Abstract
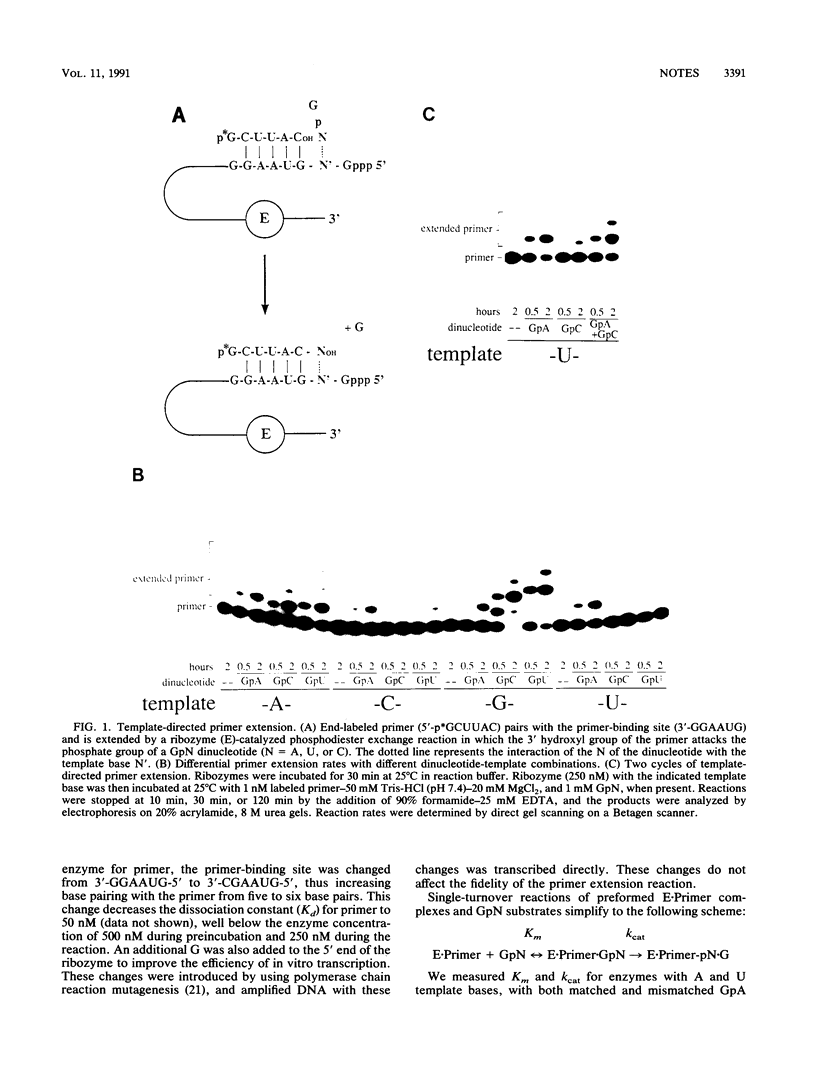
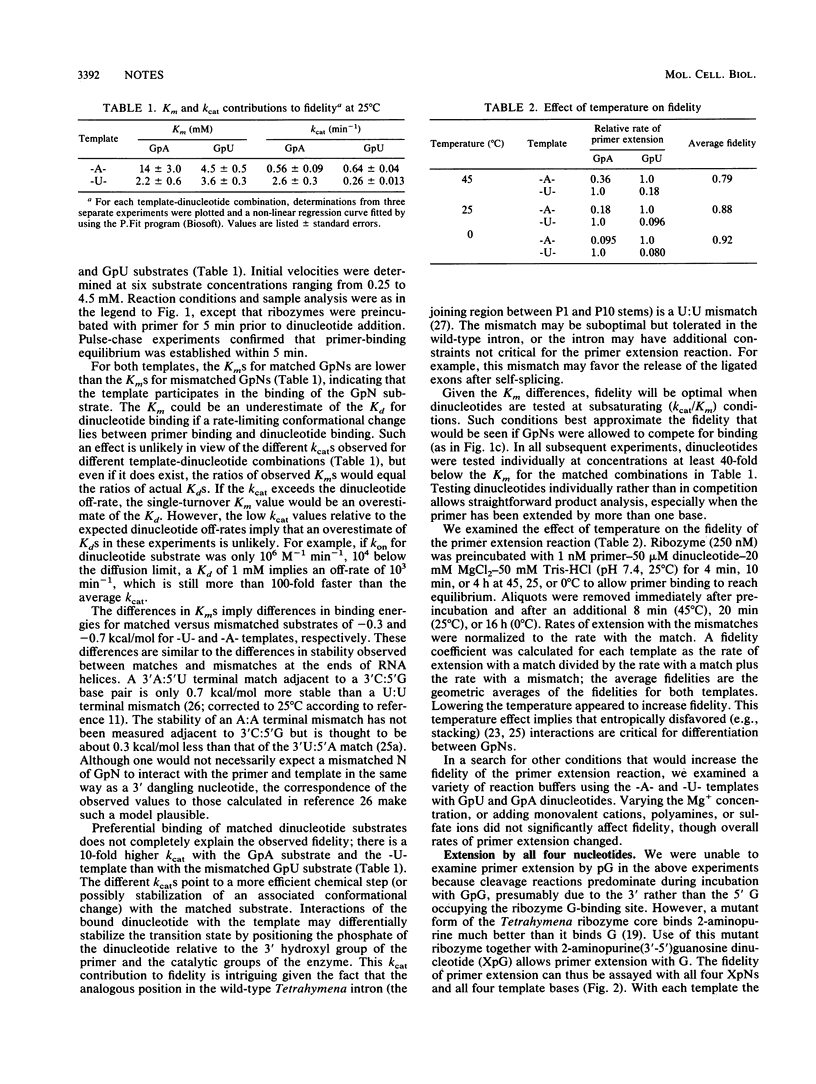
The Tetrahymena ribozyme has been shown to catalyze an RNA polymerase-like reaction in which an RNA primer is extended by the sequential addition of pN nucleotides derived from GpN dinucleotides, where N = A, C, or U. Here, we show that this reaction is influenced by the presence of a template; bases that can form Watson-Crick base pairs with a template add as much as 25-fold more efficiently than mismatched bases. A mutant enzyme with an altered guanosine binding site can catalyze template-directed primer extension with all four bases when supplied with dinucleotides of the form 2-aminopurine-pN.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Cech T. R. RNA as an RNA polymerase: net elongation of an RNA primer catalyzed by the Tetrahymena ribozyme. Science. 1988 Mar 18;239(4846):1412–1416. doi: 10.1126/science.2450400. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Ellington A. D., Tauer A. Modern metabolism as a palimpsest of the RNA world. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7054–7058. doi: 10.1073/pnas.86.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boosalis M. S., Petruska J., Goodman M. F. DNA polymerase insertion fidelity. Gel assay for site-specific kinetics. J Biol Chem. 1987 Oct 25;262(30):14689–14696. [PubMed] [Google Scholar]
- Bridson P. K., Orgel L. E. Catalysis of accurate poly(C)-directed synthesis of 3'-5'-linked oligoguanylates by Zn2+. J Mol Biol. 1980 Dec 25;144(4):567–577. doi: 10.1016/0022-2836(80)90337-x. [DOI] [PubMed] [Google Scholar]
- Cech T. R. A model for the RNA-catalyzed replication of RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4360–4363. doi: 10.1073/pnas.83.12.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Tanner N. K., Tinoco I., Jr, Weir B. R., Zuker M., Perlman P. S. Secondary structure of the Tetrahymena ribosomal RNA intervening sequence: structural homology with fungal mitochondrial intervening sequences. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3903–3907. doi: 10.1073/pnas.80.13.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. The chemistry of self-splicing RNA and RNA enzymes. Science. 1987 Jun 19;236(4808):1532–1539. doi: 10.1126/science.2438771. [DOI] [PubMed] [Google Scholar]
- Doudna J. A., Szostak J. W. RNA-catalysed synthesis of complementary-strand RNA. Nature. 1989 Jun 15;339(6225):519–522. doi: 10.1038/339519a0. [DOI] [PubMed] [Google Scholar]
- Doudna J. A., Szostak J. W., Rich A., Usman N. Chemical synthesis of oligoribonucleotides containing 2-aminopurine: substrates for the investigation of ribozyme function. J Org Chem. 1990 Oct 12;55(21):5547–5549. doi: 10.1021/jo00308a003. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Caruthers M. H., Neilson T., Turner D. H. Free energy contributions of G.U and other terminal mismatches to helix stability. Biochemistry. 1986 Jun 3;25(11):3209–3213. doi: 10.1021/bi00359a019. [DOI] [PubMed] [Google Scholar]
- Inoue T., Joyce G. F., Grzeskowiak K., Orgel L. E., Brown J. M., Reese C. B. Template-directed synthesis on the pentanucleotide CpCpGpCpC. J Mol Biol. 1984 Sep 25;178(3):669–676. doi: 10.1016/0022-2836(84)90244-4. [DOI] [PubMed] [Google Scholar]
- Inoue T., Orgel L. E. A nonenzymatic RNA polymerase model. Science. 1983 Feb 18;219(4586):859–862. doi: 10.1126/science.6186026. [DOI] [PubMed] [Google Scholar]
- Inoue T., Orgel L. E. Oligomerization of (guanosine 5'-phosphor)-2-methylimidazolide on poly(C). An RNA polymerase model. J Mol Biol. 1982 Nov 25;162(1):201–217. doi: 10.1016/0022-2836(82)90169-3. [DOI] [PubMed] [Google Scholar]
- Joyce G. F. RNA evolution and the origins of life. Nature. 1989 Mar 16;338(6212):217–224. doi: 10.1038/338217a0. [DOI] [PubMed] [Google Scholar]
- Kay P. S., Inoue T. Catalysis of splicing-related reactions between dinucleotides by a ribozyme. 1987 May 28-Jun 3Nature. 327(6120):343–346. doi: 10.1038/327343a0. [DOI] [PubMed] [Google Scholar]
- Martin C. T., Muller D. K., Coleman J. E. Processivity in early stages of transcription by T7 RNA polymerase. Biochemistry. 1988 May 31;27(11):3966–3974. doi: 10.1021/bi00411a012. [DOI] [PubMed] [Google Scholar]
- Michel F., Hanna M., Green R., Bartel D. P., Szostak J. W. The guanosine binding site of the Tetrahymena ribozyme. Nature. 1989 Nov 23;342(6248):391–395. doi: 10.1038/342391a0. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- NIYOGI S. K., STEVENS A. STUDIES OF THE RIBONUCLEIC ACID POLYMERASE FROM ESCHERICHIA COLI. IV. EFFECT OF OLIGONUCLEOTIDES ON THE RIBONUCLEIC ACID POLYMERASE REACTION WITH SYNTHETIC POLYRIBONUCLEOTIDES AS TEMPLATES. J Biol Chem. 1965 Jun;240:2593–2598. [PubMed] [Google Scholar]
- Sharp P. A. On the origin of RNA splicing and introns. Cell. 1985 Sep;42(2):397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- Turner D. H., Sugimoto N., Freier S. M. RNA structure prediction. Annu Rev Biophys Biophys Chem. 1988;17:167–192. doi: 10.1146/annurev.bb.17.060188.001123. [DOI] [PubMed] [Google Scholar]
- Waring R. B., Scazzocchio C., Brown T. A., Davies R. W. Close relationship between certain nuclear and mitochondrial introns. Implications for the mechanism of RNA splicing. J Mol Biol. 1983 Jul 5;167(3):595–605. doi: 10.1016/s0022-2836(83)80100-4. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., So A. G., Downey K. M. Mechanisms of error discrimination by Escherichia coli DNA polymerase I. Biochemistry. 1988 Jan 26;27(2):546–553. doi: 10.1021/bi00402a007. [DOI] [PubMed] [Google Scholar]




