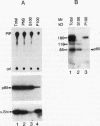
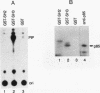
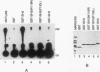
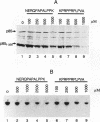
Abstract
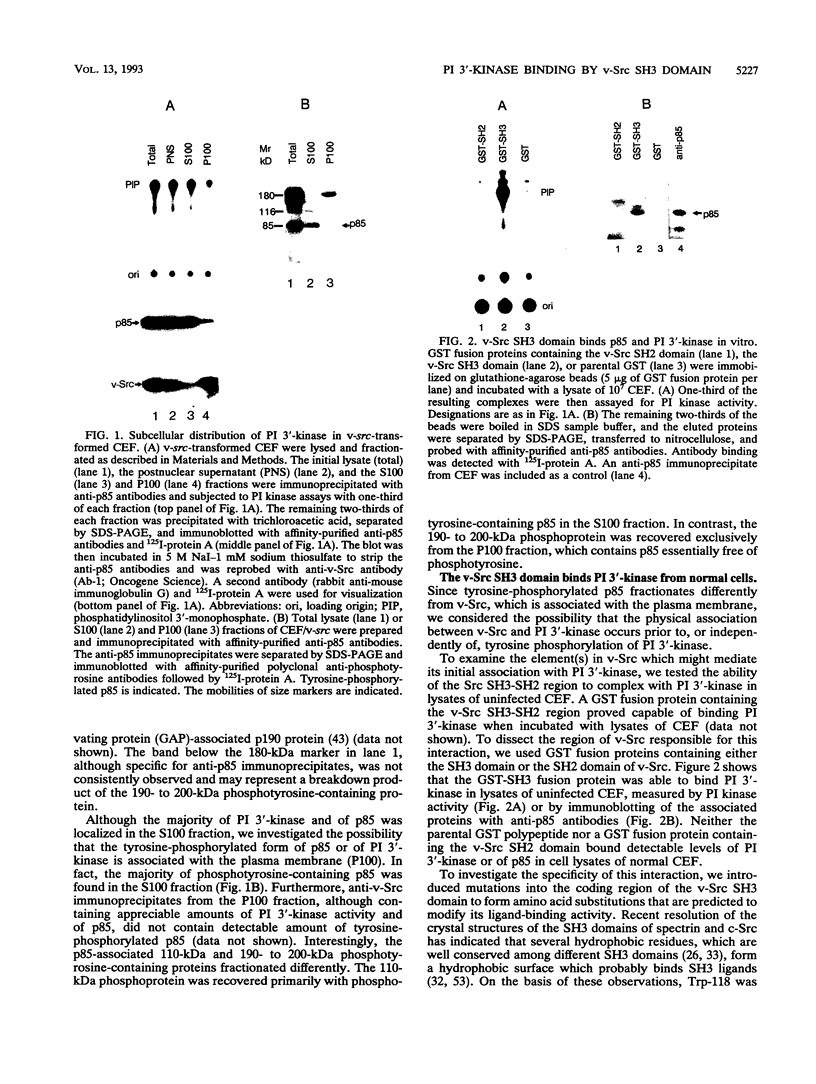
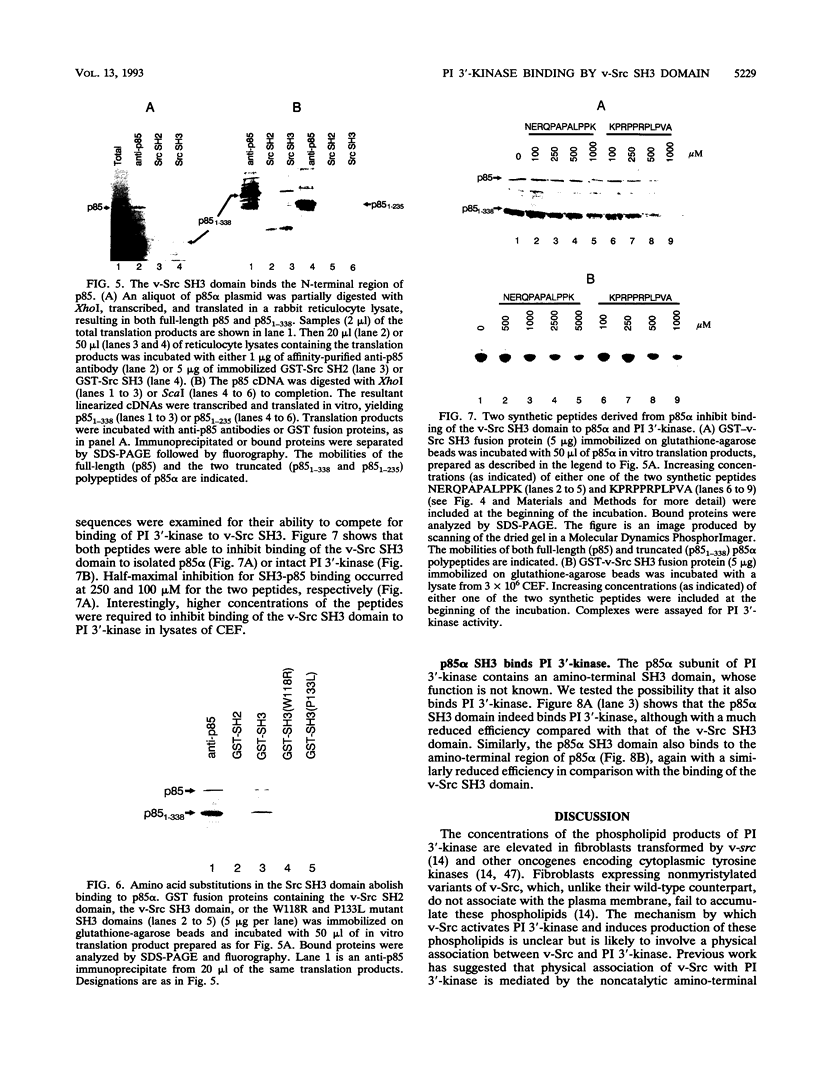
Fibroblasts transformed by v-src or by related oncogenes encoding activated tyrosine kinases contain elevated levels of polyphosphoinositides with phosphate at the D-3 position of the inositol ring, as a result of the activation of phosphatidylinositol (PI) 3'-kinase. v-src-transformed cells also contain increased levels of PI 3'-kinase activity immunoprecipitable with anti-phosphotyrosine antibodies; furthermore, PI 3'-kinase can be detected in association with the v-Src tyrosine kinase. To identify regions of v-Src that can interact with PI 3'-kinase, the v-Src SH2 and SH3 domains were expressed in bacteria and incubated with lysates of normal chicken embryo fibroblasts. In vitro, the v-Src SH3 domain, but not the SH2 domain, bound PI 3'-kinase in lysates of uninfected chicken embryo fibroblasts. Substitutions of two highly conserved SH3 residues implicated in ligand binding abolished the ability of the v-Src SH3 domain to associate with PI 3'-kinase. Furthermore, the v-Src SH3 domain bound in vitro to the amino-terminal region of the p85 alpha subunit of PI 3'-kinase. These results suggest that the v-Src SH3 domain may mediate an interaction between the v-Src tyrosine kinase and PI 3'-kinase, by direct binding to p85.
Full text
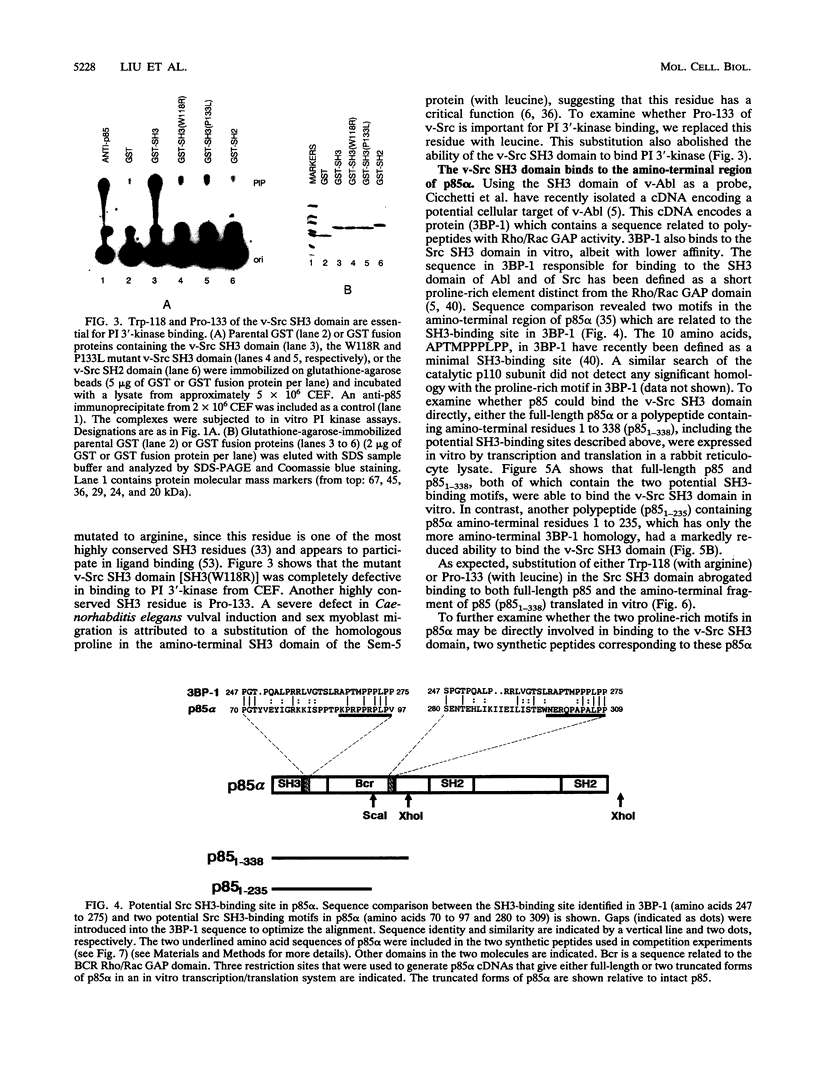
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Cicchetti P., Mayer B. J., Thiel G., Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992 Aug 7;257(5071):803–806. doi: 10.1126/science.1379745. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992 Mar 26;356(6367):340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Cohen B., Liu Y. X., Druker B., Roberts T. M., Schaffhausen B. S. Characterization of pp85, a target of oncogenes and growth factor receptors. Mol Cell Biol. 1990 Jun;10(6):2909–2915. doi: 10.1128/mcb.10.6.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Yoakim M., Piwnica-Worms H., Roberts T. M., Schaffhausen B. S. Tyrosine phosphorylation is a signal for the trafficking of pp85, an 85-kDa phosphorylated polypeptide associated with phosphatidylinositol kinase activity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4458–4462. doi: 10.1073/pnas.87.12.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J Virol. 1989 Feb;63(2):542–554. doi: 10.1128/jvi.63.2.542-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Sadowski I., Martin G. S., Pawson T. A conserved domain regulates interactions of the v-fps protein-tyrosine kinase with the host cell. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9064–9068. doi: 10.1073/pnas.84.24.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Navankasattusas S., Kavanaugh W. M., Milfay D., Fried V. A., Williams L. T. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991 Apr 5;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989 Apr;9(4):1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Requirement of phosphatidylinositol-3 kinase modification for its association with p60src. Mol Cell Biol. 1991 Apr;11(4):1972–1979. doi: 10.1128/mcb.11.4.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kornbluth S., Jong S. M., Wang L. H., Hanafusa H. Phosphatidylinositol kinase type I activity associates with various oncogene products. Oncogene Res. 1989;4(4):283–292. [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Jackson T. R., Stephens L. R. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992 Jul 9;358(6382):157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Hu P., Margolis B., Skolnik E. Y., Lammers R., Ullrich A., Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992 Mar;12(3):981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kashishian A., Kazlauskas A., Cooper J. A. Phosphorylation sites in the PDGF receptor with different specificities for binding GAP and PI3 kinase in vivo. EMBO J. 1992 Apr;11(4):1373–1382. doi: 10.1002/j.1460-2075.1992.tb05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Kashishian A., Cooper J. A., Valius M. GTPase-activating protein and phosphatidylinositol 3-kinase bind to distinct regions of the platelet-derived growth factor receptor beta subunit. Mol Cell Biol. 1992 Jun;12(6):2534–2544. doi: 10.1128/mcb.12.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B., Chen K. S. Phosphatidylinositol-3-kinase in isolated rat adipocytes. Activation by insulin and subcellular distribution. J Biol Chem. 1992 Feb 15;267(5):3423–3428. [PubMed] [Google Scholar]
- Klippel A., Escobedo J. A., Fantl W. J., Williams L. T. The C-terminal SH2 domain of p85 accounts for the high affinity and specificity of the binding of phosphatidylinositol 3-kinase to phosphorylated platelet-derived growth factor beta receptor. Mol Cell Biol. 1992 Apr;12(4):1451–1459. doi: 10.1128/mcb.12.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Liu X., Brodeur S. R., Gish G., Songyang Z., Cantley L. C., Laudano A. P., Pawson T. Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene. 1993 May;8(5):1119–1126. [PubMed] [Google Scholar]
- Marengere L. E., Pawson T. Identification of residues in GTPase-activating protein Src homology 2 domains that control binding to tyrosine phosphorylated growth factor receptors and p62. J Biol Chem. 1992 Nov 15;267(32):22779–22786. [PubMed] [Google Scholar]
- McGlade C. J., Ellis C., Reedijk M., Anderson D., Mbamalu G., Reith A. D., Panayotou G., End P., Bernstein A., Kazlauskas A. SH2 domains of the p85 alpha subunit of phosphatidylinositol 3-kinase regulate binding to growth factor receptors. Mol Cell Biol. 1992 Mar;12(3):991–997. doi: 10.1128/mcb.12.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan S. J., Smith A. D., Parker P. J. Purification and characterization of bovine brain type I phosphatidylinositol kinase. Eur J Biochem. 1990 Aug 17;191(3):761–767. doi: 10.1111/j.1432-1033.1990.tb19185.x. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Lehto V. P., Saraste M. SH3--an abundant protein domain in search of a function. FEBS Lett. 1992 Jul 27;307(1):55–61. doi: 10.1016/0014-5793(92)80901-r. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Noble M., Pauptit R., Wierenga R., Saraste M. Crystal structure of a Src-homology 3 (SH3) domain. Nature. 1992 Oct 29;359(6398):851–855. doi: 10.1038/359851a0. [DOI] [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Pawson T. Cell signalling. Conviction by genetics. Nature. 1992 Mar 26;356(6367):285–286. doi: 10.1038/356285a0. [DOI] [PubMed] [Google Scholar]
- Reedijk M., Liu X. Q., Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990 Nov;10(11):5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M., Liu X., van der Geer P., Letwin K., Waterfield M. D., Hunter T., Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3'-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992 Apr;11(4):1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith A. D., Ellis C., Lyman S. D., Anderson D. M., Williams D. E., Bernstein A., Pawson T. Signal transduction by normal isoforms and W mutant variants of the Kit receptor tyrosine kinase. EMBO J. 1991 Sep;10(9):2451–2459. doi: 10.1002/j.1460-2075.1991.tb07784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Rottapel R., Reedijk M., Williams D. E., Lyman S. D., Anderson D. M., Pawson T., Bernstein A. The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signaling proteins is induced by the Steel factor. Mol Cell Biol. 1991 Jun;11(6):3043–3051. doi: 10.1128/mcb.11.6.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Settleman J., Narasimhan V., Foster L. C., Weinberg R. A. Molecular cloning of cDNAs encoding the GAP-associated protein p190: implications for a signaling pathway from ras to the nucleus. Cell. 1992 May 1;69(3):539–549. doi: 10.1016/0092-8674(92)90454-k. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Talmage D. A., Freund R., Young A. T., Dahl J., Dawe C. J., Benjamin T. L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989 Oct 6;59(1):55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- Vallette F., Mege E., Reiss A., Adesnik M. Construction of mutant and chimeric genes using the polymerase chain reaction. Nucleic Acids Res. 1989 Jan 25;17(2):723–733. doi: 10.1093/nar/17.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varticovski L., Daley G. Q., Jackson P., Baltimore D., Cantley L. C. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol. 1991 Feb;11(2):1107–1113. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Wages D. S., Keefer J., Rall T. B., Weber M. J. Mutations in the SH3 domain of the src oncogene which decrease association of phosphatidylinositol 3'-kinase activity with pp60v-src and alter cellular morphology. J Virol. 1992 Apr;66(4):1866–1874. doi: 10.1128/jvi.66.4.1866-1874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Yoakim M., Hou W., Liu Y., Carpenter C. L., Kapeller R., Schaffhausen B. S. Interactions of polyomavirus middle T with the SH2 domains of the pp85 subunit of phosphatidylinositol-3-kinase. J Virol. 1992 Sep;66(9):5485–5491. doi: 10.1128/jvi.66.9.5485-5491.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Rosen M. K., Shin T. B., Seidel-Dugan C., Brugge J. S., Schreiber S. L. Solution structure of the SH3 domain of Src and identification of its ligand-binding site. Science. 1992 Dec 4;258(5088):1665–1668. doi: 10.1126/science.1280858. [DOI] [PubMed] [Google Scholar]