Abstract
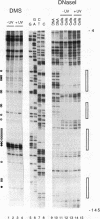
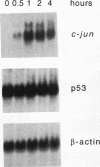
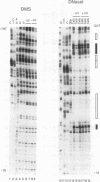
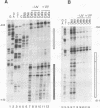
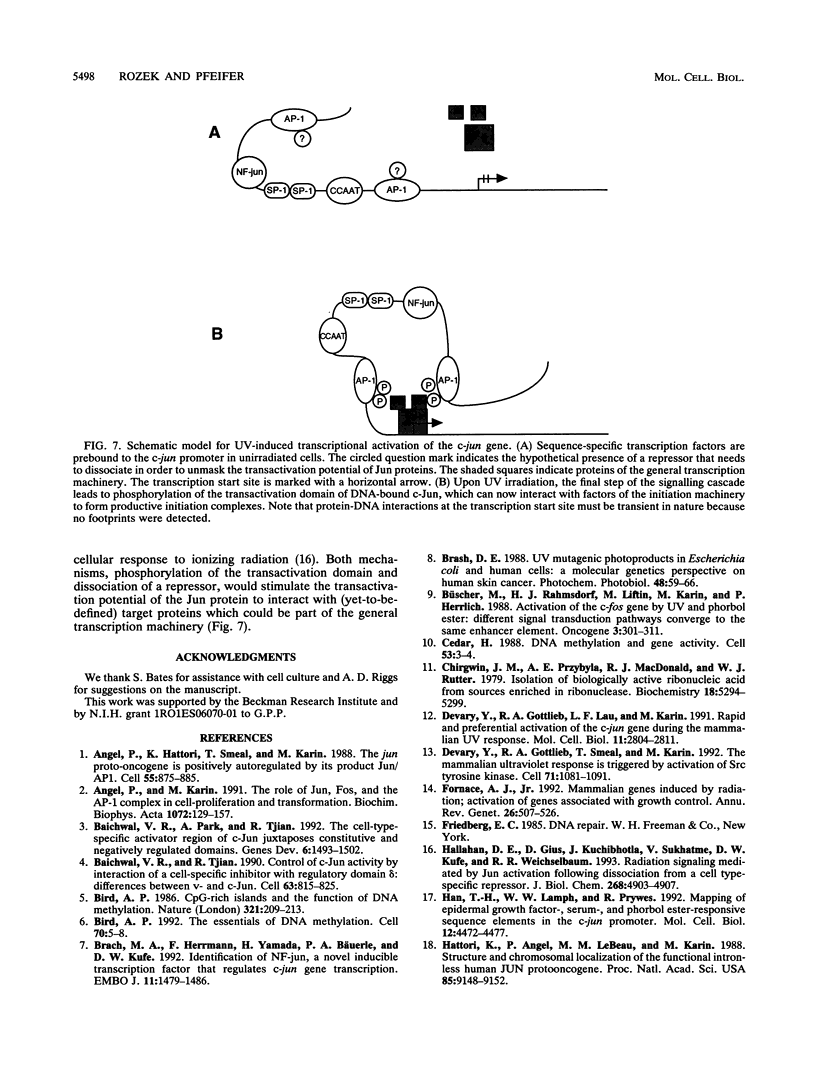
Irradiation of cells with UV light triggers a genetic response, called the UV response, which results in induction of a set of genes containing AP-1-binding sites. The c-jun gene itself, which codes for AP-1-binding activity, is strongly (> 100-fold) and rapidly activated by UV. The UV induction of c-jun is mediated by two UV response elements consisting of AP-1-like sequences within its 5' control region. We have analyzed protein-DNA interactions in vivo at the c-jun promoter in noninduced and UV-irradiated HeLa cells. In vivo footprint analysis was performed by using dimethyl sulfate on intact cells and DNase I on lysolecithihin-permeabilized cells in conjunction with ligation-mediated polymerase chain reaction to cover about 450 bp of the c-jun promoter, including the transcription start sites. We find that this region does not contain methylated cytosines and is thus a typical CpG island. In uninduced cells, in vivo protein-DNA interactions were localized to an AP-1-like sequence (nucleotides [nt] -71 to -64), a CCAAT box element (nt -91 to -87), two SP1 sequences (nt -115 to -110 and -123 to -118), a nuclear factor jun site (nt -140 to -132), and a second AP-1-like sequence (nt -190 to -183). These results indicate that complex protein-DNA interactions exist at the c-jun promoter prior to induction by an external stimulus. Surprisingly, after stimulation of c-jun expression by UV irradiation, all in vivo protein-DNA contacts remained essentially unchanged, including the two UV response elements located at the AP-1-like sequences. The UV-induced signalling cascade leads to phosphorylation of c-Jun on serines 63 and 73 (Y. Devary, R.A. Gottlieb, T. Smeal, and M. Karin, Cell 71:1081-1091, 1992). Taken together, these data suggest that modification of the transactivating domain of DNA-bound c-Jun or a closely related factor may trigger the rapid induction of the c-jun gene.
Full text
PDF

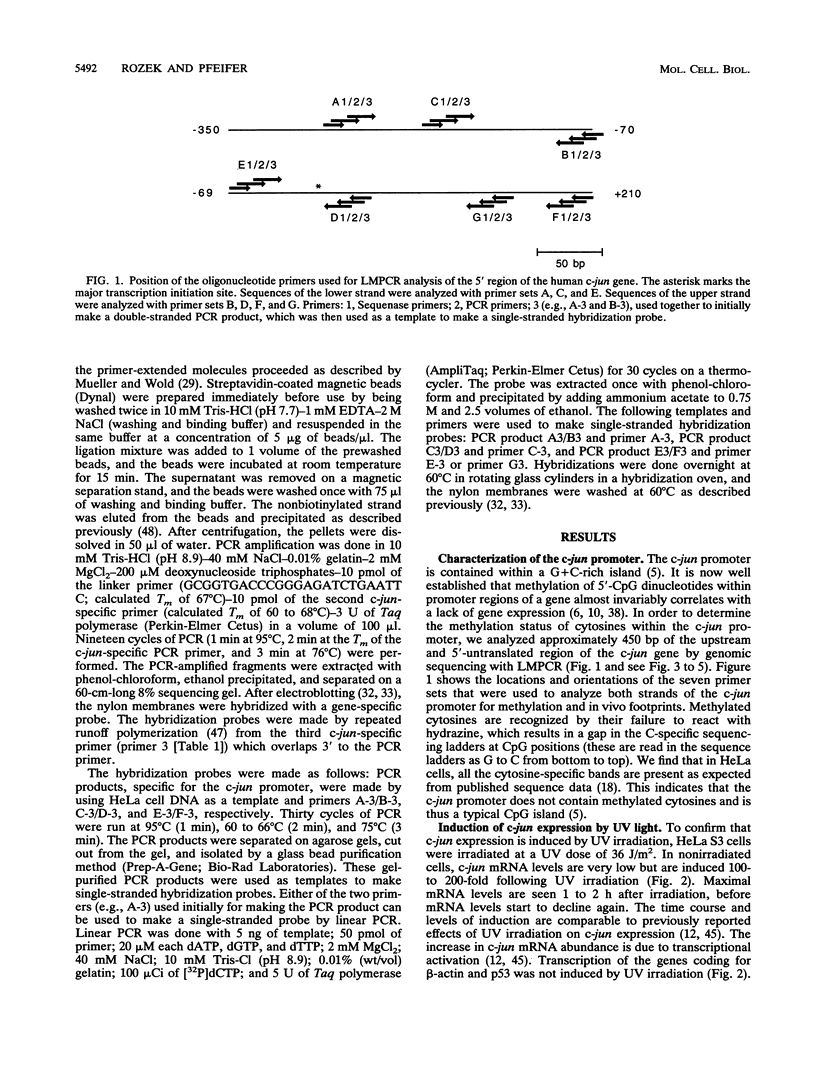
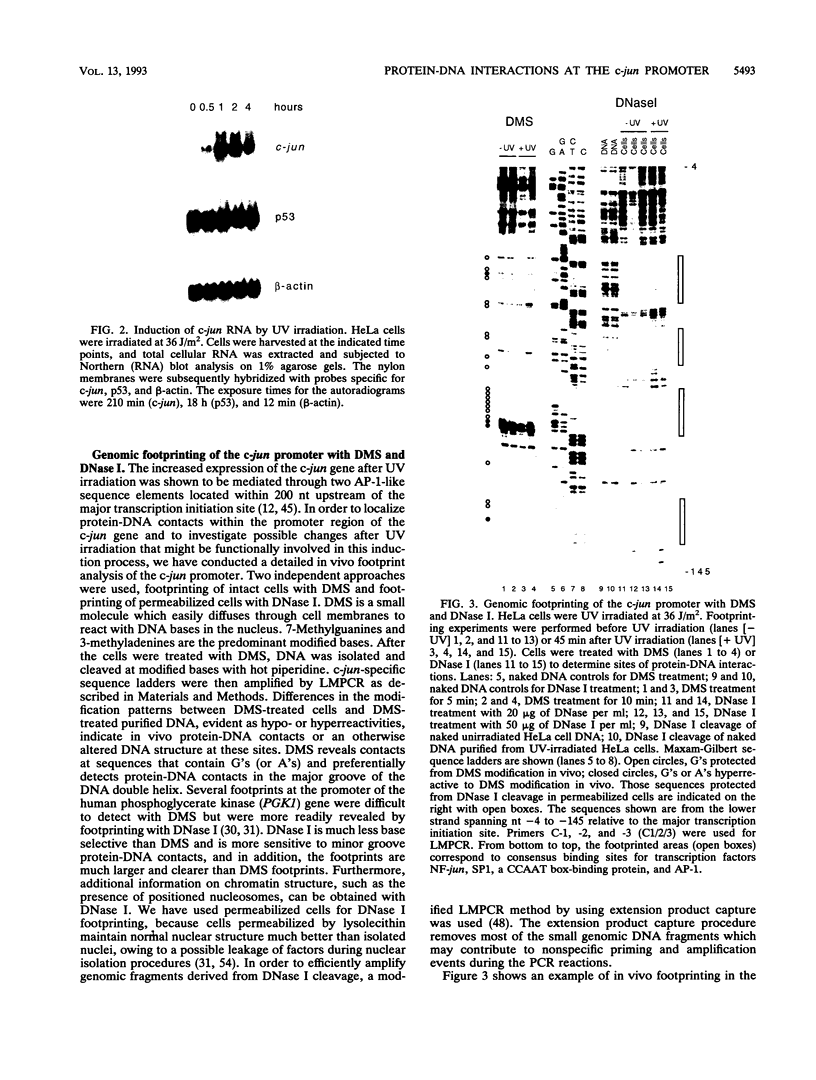
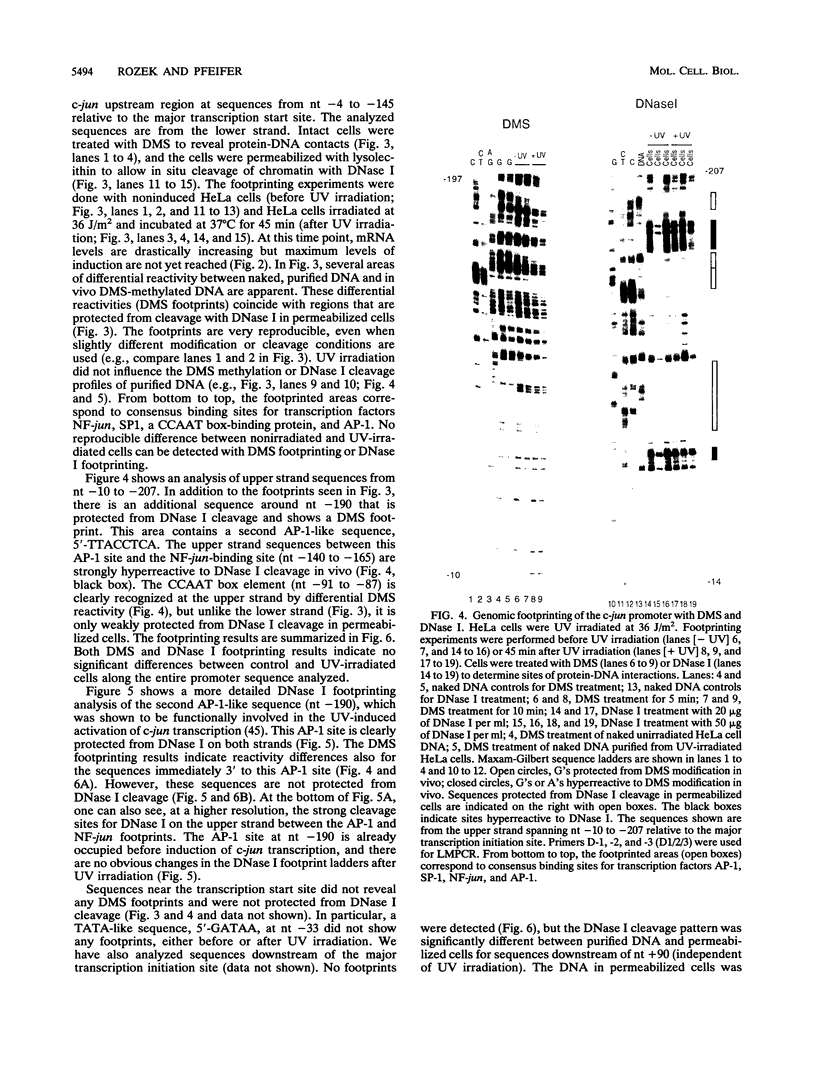
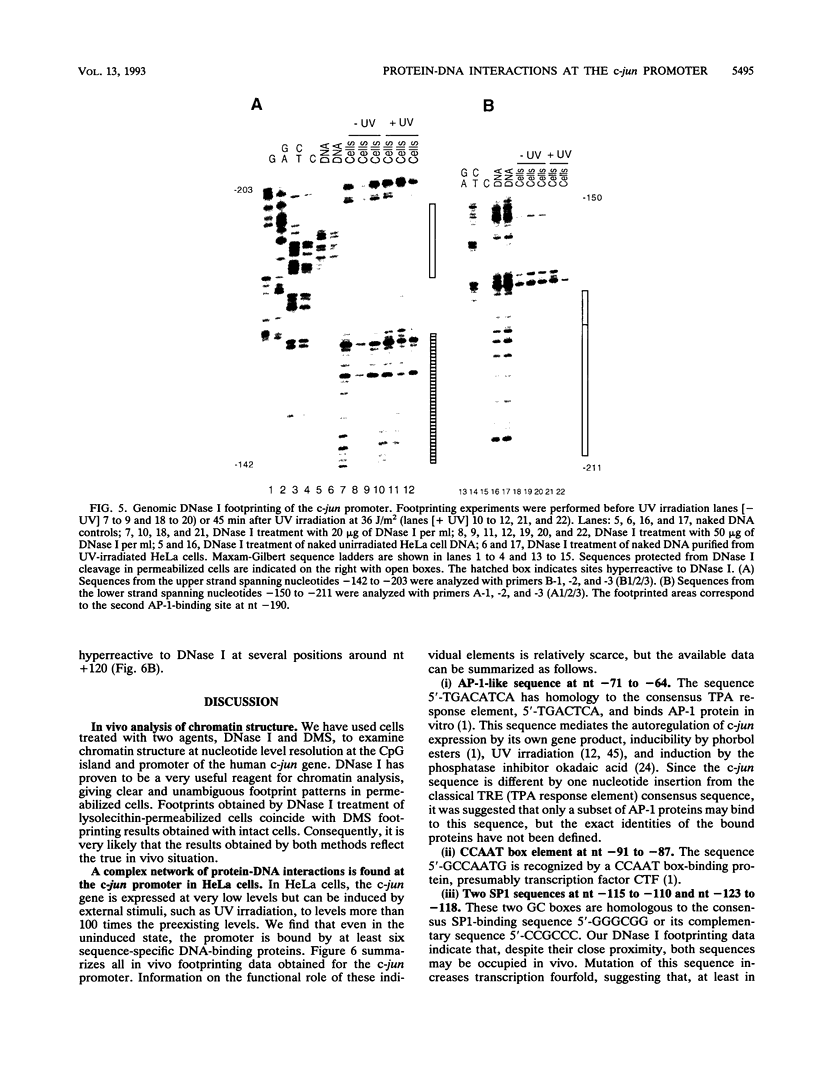



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Park A., Tjian R. The cell-type-specific activator region of c-Jun juxtaposes constitutive and negatively regulated domains. Genes Dev. 1992 Aug;6(8):1493–1502. doi: 10.1101/gad.6.8.1493. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Tjian R. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell. 1990 Nov 16;63(4):815–825. doi: 10.1016/0092-8674(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bird A. The essentials of DNA methylation. Cell. 1992 Jul 10;70(1):5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- Brach M. A., Herrmann F., Yamada H., Bäuerle P. A., Kufe D. W. Identification of NF-jun, a novel inducible transcription factor that regulates c-jun gene transcription. EMBO J. 1992 Apr;11(4):1479–1486. doi: 10.1002/j.1460-2075.1992.tb05192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E. UV mutagenic photoproducts in Escherichia coli and human cells: a molecular genetics perspective on human skin cancer. Photochem Photobiol. 1988 Jul;48(1):59–66. doi: 10.1111/j.1751-1097.1988.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Büscher M., Rahmsdorf H. J., Litfin M., Karin M., Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988 Sep;3(3):301–311. [PubMed] [Google Scholar]
- Cedar H. DNA methylation and gene activity. Cell. 1988 Apr 8;53(1):3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr Mammalian genes induced by radiation; activation of genes associated with growth control. Annu Rev Genet. 1992;26:507–526. doi: 10.1146/annurev.ge.26.120192.002451. [DOI] [PubMed] [Google Scholar]
- Hallahan D. E., Gius D., Kuchibhotla J., Sukhatme V., Kufe D. W., Weichselbaum R. R. Radiation signaling mediated by Jun activation following dissociation from a cell type-specific repressor. J Biol Chem. 1993 Mar 5;268(7):4903–4907. [PubMed] [Google Scholar]
- Han T. H., Lamph W. W., Prywes R. Mapping of epidermal growth factor-, serum-, and phorbol ester-responsive sequence elements in the c-jun promoter. Mol Cell Biol. 1992 Oct;12(10):4472–4477. doi: 10.1128/mcb.12.10.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Angel P., Le Beau M. M., Karin M. Structure and chromosomal localization of the functional intronless human JUN protooncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9148–9152. doi: 10.1073/pnas.85.23.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Fornace A. J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [PubMed] [Google Scholar]
- Ikuta T., Kan Y. W. In vivo protein-DNA interactions at the beta-globin gene locus. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10188–10192. doi: 10.1073/pnas.88.22.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Signal transduction from cell surface to nucleus in development and disease. FASEB J. 1992 May;6(8):2581–2590. doi: 10.1096/fasebj.6.8.1317309. [DOI] [PubMed] [Google Scholar]
- Kharbanda S., Datta R., Rubin E., Nakamura T., Hass R., Kufe D. Regulation of c-jun expression during induction of monocytic differentiation by okadaic acid. Cell Growth Differ. 1992 Jul;3(7):391–399. [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf H. J., Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992 Jun;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S., Stein B., van den Berg S., Kaina B., Lücke-Huhle C., Ponta H., Rahmsdorf H. J., Kraemer M., Gebel S., Herrlich P. Mechanisms of the ultraviolet light response in mammalian cells. J Cell Sci. 1989 Dec;94(Pt 4):609–615. doi: 10.1242/jcs.94.4.609. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P. Analysis of chromatin structure by ligation-mediated PCR. PCR Methods Appl. 1992 Nov;2(2):107–111. doi: 10.1101/gr.2.2.107. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Riggs A. D. Chromatin differences between active and inactive X chromosomes revealed by genomic footprinting of permeabilized cells using DNase I and ligation-mediated PCR. Genes Dev. 1991 Jun;5(6):1102–1113. doi: 10.1101/gad.5.6.1102. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Steigerwald S. D., Mueller P. R., Wold B., Riggs A. D. Genomic sequencing and methylation analysis by ligation mediated PCR. Science. 1989 Nov 10;246(4931):810–813. doi: 10.1126/science.2814502. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Tanguay R. L., Steigerwald S. D., Riggs A. D. In vivo footprint and methylation analysis by PCR-aided genomic sequencing: comparison of active and inactive X chromosomal DNA at the CpG island and promoter of human PGK-1. Genes Dev. 1990 Aug;4(8):1277–1287. doi: 10.1101/gad.4.8.1277. [DOI] [PubMed] [Google Scholar]
- Radler-Pohl A., Sachsenmaier C., Gebel S., Auer H. P., Bruder J. T., Rapp U., Angel P., Rahmsdorf H. J., Herrlich P. UV-induced activation of AP-1 involves obligatory extranuclear steps including Raf-1 kinase. EMBO J. 1993 Mar;12(3):1005–1012. doi: 10.1002/j.1460-2075.1993.tb05741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. M., Shen C. K. Protein-DNA interactions in vivo of an erythroid-specific, human beta-globin locus enhancer. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8676–8680. doi: 10.1073/pnas.88.19.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Pfeifer G. P. X-chromosome inactivation and cell memory. Trends Genet. 1992 May;8(5):169–174. doi: 10.1016/0168-9525(92)90219-t. [DOI] [PubMed] [Google Scholar]
- Ronai Z. A., Lambert M. E., Weinstein I. B. Inducible cellular responses to ultraviolet light irradiation and other mediators of DNA damage in mammalian cells. Cell Biol Toxicol. 1990 Jan;6(1):105–126. doi: 10.1007/BF00135030. [DOI] [PubMed] [Google Scholar]
- Ronai Z. A., Weinstein I. B. Identification of ultraviolet-inducible proteins that bind to a TGACAACA sequence in the polyoma virus regulatory region. Cancer Res. 1990 Sep 1;50(17):5374–5381. [PubMed] [Google Scholar]
- Rychlik W., Rhoads R. E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989 Nov 11;17(21):8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Shah G., Ghosh R., Amstad P. A., Cerutti P. A. Mechanism of induction of c-fos by ultraviolet B (290-320 nm) in mouse JB6 epidermal cells. Cancer Res. 1993 Jan 1;53(1):38–45. [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D., Grover-Bardwick A., Heidecker G., Rapp U. R., Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992 Aug;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Angel P., van Dam H., Ponta H., Herrlich P., van der Eb A., Rahmsdorf H. J. Ultraviolet-radiation induced c-jun gene transcription: two AP-1 like binding sites mediate the response. Photochem Photobiol. 1992 Mar;55(3):409–415. doi: 10.1111/j.1751-1097.1992.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törmänen V. T., Pfeifer G. P. Mapping of UV photoproducts within ras proto-oncogenes in UV-irradiated cells: correlation with mutations in human skin cancer. Oncogene. 1992 Sep;7(9):1729–1736. [PubMed] [Google Scholar]
- Törmänen V. T., Swiderski P. M., Kaplan B. E., Pfeifer G. P., Riggs A. D. Extension product capture improves genomic sequencing and DNase I footprinting by ligation-mediated PCR. Nucleic Acids Res. 1992 Oct 25;20(20):5487–5488. doi: 10.1093/nar/20.20.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlap T., Franklin C. C., Wagner F., Kraft A. S. Upstream regions of the c-jun promoter regulate phorbol ester-induced transcription in U937 leukemic cells. Nucleic Acids Res. 1992 Feb 25;20(4):897–902. doi: 10.1093/nar/20.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih F., Stewart A. F., Boshart M., Nitsch D., Schütz G. In vivo monitoring of a cAMP-stimulated DNA-binding activity. Genes Dev. 1990 Aug;4(8):1437–1449. doi: 10.1101/gad.4.8.1437. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gralla J. D. In situ nucleoprotein structure involving origin-proximal SV40 DNA control elements. Nucleic Acids Res. 1990 Apr 11;18(7):1797–1803. doi: 10.1093/nar/18.7.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam H., Duyndam M., Rottier R., Bosch A., de Vries-Smits L., Herrlich P., Zantema A., Angel P., van der Eb A. J. Heterodimer formation of cJun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 1993 Feb;12(2):479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]