC-2 showed no evidence of epilepsy, and instead manifested degeneration of photoreceptors in the retina and seminiferous tubules in the testicles106, which might be due to defective transepithelial ion transport in retinal pigment epithelium and Sertoli cells. Recent analysis of adult Clcn2 knockout mice revealed spongiform vacuolation of white matter in the central nervous system, which might be related to defective oligodendrocyte function107. In humans, early data suggested an association between ClC-2 mutations and idiopathic generalized epilepsy108. However, ClC-2 mutations in subjects with epilepsy do not alter ClC-2 function in vitro, and the same amino-acid changes are found in subjects without epilepsy107,109.
ClC-Ka and ClC-Kb
ClC-Ka and ClC-Kb are renal chloride channels that are expressed in the thin and thick ascending limb of Henle’s loop, respectively, where they provide a transcellular route for chloride reabsorption9,110 (FIG. 6b). ClC-Kb is also highly expressed in other nephron segments including the distal convoluted tubule. Both renal ClCs show little voltage dependence owing to the presence of a valine instead of the gating glutamate found in other ClCs. Loss-of-function mutations in ClC-Kb cause Bartter’s syndrome type 3, which is characterized by impaired NaCl reabsorption with hypokalaemia, hypochloraemia and alkalosis111. No disease-causing ClC-Ka mutations have been reported. Mice lacking ClC-K1 (the murine homologue of ClC-Ka) manifest nephrogenic diabetes insipidus with defective urinary-concentrating ability112. A more severe renal salt-wasting syndrome (Bartter’s syndrome type 4) is caused by mutations in an unrelated protein, barttin, which is needed for correct plasma membrane expression of ClC-Ka and ClC-Kb113. This syndrome is therefore the functional equivalent of CLCNKA/CLCNKB double knockout along the entire nephron, thereby explaining the greater severity of symptoms. In addition to renal disease, subjects with barttin mutations manifest hearing impairment114, which is related to the involvement of ClC-Ka, ClC-Kb and barttin in marginal cells of the stria vascularis in the inner ear that secrete potassium into the endolymph. Potassium secretion requires the coordinated action of the basolateral Na+/K+-ATPase and Na+/K+/2Cl− cotransporter to pump potassium inside the cell; potassium is then secreted across the apical membrane through KCNQ1 channels, whereas chloride is recycled back across the basolateral membrane through ClC-Ka and ClC-Kb. The presence of both ClC channels in the same cells explains why subjects with only ClC-Kb mutations do not manifest hearing impairment. Interestingly, gain-of-function polymorphisms in ClC-Ka and ClC-Kb have been associated with hypertension. The common variant of ClC-Kb, T481S, shows greater chloride channel function than does wild-type ClC-Kb, and is associated with increased blood pressure115,116. Single nucleotide polymorphisms in ClC-Ka are associated with salt-sensitive hypertension117. These findings support the use of renal ClC inhibitors as novel antihypertensive agents, as previously proposed111.
Intracellular ClCs
Mutations in intracellular ClCs also cause human disease, by a common mechanism involving defective acidification of intracellular organelles. Loss-of-function mutations in ClC-5 cause Dent’s disease (FIG. 6c), in which impaired receptor-mediated endocytosis and endosomal acidification in renal proximal tubule cells result in proteinuria and kidney stones118,119. Impairment of parathyroid hormone endocytosis by proximal tubule cells causes kidney stones by alteration in calcium homeostasis and hyperphosphaturia120. ClC-7 is expressed ubiquitously in late endosomes and lysosomes in many cell types. Loss-of-function mutations in ClC-7 cause osteopetrosis121 (TABLE 1). ClC-7 is transported by exocytosis to the ruffled border, an extension of the osteoclast plasma membrane that faces the resorption lacuna (FIG. 6d), where it facilitates the secretion of HCl into the lacuna required for bone demineralization. As found for ClC-5 and ClC-4, ClC-7 also functions as a 2Cl−/1H+ antiporter88.
CIC inhibitors and potential drug targets
There is a lack of selective, potent inhibitors of ClC proteins9. ClC-1 is inhibited at high micromolar concentrations by 9-anthracene-carboxylic acid (9-AC) and by 2(p-chlorophenoxy) propionic acid (CPP) analogues122. The differential inhibition of ClC-0, ClC-1 and ClC-2 by 9-AC, together with structural information from bacterial ClCs, suggested the putative 9-AC inhibitor binding site in ClC-1 (REF. 123). ClC-2 is inhibited by conventional chloride channel blockers such as 4,4′-diisothiocyanostilbene2,2′-disulphonic acid (DIDS), 9-AC and diphenylcarboxylate (DPC) at millimolar concentrations, but is more sensitive to 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) (90% inhibition at ~0.5 mM)124, a compound that blocks multiple types of chloride channels, including CFTR, at similar concentrations. ClC-2 is inhibited by cadmium and zinc (IC50 values of ~40 µM and 300 µM, respectively), a characteristic that has been exploited to inhibit native ClC-2 currents in different cell types125, although these metals are not selective in their action. The renal ClCs have been studied in more detail in terms of pharmacological modulators. Although ClC-Ka and ClC-Kb are highly homologous, ClC-Ka is more sensitive to DIDS and CPP126, which suggested the feasibility of developing selective blockers. Recently, phenylbenzofuran carboxylic acids were reported to block ClC-Ka and ClC-Kb with an IC50 value of less than 10 µM127. It was also shown that fenamates like niflumic acid act biphasically in the 0.1–1 mM range, with lower concentrations causing an approximately threefold increase in current, and higher concentrations causing inhibition128. Decrease of the dihedral angle between aromatic moieties switched activity from inhibition to activation, suggesting the possibility of developing ClC openers127.
Wild-type ClC proteins represent potentially important drug targets. Inhibitors of renal ClCs might be useful as diuretic agents to treat hypertension and congestive heart failure. Selective action on ClC-Ka or ClC-Kb would avoid hearing impairment. ClC-7 is a potentially important drug target. Pharmacological inhibition of ClC-7 could reduce the activity of osteoclasts in osteoporosis, thereby preventing or reversing disease progression129. The chloride-channel inhibitor NS53736 was reported to inhibit bone resorption in a rat ovariectomy model of osteoporosis, resulting in increased bone density130, and inhibited osteoclast-mediated pitting on bone slices in vitro. The authors proposed, without direct evidence, a mechanism involving ClC-7 inhibition, although it was reported in the same study that NS53736 inhibited volume-sensitive chloride channels.
Little is known about the pharmacology of intracellular ClCs, which, unlike plasma-membrane channels, cannot easily be studied at the functional level. For these proteins, novel screening methods are needed, perhaps based on measurement of Cl−/H+ transport in intracellular compartments. Alternatively, engineered protein sequences might allow their targeting to the plasma membrane and facilitate their functional assessment.
Most ClC mutations associated with genetic diseases cause loss of function and so are difficult drug targets. In principle, dominant mutations like those causing Thomsen-type myotonia might be more amenable to pharmacological intervention, as modulators that are able to suppress the dominant negative effect of such mutations might restore ion transport.
At present, there is only one approved drug for which the target might be a ClC protein. Lubiprostone (Amitiza; Sucampo Pharmaceuticals/Takeda) has been marketed for the treatment of chronic constipation. The rationale behind this indication is based on evidence that lubiprostone activates ClC-2, which presumably causes an increase in intestinal fluid secretion as discussed above with regard to CFTR secretory mechanisms. However, the evidence supporting lubiprostone action and ClC-2 expression at the luminal membrane of enterocytes is weak131. There is conflicting evidence that ClC-2 is expressed at a basolateral location132,133, where ClC-2 activation would be pro-absorptive rather than pro-secretory. In support of this conclusion is the finding that Clcn2 gene disruption in mice improves the survival of mice with cystic fibrosis134, the inverse of what would be expected were ClC-2 pro-secretory. The small benefit of lubiprostone in increasing the average frequency of bowel movements in clinical studies135 might be related to a substantial subpopulation of subjects with gastrointestinal side effects rather than to a pro-secretory mechanism of action. Recent results suggest that lubiprostone’s effects could be due in part to activation of CFTR136.
Targeting ligand-gated chloride channels
Inhibitory synaptic transmission involves ionotropic GABA and glycine receptors, which are of great importance in the brain and spinal cord, respectively. In GABAA, GABAC and glycine receptors, binding of the neurotransmitter triggers the opening of a chloride-permeable pore137–140. GABAB receptors are unrelated proteins, belonging to the superfamily of G-protein-coupled receptors. All GABA- and glycine-activated chloride channels are pentameric proteins formed by the assembly of different subunits (FIG. 1d). For glycine receptors, there are four types of α subunit (α1–4) and one type of β subunit, with the usual stoichiometry being 2α1:3β138. There are many more types of GABA subunits: α1–6, β1–3, γ1–3, δ, ε, π, θ and ρ1–3. Different combinations of these subunits generate GABAA and GABAC receptors. GABAA receptors are typically formed by the assembly of two α, two β and one γ subunit, usually 2α1:2β2:1γ2 (REF. 141). By contrast, GABAC receptors are homomers or heteromers of ρ1, ρ2 and ρ3 subunits only. From the various subunit types, a large number of GABA receptor isoforms can be generated. Although this large structural diversity presents a challenge to drug development, it represents a potentially unique opportunity for the development of selective modulators.
The subunits of ligand-activated chloride channels all share similar topology, with a large, extracellular amino-terminal domain (ECD) that contributes to the neurotransmitter binding site, four transmembrane domains and a short extracellular carboxyl terminus. The ECD contains a cysteine disulphide bridge separated by 13 amino acids, a structure named the cys-loop, which is also present in nicotinic acetylcholine receptors and 5-hydroxytryptamine 3 (5HT3) receptors. The GABA or glycine binding site is formed at the interface between the ECDs of an α and a β subunit. The second transmembrane domain of each subunit is thought to constitute part of the channel pore involved in chloride translocation and gating140,141. unlike the ClCs, in which each subunit forms a separate pore, a common pore in GABA- and glycine-activated channels is formed at the centre of the pentamer by the assembly of the subunits140,141.
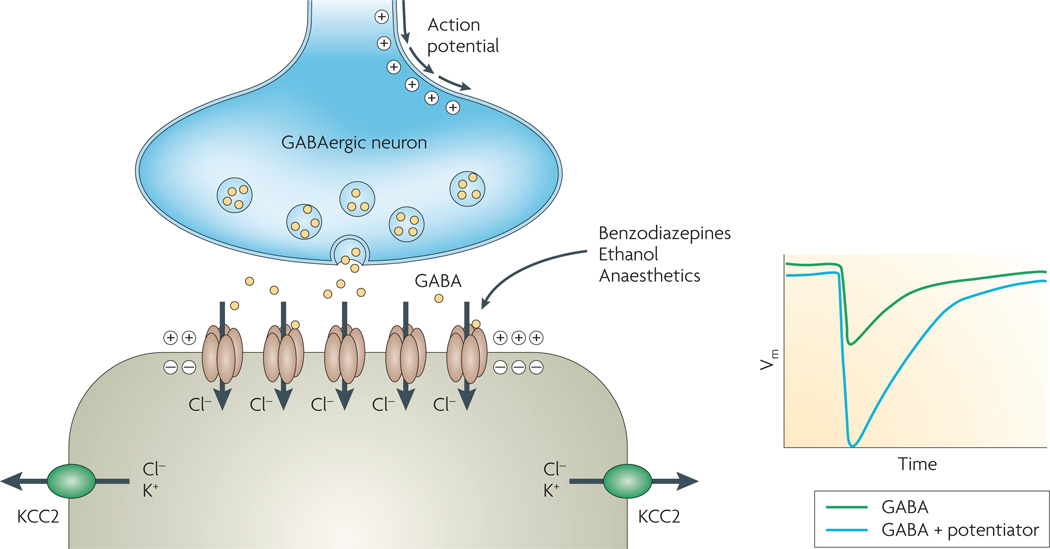
The inhibitory function of GABA and glycine receptors is a consequence of the electrochemical potential for chloride. ligand-gated chloride channels are excitatory in embryonic life and inhibitory in the developed nervous system9,142,143. In differentiated neurons, the activity of the K+/Cl− cotransporter (KCC2) lowers intracellular chloride concentration so that the equilibrium potential for chloride is more negative than the membrane potential (FIG. 7). Accordingly, opening of GABA- or glycine-activated chloride channels in the postsynaptic membrane causes chloride influx and membrane hyperpolarization, suppressing excitability. In embryonic neurons, chloride concentration is higher because of reduced KCC2 expression, such that opening of ligand-gated chloride channels causes membrane depolarization and opening of calcium channels9,142,143.
Figure 7. Ligand-gated chloride channels.
Schematic of GABA (γ-aminobutyric acid) inhibitory synapse. Release of GABA from presynaptic membrane triggers the transient activation of ionotropic GABA receptors. The low intracellular chloride concentration in the postsynaptic neuron, generated by the action of the K+/Cl− cotransporter (KCC2), drives chloride influx through GABA-activated chloride channels causing membrane hyperpolarization. Benzodiazepines, anaesthetics, ethanol and other compounds act on GABA receptors to potentiate neurotransmitter effect. Vm, transmembrane potential.
Mutations in ligand-gated chloride channels cause human genetic diseases. Startle disease (hyperekplexia) results from mutations in the α1 subunit of the glycine receptor, and is characterized by an exaggerated reaction to unexpected acoustic or tactile stimuli that triggers attacks of muscle rigidity144. Mutations in GABA receptor subunits are associated with epilepsy145–147. GABA receptor dysfunction has also been associated with depression and affective disorders148.
Given their site of expression and role in the nervous system, GABA and glycine receptors are important pharmacological targets for natural toxic substances as well as for drugs with anti-epileptic, anxiolytic, hypnotic and muscle-relaxant activities138–140. GABAA receptors are targets of widely used sedative and hypnotic drugs including barbiturates and benzodiazepines, which interact with GABAA receptors at the interface of α and γ subunits and potentiate the GABA signal by an allosteric mechanism. The same site is targeted by inverse agonists such as β-carbolines, which have an effect opposite to that of anxiolytic benzodiazepines. Both agonists and inverse agonists are found in the same chemical class. Inverse agonists selective for the α5 subunit of the GABAA receptor, which is highly expressed in the hippocampus, were reported to enhance cognition in animals without anxiogenic and convulsant effects149. Such triazolophthalazines might therefore be useful in Alzheimer’s disease and other dementias. Another example of a subunit-specific drug is gaboxadol, a selective agonist of GABAA receptors containing δ subunits, which are localized extrasynaptically in thalamic neurons. Gaboxadol improved sleep duration and quality in Phase III clinical trials150, although its further development was discontinued.
GABAC receptors, composed only of ρ subunits, have a different pharmacological profile from GABAA receptors, with insensitivity to barbiturates and benzodiazepines, and selective sensitivity to the competitive antagonists (1,2,5,6-tetrahydropyridine-4-yl-) methyl-phosphinic acid (TPMPA) and 4,5,6,7-tetrahydroisoxazole[4,5-c] pyridine-3-ol (THIP)151. The unique structural composition of GABAC receptors might be exploited to develop selective pharmacological modulators for visual, sleep and cognitive disorders151,152.
Despite their importance, glycine-activated chloride channels are not targets of existing drugs140. Glycine receptors, which are also activated physiologically by taurine and β-alanine, are antagonized by the convulsant toxin strychnine. The importance of glycine receptors as drug targets has been demonstrated in animal models by showing their involvement in inflammatory pain sensitization. The α3 subunit of glycine receptors is inhibited by prostaglandin E2-dependent phosphorylation153. Consequently, mice lacking functional α3 subunit show reduced pain perception. Glycine receptors are highly sensitive to 5HT3 receptor antagonists such as tropisetron140, which may suggest avenues for the development of more selective modulators.
GABA and glycine receptors are also involved in the action of substances of abuse including ethanol154 and inhaled solvents155. The n-alcohols bind to an aqueous cavity in the transmembrane segments, causing potentiation of the inhibitory signal156. It has been proposed that this cavity undergoes volume changes during receptor activation, with occupancy by n-alcohols promoting channel opening. Inhaled solvents such as toluene and trichloroethylene also enhance chloride currents through GABAA and glycine receptors with a similar site of action155.
Targeting volume-sensitive chloride channels
Volume-sensitive chloride channels have been studied for about two decades using electrophysiological and radio-isotope methods157. They are ubiquitous and typically activated by cell swelling in response to hypotonic shock. Volume-sensitive chloride channels generally show outward rectification in their instantaneous current–voltage relationship, with larger currents produced immediately after changes in membrane potential to larger positive values. The currents show time-dependent inactivation at positive membrane potentials and, of note, channel inactivation is accelerated at more positive membrane potentials9,158–160. There is evidence that volume-sensitive chloride channels are permeable to organic osmolytes such as taurine161,162, but conflicting data suggest separate channels for chloride and taurine163.
Despite considerable effort, the molecular identity of volume-sensitive chloride channels has yet to be established. Various claims have been made and subsequently disproved. First, Valverde et al.164 reported that P-glycoprotein (also known as MDR1, ABCB1) over-expression or silencing modulated volume-sensitive chloride-channel activity. However, subsequent studies found no relationship between P-glycoprotein expression and chloride-channel activity9. A second candidate was the protein pICln identified by expression cloning165–167, but several groups could not confirm the membrane localization of pICln or its link with volume-sensitive chloride channels168–170. It seems more likely that pICln is an intracellular protein with a role in spliceosome function171. Finally, ClC-3 was proposed as a volume-sensitive chloride channel, on the basis of outwardly rectifying volume-sensitive chloride currents following overexpression and analysis of site-directed mutants172. However, other investigators have challenged this claim. Mice deficient in ClC-3 showed degeneration of cells in hippocampus and retina, without alteration of volume-sensitive chloride currents173. Also, overexpression of ClC-3 did not alter chloride currents of any type174. ClC-3 is probably an intracellular chloride channel involved in endosomal acidification173,175.
One of the difficulties in studying volume-sensitive chloride channels is the general lack of selective channel blockers or activators. Volume-sensitive chloride channels are inhibited at micromolar concentrations by the nonspecific compounds niflumic acid, NPPB, 1,9-dideoxyforskolin and tamoxifen9,176. A more selective inhibitor is 4-(2-butyl-6,7-dichlor-2-cyclopentyl-indan-1-on-5-yl) oxybutyric acid (DCPIB)177, which blocks volume-sensitive chloride channels at concentrations that do not inhibit CFTR, ClC and CaCC. Selective, potent inhibitors would be of obvious utility in the identification of volume-sensitive chloride channels and the study of their presumed physiological role in cell-volume regulation. Regulatory volume decrease in cells following exposure to a hypotonic solution involves the activation of potassium and chloride channels, resulting in net cellular potassium chloride release and secondary osmotic water efflux. Release of taurine and other osmolytes through the same channels might also contribute to this process. It has been reported that volume-sensitive chloride channels are involved in the cell-volume decrease underlying apoptosis178. Using chloride channel blockers such as NPPB and phloretin, it was found that normotonic cell shrinkage occurs early during apoptosis and that its pharmacological inhibition could prevent subsequent cytochrome c release and caspase activation179. Selective inhibitors of volume-sensitive chloride channels might therefore be useful in treating degenerative disorders involving apoptotic cell death. However, no specific ligands for these channels have been reported to date for in vivo applications.
Prospects for chloride-channel modulators
There is now an extensive body of information on chloride-channel function, biological roles and genetics. However, there remain notable gaps such as the lack of molecular identification of volume-sensitive chloride channels. The recent identification of TMEM16A as a strong candidate for a CaCC72–74 may reveal new physiological functions of CaCCs and CaCC-related diseases. Other members of the TMEM16A family, which number ten in mammals180, might also function as chloride channels. Because of the importance of CaCC activity in smooth muscle cells, TMEM16A inhibitors may be useful in treating hypertension and asthma. The overexpression of TMEM16A in various human tumours — which might be related to its involvement in proliferation, invasion and/or cellular homeostasis — suggests that TMEM16 inhibitors could be used in tumour therapy. Also of considerable promise are ClC-subtype-selective inhibitors as diuretics and for treatment of osteoporosis. Finally, GABA-activated ionotropic channels in the central nervous system, which are composed of multiple subunit combinations, represent potential targets for more selective agents in the treatment of anxiety, depression, epilepsy, insomnia and cognitive disorders.
Insights emerging from basic research indicate broad opportunities and challenges for chloride-channel-based therapies. Compounds under development offer promise for the treatment of cystic fibrosis, PKD and secretory diarrhoeas, for which the clinical trial outcomes are eagerly anticipated. The availability of efficient assays for primary screening of chloride-channel modulators offers drug discovery opportunities for many chloride channels, with the caveat that screening requires stable, plasma-membrane expression of functional channels. For example, screening for renal ClC modulators requires channel co-expression with its partner barttin, and screening of ligand-activated chloride channels requires cell lines stably expressing channels with precise subunit stoichiometry. ClC-7 screening is particularly challenging because its intracellular location precludes the use of the conventional screening methods developed for plasma membrane chloride channels. Notwithstanding these technical challenges, there remain compelling and relatively unexplored opportunities in chloride-channel-based drug discovery.
Glossary
- Cystic fibrosis
One of the most prevalent life-shortening genetic diseases among Caucasians, characterized by recurrent lung infections, sterility and intestinal malabsorption.
- Meconium ileus
Often one of the early symptoms of cystic fibrosis consisting of intestinal obstruction by thickened meconium (fetal stool).
- Inwardly rectifying
The intrinsic ability of an ion channel to allow higher ion flux at negative (inwardly rectifying) or positive (outwardly rectifying) membrane potentials, even in the presence of a symmetrical concentration of the permeant ion.
- Glycocalyx
The network of polysaccharides present on the surface of many cell types.
- Intestinal loop model
A surgical technique to study fluid absorption/secretion in an isolated intestinal loop of a laboratory animal.
- Airway surface liquid
The layer of fluid (thickness 7–10 µm) covering the airways that allows efficient beating of cilia for mucociliary clearance.
- Polyspermia
Penetration of more than one spermatozoon into an oocyte at the time of fertilization.
- Best vitelliform macular dystrophy
Early onset degeneration of the macula in retina causing loss of vision.
- Electrical organ
A specialized muscle-derived structure of fish belonging to the genus Torpedo that is able to produce electrical discharges of up to 220 volts.
- Shunt conductance
A pathway that allows flow of ions (often chloride) in parallel with another pathway for ions of opposite charge.
- Osteopetrosis
A genetic disease caused by osteoclast loss of function with unbalanced bone growth leading to dense but brittle bones.
- Endolymph
The potassium-rich fluid contained in the membranous labyrinth of the inner ear.
Footnotes
DATABASES
OMIM: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
Autosomal dominant polycystic kidney disease | Bartter’s syndrome type 3 | Bartter’s syndrome type 4 | Becker-type myotonia | Cystic fibrosis | Dent’s disease | Startle disease | Thomsen-type myotonia
UniProtKB: http://ca.expasy.org/sprot
CFTR | ClC-1 | ClC-2 | ClC-3 | ClC-4 | ClC-5 | ClC-6 | ClC-7 | ClC-Ka | ClC-Kb
FURTHER INFORMATION
Cystic Fibrosis Foundation Patient Registry Annual Data
Report 2006: http://www.cff.org/LivingWithCF/QualityImprovement/PatientRegistryReport/
FDA list of orphan designations and approvals: http://www.fda.gov/orphan/designat/list.htm
Vertex web site: http://www.vrtx.com/current-projects/drug-candidates/vx-770.html
ALL Links Are AcTive in THe onLine PDF
References
- 1.Norton RS, Olivera BM. Conotoxins down under. Toxicon. 2006;48:780–798. doi: 10.1016/j.toxicon.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Catterall WA, et al. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Wachter RM, Elsliger MA, Kallio K, Hanson GT, Remington SJ. Structural basis of spectral shifts in the yellow-emission variants of green fluorescent protein. Structure. 1998;6:1267–1277. doi: 10.1016/s0969-2126(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 4.Jayaraman S, Haggie P, Wachter RM, Remington SJ, Verkman AS. Mechanism and cellular applications of a green fluorescent protein-based halide sensor. J. Biol. Chem. 2000;275:6047–6050. doi: 10.1074/jbc.275.9.6047. [DOI] [PubMed] [Google Scholar]
- 5.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]
- 6. Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. Identification of the first chloride-channel inhibitor with nanomolar potency and high selectivity. Proof of concept that CFTR inhibitors are useful for diarrhoea treatment.
- 7.Galietta LV, Jayaraman S, Verkman AS. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am. J. Physiol. 2001;281:C1734–C1742. doi: 10.1152/ajpcell.2001.281.5.C1734. [DOI] [PubMed] [Google Scholar]
- 8.De La Fuente R, Namkung W, Mills A, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol. Pharmacol. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- 9.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 10.Amaral MD, Kunzelmann K. Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends Pharmacol. Sci. 2007;28:334–341. doi: 10.1016/j.tips.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Sheppard D, Welsh M. Structure and function of the CFTR chloride channel. Physiol. Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 12.Gadsby D, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rommens JM, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 14.Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut. 2007;56:1153–1163. doi: 10.1136/gut.2004.062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiagarajah JR, Verkman AS. CFTR pharmacology and its role in intestinal fluid secretion. Curr. Opin. Pharmacol. 2003;3:594–599. doi: 10.1016/j.coph.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Farthing MJ. Antisecretory drugs for diarrheal disease. Dig. Dis. 2006;24:47–58. doi: 10.1159/000090308. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Findlay IA, Sheppard DN. The relationship between cell proliferation, Cl− secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int. 2004;66:1926–1938. doi: 10.1111/j.1523-1755.2004.00967.x. [DOI] [PubMed] [Google Scholar]
- 18.Davidow CJ, Maser RL, Rome LA, Calvet JP, Grantham JJ. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 1996;50:208–218. doi: 10.1038/ki.1996.304. [DOI] [PubMed] [Google Scholar]
- 19.Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol. Rev. 1999;79:S109–S144. doi: 10.1152/physrev.1999.79.1.S109. [DOI] [PubMed] [Google Scholar]
- 20.Taddei A, et al. Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker. FEBS Lett. 2004;558:52–56. doi: 10.1016/S0014-5793(04)00011-0. [DOI] [PubMed] [Google Scholar]
- 21.Caci E, et al. Evidence for direct CFTR inhibition by CFTRinh-172 based on arginine 347 mutagenesis. Biochem. J. 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 22.Sonawane ND, Muanprasat C, Nagatani R, Jr, Song Y, Verkman AS. In vivo pharmacology and antidiarrheal efficacy of a thiazolidinone CFTR inhibitor in rodents. J. Pharm. Sci. 2005;94:134–143. doi: 10.1002/jps.20228. [DOI] [PubMed] [Google Scholar]
- 23.Sonawane N, Verkman AS. Thiazolidinone CFTR inhibitors with improved water solubility identified by structure-activity analysis. Bioorg. Med. Chem. 2008;16:8187–8195. doi: 10.1016/j.bmc.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muanprasat C, et al. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J. Gen. Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonawane ND, Hu J, Muanprasat C, Verkman AS. Luminally-active, nonabsorbable CFTR inhibitors as potential therapy to reduce intestinal fluid losses in cholera. FASEB J. 2006;20:130–132. doi: 10.1096/fj.05-4818fje. [DOI] [PubMed] [Google Scholar]
- 26.Sonawane N, Zhao D, Zegarra-Mora O, Galietta LJV, Verkman AS. Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology. 2007;132:1234–1244. doi: 10.1053/j.gastro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Sonawane N, Zhao D, Zegarra-Mora O, Galietta LJV, Verkman AS. Nanomolar CFTR inhibition by pore-occluding divalent polyethylene glycol-malonic acid hydrazides. Chem. Biol. 2008;15:718–728. doi: 10.1016/j.chembiol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology. 2004;126:511–519. doi: 10.1053/j.gastro.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Yoder BK, Mulroy S, Eustace H, Boucher C, Sandford R. Molecular pathogenesis of autosomal dominant polycystic kidney disease. Expert. Rev. Mol. Med. 2006;8:1–22. doi: 10.1017/S1462399406010362. [DOI] [PubMed] [Google Scholar]
- 30.Grantham J, Chapman A, Torres V. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin. J. Am. Soc. Nephrol. 2006;1:148–157. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
- 31.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am Soc. Nephrol. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin MH, Verkman AS. CFTR-regulated chloride transport at the ocular surface in living mice measured by potential differences. Invest. Opthalmol. Vis. Sci. 2005;46:1428–1434. doi: 10.1167/iovs.04-1314. [DOI] [PubMed] [Google Scholar]
- 33.Ma T, et al. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J. Biol. Chem. 2002;277:37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- 34.Galietta LJ, et al. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J. Biol. Chem. 2001;276:19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 35.Denning GM, et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 36.Du K, Sharma M, Lukacs GL. The ΔF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nature Struct. Mol. Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- 37.Dalemans W, et al. Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 38.Haws CM, et al. Delta F508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am. J. Physiol. 1996;270:C1544–C1555. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- 39.Gregory RJ, et al. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol. Cell. Biol. 1991;11:3886–3893. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations — correlation with incidence data and application to screening. Hum. Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 41.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol. Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 42.Verkman AS, Song Y, Thiagarajah J. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. 2005;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 43.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J. Intern. Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 44.Yang H, et al. Nanomolar affinity small molecule correctors of defective ΔF508-CFTR chloride channel gating. J. Biol. Chem. 2003;278:35079–35085. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 45.Pedemonte N, et al. Phenylglycine and sulfonamide correctors of defective ΔF508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol. Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 46.Van Goor F, et al. Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am. J. Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 47.Egan ME, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 48.Norez C, et al. Rescue of functional ΔF508-CFTR channels in cystic fibrosis epithelial cells by the α-glucosidase inhibitor miglustat. FEBS Lett. 2006;580:2081–2086. doi: 10.1016/j.febslet.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Dormer RL, et al. Sildenafil (Viagra) corrects ΔF508-CFTR location in nasal epithelial cells from patients with cystic fibrosis. Thorax. 2005;60:55–59. doi: 10.1136/thx.2003.019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lubamba B, et al. Preclinical evidence that sildenafil and vardenafil activate chloride transport in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:506–515. doi: 10.1164/rccm.200703-344OC. [DOI] [PubMed] [Google Scholar]
- 51.Berger A, et al. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl− channel activity. J. Biol. Chem. 2005;280:5221–5226. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- 52.Song Y, et al. Evidence against the rescue of defective ΔF508-CFTR cellular processing by curcumin in cell culture and mouse models. J. Biol. Chem. 2004;279:40629–40633. doi: 10.1074/jbc.M407308200. [DOI] [PubMed] [Google Scholar]
- 53.Loo T, Bartlett M, Clarke D. Thapsigargin or curcumin does not promote maturation of processing mutants of the ABC transporters, CFTR, and P-glycoprotein. Biochem. Biophys. Res. Commun. 2004;325:580–585. doi: 10.1016/j.bbrc.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 54.Grubb B, et al. SERCA pump inhibitors do not correct biosynthetic arrest of ΔF508 CFTR in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2006;34:355–363. doi: 10.1165/rcmb.2005-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing deltaF508-CFTR. J. Clin. Invest. 1997;100:2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeitlin PL, et al. Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol. Ther. 2002;6:119–126. doi: 10.1006/mthe.2002.0639. [DOI] [PubMed] [Google Scholar]
- 57. Pedemonte N, et al. Small molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. Identification of the first small-molecule pharmacological chaperones to correct mutant CFTR misfolding.
- 58.Yu GJ, et al. Potent s-cis-locked bithiazole correctors of ΔF508-CFTR cellular processing for cystic fibrosis therapy. J. Med. Chem. 2008;51:6044–6054. doi: 10.1021/jm800533c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amaral M. Processing of CFTR: traversing the cellular maze — how much CFTR needs to go through to avoid cystic fibrosis? Pediatr. Pulmonol. 2005;39:479–491. doi: 10.1002/ppul.20168. [DOI] [PubMed] [Google Scholar]
- 60.Wilschanski M, et al. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N. Engl. J. Med. 2003;349:1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 61.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 62. Kerem E, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet. 2008;372:719–727. doi: 10.1016/S0140-6736(08)61168-X. Describes Phase II data supporting possible efficacy of a read-through therapy for cystic fibrosis.
- 63. Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu. Rev. Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. An excellent review of CaCCs.
- 64.Leblanc N, et al. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can. J. Physiol. Pharmacol. 2005;83:541–556. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 65.Frings S, Reuter D, Kleene SJ. Neuronal Ca2+-activated Cl− channels — homing in on an elusive channel species. Prog. Neurobiol. 2000;60:247–289. doi: 10.1016/s0301-0082(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 66.Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl Acad. Sci. USA. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qu Z, Wei RW, Mann W, Hartzell HC. Two bestrophins cloned from Xenopus laevis oocytes express Ca2+-activated Cl− currents. J. Biol. Chem. 2003;278:49563–49572. doi: 10.1074/jbc.M308414200. [DOI] [PubMed] [Google Scholar]
- 68.Cunningham SA, et al. Cloning of an epithelial chloride channel from bovine trachea. J. Biol. Chem. 1995;270:31016–31026. doi: 10.1074/jbc.270.52.31016. [DOI] [PubMed] [Google Scholar]
- 69.Huang P, et al. Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J. Biol. Chem. 2001;276:20093–20100. doi: 10.1074/jbc.M009376200. [DOI] [PubMed] [Google Scholar]
- 70.Eggermont J. Calcium-activated chloride channels: (un)known, (un)loved? Proc. Am. Thorac. Soc. 2004;1:22–27. doi: 10.1513/pats.2306010. [DOI] [PubMed] [Google Scholar]
- 71.Kunzelmann K, Milenkovic VM, Spitzner M, Soria RB, Schreiber R. Calcium-dependent chloride conductance in epithelia: is there a contribution by Bestrophin? Pflugers Arch. 2007;454:879–889. doi: 10.1007/s00424-007-0245-z. [DOI] [PubMed] [Google Scholar]
- 72.Tsunenari T, et al. Structure-function analysis of the bestrophin family of anion channels. J. Biol. Chem. 2003;278:41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qu Z, Fischmeister R, Hartzell C. Mouse bestrophin-2 is a bona fide Cl− channel: identification of a residue important in anion binding and conduction. J. Gen. Physiol. 2004;123:327–340. doi: 10.1085/jgp.200409031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu K, Xiao Q, Cui G, Lee A, Hartzell HC. The best disease-linked Cl− channel hBest1 regulates CaV1 (L-type) Ca2+ channels via src-homology-binding domains. J. Neurosci. 2008;28:5660–5670. doi: 10.1523/JNEUROSCI.0065-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 76.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 77.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu. Rev. Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 79.Rufo PA, et al. Diarrhea-associated HIV-1 APIs potentiate muscarinic activation of Cl− secretion by T84 cells via prolongation of cytosolic Ca2+ signaling. Am. J. Physiol. 2004;264:C998–C1008. doi: 10.1152/ajpcell.00357.2003. [DOI] [PubMed] [Google Scholar]
- 80.Schultheiss G, Siefjediers A, Diener M. Muscarinic receptor stimulation activates a Ca2+-dependent Cl− conductance in rat distal colon. J. Membr. Biol. 2005;204:117–127. doi: 10.1007/s00232-005-0757-4. [DOI] [PubMed] [Google Scholar]
- 81.Hegab AE, et al. Niflumic acid and AG-1478 reduce cigarette smoke-induced mucin synthesis: the role of hCLCA1. Chest. 2007;131:1149–1156. doi: 10.1378/chest.06-2031. [DOI] [PubMed] [Google Scholar]
- 82.Deterding RR, et al. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007;176:362–369. doi: 10.1164/rccm.200608-1238OC. [DOI] [PubMed] [Google Scholar]
- 83.Grasemann H, et al. Inhalation of Moli1901 in patients with cystic fibrosis. Chest. 2007;131:1461–1466. doi: 10.1378/chest.06-2085. [DOI] [PubMed] [Google Scholar]
- 84. Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990;348:510–514. doi: 10.1038/348510a0. Describes the original cloning of a ClC-type chloride channel.
- 85. Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. Demonstration that a ClC-type protein functions as an electrogenic Cl−/H+ antiporter. This mechanism of transport was later found in eukaryotic intracellular ClCs.
- 86.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 87.Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 88.Graves AR, Curran PK, Smith CL, Mindell JA. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008;453:788–792. doi: 10.1038/nature06907. [DOI] [PubMed] [Google Scholar]
- 89.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 90.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 91.Miller C, White MM. Dimeric structure of single chloride channels from Torpedo electroplax. Proc. Natl Acad. Sci. USA. 1984;81:2772–2775. doi: 10.1073/pnas.81.9.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ludewig U, Pusch M, Jentsch TJ. Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature. 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- 93.Saviane C, Conti F, Pusch M. The muscle chloride channel ClC-1 has a double-barreled appearance that is differentially affected in dominant and recessive myotonia. J. Gen. Physiol. 1999;113:457–468. doi: 10.1085/jgp.113.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weinreich F, Jentsch TJ. Pores formed by single subunits in mixed dimers of different CLC chloride channels. J. Biol. Chem. 2001;276:2347–2353. doi: 10.1074/jbc.M005733200. [DOI] [PubMed] [Google Scholar]
- 95.Estévez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ. Functional and structural conservation of CBS domains from CLC chloride channels. J. Physiol. 2004;557:363–378. doi: 10.1113/jphysiol.2003.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J. Physiol. 2007;578:633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rychkov GY, et al. Concentration and pH dependence of skeletal muscle chloride channel ClC-1. J. Physiol. 1996;497:423–435. doi: 10.1113/jphysiol.1996.sp021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ. Gating of the voltage-dependent chloride channel CIC-0 by the permeant anion. Nature. 1995;373:527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- 99.Klocke R, Steinmeyer K, Jentsch TJ, Jockusch H. Role of innervation, excitability, and myogenic factors in the expression of the muscular chloride channel ClC-1. A study on normal and myotonic muscle. J. Biol. Chem. 1994;269:27635–27639. [PubMed] [Google Scholar]
- 100.Pusch M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum. Mutat. 2002;19:423–434. doi: 10.1002/humu.10063. [DOI] [PubMed] [Google Scholar]
- 101.Charlet-B N, et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol. Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 102.Mankodi A, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell. 2002;10:35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- 103.Thiemann A, Gründer S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- 104.Gründer S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- 105.Staley K, Smith R, Schaack J, Wilcox C, Jentsch TJ. Alteration of GABAA receptor function following gene transfer of the CLC-2 chloride channel. Neuron. 1996;17:543–551. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 106.Bösl MR, et al. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J. 2001;20:1289–1299. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blanz J, et al. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J. Neurosci. 2007;27:6581–6589. doi: 10.1523/JNEUROSCI.0338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haug K, et al. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nature Genet. 2003;33:527–532. doi: 10.1038/ng1121. [DOI] [PubMed] [Google Scholar]
- 109.Niemeyer MI, et al. Functional evaluation of human ClC-2 chloride channel mutations associated with idiopathic generalized epilepsies. Physiol. Genomics. 2004;19:74–83. doi: 10.1152/physiolgenomics.00070.2004. [DOI] [PubMed] [Google Scholar]
- 110.Krämer BK, Bergler T, Stoelcker B, Waldegger S. Mechanisms of disease: the kidney-specific chloride channels ClCKA and ClCKB, the Barttin subunit, and their clinical relevance. Nature Clin. Pract. Nephrol. 2008;4:38–46. doi: 10.1038/ncpneph0689. [DOI] [PubMed] [Google Scholar]
- 111.Simon DB, et al. Mutations in the chloride channel gene CLCNKB, cause Bartter’s syndrome type III. Nature Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 112.Matsumura Y, et al. Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nature Genet. 1999;21:95–98. doi: 10.1038/5036. [DOI] [PubMed] [Google Scholar]
- 113.Estévez R, et al. Barttin is a Cl− channel beta-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- 114.Birkenhäger R, et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nature Genet. 2001;29:310–314. doi: 10.1038/ng752. [DOI] [PubMed] [Google Scholar]
- 115.Jeck N, et al. Activating mutation of the renal epithelial chloride channel ClC-Kb predisposing to hypertension. Hypertension. 2004;43:1175–1181. doi: 10.1161/01.HYP.0000129824.12959.f0. [DOI] [PubMed] [Google Scholar]
- 116.Jeck N, Waldegger P, Doroszewicz J, Seyberth H, Waldegger S. A common sequence variation of the CLCNKB gene strongly activates ClC-Kb chloride channel activity. Kidney Int. 2004;65:190–197. doi: 10.1111/j.1523-1755.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- 117.Barlassina C, et al. Common genetic variants and haplotypes in renal CLCNKA gene are associated to salt-sensitive hypertension. Hum. Mol. Genet. 2007;16:1630–1638. doi: 10.1093/hmg/ddm112. [DOI] [PubMed] [Google Scholar]
- 118.Lloyd SE, et al. A common molecular basis for three inherited kidney stone diseases. Nature. 1996;379:445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- 119.Günther W, Piwon N, Jentsch TJ. The ClC-5 chloride channel knock-out mouse — an animal model for Dent’s disease. Pflugers Arch. 2003;445:456–462. doi: 10.1007/s00424-002-0950-6. [DOI] [PubMed] [Google Scholar]
- 120.Piwon N, Günther W, Schwake M, Bösl MR, Jentsch TJ. ClC-5 Cl− channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- 121.Kornak U, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 122.Pusch M, et al. Pharmacological characterization of chloride channels belonging to the ClC family by the use of chiral clofibric acid derivatives. Mol. Pharmacol. 2000;58:498–507. doi: 10.1124/mol.58.3.498. [DOI] [PubMed] [Google Scholar]
- 123.Estévez R, Schroeder BC, Accardi A, Jentsch TJ, Pusch M. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron. 2003;38:47–59. doi: 10.1016/s0896-6273(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 124.Furukawa T, Ogura T, Katayama Y, Hiraoka M. Characteristics of rabbit ClC-2 current expressed in Xenopus oocytes and its contribution to volume regulation. Am. J. Physiol. 1998;274:C500–C512. doi: 10.1152/ajpcell.1998.274.2.C500. [DOI] [PubMed] [Google Scholar]
- 125.Clark S, Jordt SE, Jentsch TJ, Mathie A. Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons. J. Physiol. 1998;506:665–678. doi: 10.1111/j.1469-7793.1998.665bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Picollo A, et al. Molecular determinants of differential pore blocking of kidney CLC-K chloride channels. EMBO Rep. 2004;5:584–589. doi: 10.1038/sj.embor.7400169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liantonio A, et al. Molecular switch for CLC-K Cl− channel block/activation: optimal pharmacophoric requirements towards high-affinity ligands. Proc. Natl Acad. Sci. USA. 2008;105:1369–1373. doi: 10.1073/pnas.0708977105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liantonio A, et al. Activation and inhibition of kidney CLC-K chloride channels by fenamates. Mol. Pharmacol. 2006;69:165–173. doi: 10.1124/mol.105.017384. [DOI] [PubMed] [Google Scholar]
- 129.Schaller S, Henriksen K, Sørensen MG, Karsdal MA. The role of chloride channels in osteoclasts: ClC-7 as a target for osteoporosis treatment. Drug News Perspect. 2005;18:489–495. doi: 10.1358/dnp.2005.18.8.944546. [DOI] [PubMed] [Google Scholar]
- 130.Schaller S, et al. The chloride channel inhibitor NS3736 prevents bone resorption in ovariectomized rats without changing bone formation. J. Bone Miner. Res. 2004;19:1144–1153. doi: 10.1359/JBMR.040302. [DOI] [PubMed] [Google Scholar]
- 131.Cuppoletti J, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am. J. Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 132.Peña-Münzenmayer G, et al. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J. Cell Sci. 2005;118:4243–4252. doi: 10.1242/jcs.02525. [DOI] [PubMed] [Google Scholar]
- 133.Gyömörey K, Yeger H, Ackerley C, Garami E, Bear CE. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am. J. Physiol. 2000;279:C1787–C1794. doi: 10.1152/ajpcell.2000.279.6.C1787. [DOI] [PubMed] [Google Scholar]
- 134.Zdebik AA, Cuffe JE, Bertog M, Korbmacher C, Jentsch TJ. Additional disruption of the ClC-2 Cl− channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J. Biol. Chem. 2004;279:22276–22283. doi: 10.1074/jbc.M309899200. [DOI] [PubMed] [Google Scholar]
- 135.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment. Pharmacol. Ther. 2007;25:1351–1361. doi: 10.1111/j.1365-2036.2007.03320.x. [DOI] [PubMed] [Google Scholar]
- 136.Bao HF, et al. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am. J. Physiol. 2008;295:G234–G251. doi: 10.1152/ajpgi.00366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 138.Cascio M. Modulating inhibitory ligand-gated ion channels. AAPS J. 2006;8:E353–E361. doi: 10.1007/BF02854906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bowery NG, Smart TG. GABA and glycine as neurotransmitters: a brief history. Br. J. Pharmacol. 2006;147:S109–S119. doi: 10.1038/sj.bjp.0706443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Webb TI, Lynch JW. Molecular pharmacology of the glycine receptor chloride channel. Curr. Pharm. Des. 2007;13:2350–2367. doi: 10.2174/138161207781368693. [DOI] [PubMed] [Google Scholar]
- 141.Kash TL, Trudell JR, Harrison NL. Structural elements involved in activation of the γ-aminobutyric acid type A (GABAA) receptor. Biochem. Soc. Trans. 2004;32:540–546. doi: 10.1042/BST0320540. [DOI] [PubMed] [Google Scholar]
- 142.DeFazio RA, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hübner CA, et al. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 144.Shiang R, et al. Point mutations in the gene encoding the α-1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nature Genet. 1993;5:351–357. doi: 10.1038/ng1293-351. [DOI] [PubMed] [Google Scholar]
- 145.Baulac S, et al. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ-2-subunit gene. Nature Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 146.Cossette P, et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nature Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 147.Maljevic S, et al. A mutation in the GABAA receptor α1-subunit is associated with absence epilepsy. Ann. Neurol. 2006;59:983–987. doi: 10.1002/ana.20874. [DOI] [PubMed] [Google Scholar]
- 148.Wong CG, Bottiglieri T, Snead OC. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003;54:S3–S12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- 149.Sternfeld F, et al. Selective, orally active γ-aminobutyric acidA α-5 receptor inverse agonists as cognition enhancers. J. Med. Chem. 2004;47:2176–2179. doi: 10.1021/jm031076j. [DOI] [PubMed] [Google Scholar]
- 150.Wafford KA, Ebert B. Gaboxadol — a new awakening in sleep. Curr. Opin. Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 151.Chebib M. GABAC receptor ion channels. Clin. Exp. Pharmacol. Physiol. 2004;31:800–804. doi: 10.1111/j.1440-1681.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- 152.Johnston GA, Chebib M, Hanrahan JR, Mewett KN. GABAC receptors as drug targets. Curr. Drug Targets CNS Neurol. Disord. 2003;2:260–268. doi: 10.2174/1568007033482805. [DOI] [PubMed] [Google Scholar]
- 153.Harvey RJ, et al. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 154.Ye JH, et al. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J. Pharmacol. Exp. Ther. 2001;296:77–83. [PubMed] [Google Scholar]
- 155.Beckstead MJ, Weiner JL, Eger EI, Gong DH, Mihic SJ. Glycine and γ-aminobutyric acidA receptor function is enhanced by inhaled drugs of abuse. Mol. Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- 156.Tao L, Ye JH. Inhibition of glycine receptor function of native neurons by aliphatic n-alcohols. Br. J. Pharmacol. 2002;136:629–635. doi: 10.1038/sj.bjp.0704775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stutzin A, Hoffmann EK. Swelling-activated ion channels: functional regulation in cell-swelling, proliferation and apoptosis. Acta Physiol. (Oxf) 2006;187:27–42. doi: 10.1111/j.1748-1716.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 158.Kubo M, Okada Y. Volume-regulatory Cl− channel currents in cultured human epithelial cells. J. Physiol. 1992;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jackson PS, Strange K. Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. J. Gen. Physiol. 1995;105:661–676. doi: 10.1085/jgp.105.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- 161.Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am. J. Physiol. 1993;265:C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- 162.Sánchez-Olea R, Morales M, García O, Pasantes-Morales H. Cl− channel blockers inhibit the volume-activated efflux of Cl− and taurine in cultured neurons. Am. J. Physiol. 1996;270:C1703–C1708. doi: 10.1152/ajpcell.1996.270.6.C1703. [DOI] [PubMed] [Google Scholar]
- 163.Shennan DB. Swelling-induced taurine transport: relationship with chloride channels, anion-exchangers and other swelling-activated transport pathways. Cell Physiol. Biochem. 2008;21:15–28. doi: 10.1159/000113743. [DOI] [PubMed] [Google Scholar]
- 164.Valverde MA, et al. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992;355:830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- 165.Paulmichl M, et al. New mammalian chloride channel identified by expression cloning. Nature. 1992;356:238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- 166.Gschwentner M, et al. Antisense oligonucleotides suppress cell-volume-induced activation of chloride channels. Pflugers Arch. 1995;430:464–470. doi: 10.1007/BF00373882. [DOI] [PubMed] [Google Scholar]
- 167.Ritter M, et al. Cell swelling stimulates cytosol to membrane transposition of ICln. J. Biol. Chem. 2003;278:50163–50174. doi: 10.1074/jbc.M300374200. [DOI] [PubMed] [Google Scholar]
- 168.Eggermont J, et al. Is there a link between protein pICln and volume-regulated anion channels? Biochem. J. 1998;331:347–349. doi: 10.1042/bj3310347u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Krapivinsky GB, Ackerman MJ, Gordon EA, Krapivinsky LD, Clapham DE. Molecular characterization of a swelling-induced chloride conductance regulatory protein, pICln. Cell. 1994;76:439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 170.Buyse G, et al. Expression of human pICln and ClC-6 in Xenopus oocytes induces an identical endogenous chloride conductance. J. Biol. Chem. 1997;272:3615–3621. doi: 10.1074/jbc.272.6.3615. [DOI] [PubMed] [Google Scholar]
- 171.Pu WT, Krapivinsky GB, Krapivinsky L, Clapham DE. pICln inhibits snRNP biogenesis by binding core spliceosomal proteins. Mol. Cell. Biol. 1999;19:4113–4120. doi: 10.1128/mcb.19.6.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 173.Stobrawa SM, et al. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 174.Weylandt KH, et al. Human ClC-3 is not the swelling-activated chloride channel involved in cell volume regulation. J. Biol. Chem. 2001;276:17461–17467. doi: 10.1074/jbc.M011667200. [DOI] [PubMed] [Google Scholar]
- 175.Hara-Chikuma M, et al. ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. J. Biol. Chem. 2005;280:1241–1247. doi: 10.1074/jbc.M407030200. [DOI] [PubMed] [Google Scholar]
- 176.Nilius B, et al. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 177.Decher N, et al. DCPIB is a novel selective blocker of I(Cl, swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br. J. Pharmacol. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Okada Y, et al. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 2006;209:21–29. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]
- 179.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl Acad. Sci. USA. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rock JR, Harfe BD. Expression of TMEM16 paralogs during murine embryogenesis. Dev. Dyn. 2008;237:2566–2574. doi: 10.1002/dvdy.21676. [DOI] [PubMed] [Google Scholar]
- 181.Mansoura MK, Biwersi J, Ashlock MA, Verkman AS. Fluorescent chloride indicators to assess the efficacy of CFTR cDNA delivery. Hum. Gene Ther. 1999;10:861–875. doi: 10.1089/10430349950018274. [DOI] [PubMed] [Google Scholar]
- 182.Verkman AS. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Disease. Alvarez-Leefmans FJ, Delpire E, editors. Elsevier; in the press. [Google Scholar]
- 183.Molokanova E, Savchenko A. Bright future of optical assays for ion channel drug discovery. Drug Discov. Today. 2008;13:14–22. doi: 10.1016/j.drudis.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 184.Baker BJ, et al. Genetically encoded fluorescent sensors of membrane potential. Brain Cell. Biol. 2008;36:53–67. doi: 10.1007/s11068-008-9026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]