Abstract
Platelets have recently been shown to drive liver injury in murine models of viral hepatitis and promote liver regeneration through the release of serotonin. Despite their emerging role in inflammatory liver disease, little is known about the mechanisms by which platelets bind to the hepatic vasculature. Therefore, we referenced public expression data to determine the profile of potential adhesive receptors expressed by hepatic endothelium. We then used a combination of tissue-binding and flow-based endothelial-binding adhesion assays to show that resting platelets bind to human hepatic sinusoidal endothelial cells and that the magnitude of adhesion is greatly enhanced by thrombin-induced platelet activation. Adhesion was mediated by the integrins Gp1b, αIIbβIII, and αvβ3, as well as immobilized fibrinogen. Platelet binding to hepatic endothelial cells resulted in NF-κB activation and increased chemokine secretion. The functional relevance of platelet binding was confirmed by experiments that showed markedly increased binding of neutrophils and lymphocytes to hepatic endothelial cells under shear conditions replicating those found in the hepatic sinusoid, which was in part dependent on P-selectin expression. Thus the ability of platelets to activate endothelium and promote leukocyte adhesion may reflect an additional mechanism through which they promote liver injury.
Keywords: sinusoid, human integrin, liver
hepatic inflammation in response to liver injury drives chronic hepatitis, fibrogenesis, and cirrhosis (8, 13, 17, 24, 49). Persistent inflammation at any site, including the liver, is the result of an accumulation of leukocytes, which become organized into stable inflammatory infiltrates (2). For this to occur, leukocytes must be recruited into tissue from the circulation, following adhesion to endothelium from flow and transmigration through the endothelial barrier. The complex microvascular anatomy of the liver allows leukocytes to bind endothelium at three anatomical sites, the portal tract, the sinusoids, or the terminal hepatic veins (63) although the majority of recruitment occurs through the sinusoids (71). In response to injury, recruitment increases as a consequence of endothelial activation (9, 25).
Platelet adhesion to the vascular wall occurs at sites of endothelial cell loss to prevent bleeding and vascular leak. However, recently it has become apparent that platelets can, under some circumstances, bind to endothelial cells, where they can support leukocyte recruitment to the vessel wall (28, 30) (reviewed in Ref. 62). In L-selectin- deficient mice, activated platelets can reconstitute P-selectin-dependent lymphocyte homing to lymph node high endothelial venules (14). Hepatic endothelial cells fail to express P-selectin even when inflamed, and a similar mechanism could provide a P-selectin substrate within hepatic sinusoids in the absence of endothelial P-selectin (1, 68). Platelets are sequestered in the liver following experimental transplantation (12, 73) and correlate with graft survival (12). Similarly increased binding of platelets to sinusoidal endothelium and enhanced neutrophil recruitment is observed in mice exposed to LPS (27) and following I/R injury (49), and serum from cirrhotic patients contains elevated levels of von Willebrand factor (vWF) and promotes platelet binding to collagen (41). Despite the recent evidence implicating platelets in liver injury and inflammation, little is known about the molecular regulation of platelet adhesion to hepatic endothelium within the low-perfusion sinusoidal bed. We now report the molecular regulation of platelet adhesion to human hepatic endothelium and demonstrate that adherent platelets activate NF-κB-dependent endothelial chemokine and adhesion molecule expression and act as a bridge to enhance leukocyte recruitment.
MATERIALS AND METHODS
Platelet isolation.
Platelets were isolated using standard methods from whole blood of healthy volunteers who had not taken aspirin or other platelet-modulating agents in the previous 10 days (28). Platelet-rich plasma (PRP) was prepared by centrifuging EDTA anticoagulated blood at room temperature for 5 min at 290 g, and platelets were pelleted by centrifuging the PRP at room temperature for 5 min at 1,800 g. The platelet-rich pellet was washed in PBS without calcium chloride and magnesium chloride (Sigma) containing 5 mM glucose and 6 mM theophylline (both Sigma) and resuspended to a final concentration of 1 × 108 platelets/ml in Ca/Mg-free PBS for static adhesion assays or serum-free and protein-free hybridoma medium, containing 0.15% bovine serum albumin (both Sigma) for flow-based adhesion assays.
Human liver tissue.
All tissue samples used were obtained from The Liver Unit, Queen Elizabeth Hospital in Birmingham with informed consent and approval from the Birmingham Ethics Committee. Normal liver samples were surplus to surgical requirements, and diseased livers were explanted during transplantation or regraft surgery for alcoholic liver disease (ALD) or primary biliary cirrhosis (PBC).
Endothelial cell isolation and culture.
Primary cultures of human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords obtained from Birmingham Women's Hospital and prepared according to previously described methodology (26). Human hepatic sinusoidal endothelial cells (HSEC) were isolated in house from ∼50 g of human liver tissue. Isolation was performed as previously described (33, 34). Briefly, nonparenchymal cells were collected after collagenase digestion of mechanically disaggregated liver and were further purified by density gradient centrifugation over Percoll. Endothelial cells were isolated from the resultant heterogeneous cell mixture by positive immunomagnetic selection using antibodies raised against CD31 (DAKO) and magnetic beads (Dynal) conjugated with goat anti-mouse antibody according to the manufacturer's protocol. All endothelial cells were maintained in complete media comprising Human Endothelial-SFM basal growth medium (Invitrogen) containing 104 U/ml penicillin and 10 μl/ml streptomycin, 10 ng/ml epidermal growth factor (R & D Systems), 10 μg/ml hydrocortisone (Sigma), and either 10% heat-inactivated human serum (TCS Biologicals, for HSEC) or 10% fetal calf serum (Invitrogen, for HUVEC). All endothelial cells were plated out into collagen-coated culture flasks (Sigma) and maintained at 37°C in a humidified 3% CO2 incubator until confluent. The endothelial cells were used only up to passage 6, and phenotypic identity and purity were confirmed by staining for endothelial markers (35).
SAGE analysis of sinusoidal endothelial cell phenotype.
To find genes that might be used by platelets to bind liver endothelium in the absence of vWF, and that are differentially expressed in liver endothelium vs. other types of endothelium, we searched SAGE libraries (55, 65) from the Gene Expression Omnibus database (GEO, Ref. 45). One library from liver containing 77,759 tags was contrasted with five libraries from other sources of endothelium, totaling 241,804 tags. Table 1 lists the SAGE libraries used. To analyze the data, the source code algorithm for the SAGEmap xProfiler tool was downloaded and run locally from the NCBI (ftp://ftp.ncbi.nih.gov/pub/sage/obsolete/source/cpp). All the default settings were used except a posterior probability of ≤0.8 cutoff was used, and the c statistic was set at 3 as previously (29). All SAGE libraries were short 10-bp tags, and tag-to-gene mappings were made using the latest file available from the SAGE download site (46).
Table 1.
SAGE libraries used
| Liver libraries |
||||
|---|---|---|---|---|
| Anchoring Enzyme | Final GEO Accession | Final Count | SAGE Library | Origin |
| NlaIII | GSM384018 | 77759 | SAGE Vascular endothelium normal liver associated AP NLEC1 | Normal liver |
| Other endothelial libraries | ||||
| NlaIII | GSM16892 | 68987 | Human Glomerular Endothelial Cell | Glomerular |
| NlaIII | GSM384019 | 51642 | SAGE Vascular normal CS control | Microvascular |
| NlaIII | GSM384017 | 57023 | SAGE Vascular endothelium normal breast associated P1H12+ AP 1 | Breast normal endothelium |
| NlaIII | GSM62243 | 25706 | Human Pulmonary Artery Endothelial Cell (Control) | Primary human pulmonary artery endothelial cells |
| NlaIII | GSM62240 | 38446 | Human Aortic Endothelial Cell Exposure (Control) | Heart |
Investigation of platelet adhesion to liver sections.
Platelet adhesion to human liver sections was investigated using an immunofluorescent static-adhesion assay. The methodology used was an adaptation (33) of the protocol described by Stamper and Woodruff (60). Briefly, fresh frozen, 5 μM liver sections were incubated with 100 μl of platelet suspension at a final concentration of 1 × 108 platelets/ml for 1 h before washing to remove nonadherent platelets and ethanol fixation. The sections were then treated with an antibody raised against platelet glycoprotein IIb (CD41, DAKO, 1/100 dilution) for 45 min, followed by a secondary FITC-labeled antibody (goat-anti mouse FITC, DAKO, 1/50 dilution). Finally, the sections were mounted in 90% glycerol containing 2.5% diazabicyclo-octane (Sigma) to retard fading. On occasion, an immunohistochemical detection method was used to visualize platelet binding to liver sections. Here CD41 antibody binding was detected using a species-specific secondary antibody-peroxidase reagent kit (ImmPRESS, Vector Laboratories) according to the manufacturer's instructions. Antibody binding was visualized with a peroxidase substrate kit (ImmPACT DAB, Vector Laboratories), and sections were counterstained with hematoxylin. Sections were examined using an Axiovert fluorescent microscope, and images were captured using a digital camera and Axiovision software (Zeiss). Three different liver samples and three platelet donors were used for each series of experiments. Platelet binding to endothelial cells was scored by qualitative microscopic examination of multiple high-power fields in each sample. Staining was quantified by measuring six random nonoverlapping views per sample centered on vascular structures at ×200 magnification using threshold analysis and calculation of percentage of area occupied by platelets with ImageJ software (rsbweb.nih.gov/ij/).
Investigation of platelet adhesion to cultured endothelial cells in a static assay.
Endothelial cells were grown to confluency on gelatin-coated 2.5-mm-diameter coverslips (Thermanox, Fisher Scientific). The coverslips were fixed in ethanol, and endothelial cells were placed uppermost on a microscopic slide and treated with platelet suspension as described above (1 × 108 platelets/ml, 100 μl per coverslip). The adherent platelets were then visualized and photographed using fluorescently labeled CD41 antibody as described above. However, this method only permitted qualitative assessment of the degree of platelet binding. Therefore, for a more quantitative assessment of binding, the number of platelets bound to the luminal side of the endothelial cells was counted using phase-contrast microscopy. Then, a platelet/endothelial cell ratio was calculated using the average of the number of adherent platelets binding to the endothelial cells in a high-power microscope field of view.
Investigation of platelet adhesion to cultured endothelial cells in a flow-based assay.
To investigate platelet adhesion to hepatic endothelial cells under conditions of physiological blood flow, we used a flow-based adhesion assay system (36). Briefly, platelet solution (1 × 108 /ml) or wash buffer was perfused over the surface of the endothelial cells at a wall shear stress of 0.1 Pa for 5 min. This shear stress is representative of that present in the human liver sinusoid (31, 32). After perfusion with wash buffer to wash out any remaining unbound platelets, adhesion of platelets was visualized by phase-contrast video microscopy. Subsequently, platelet adhesion per endothelial cell was calculated as described above. On occasion, after perfusion of platelets for 5 min and washing to remove unbound cells, we then perfused 1 × 106/ml freshly isolated neutrophils or peripheral blood lymphocytes isolated as described previously (30). Leukocytes were perfused for 5 min before quantification of adherent cells/mm2 per 106 cells perfused (36). Where indicated, we also used a specific inhibitor of P-selectin (KF 38789, 10 μM, Tocris Bioscience) to determine selectivity of neutrophil binding.
Blocking antibodies and peptides.
To investigate which molecules were responsible for platelet adhesion to endothelial cells, we employed antibodies and blocking peptides in the assay systems described above. Reagents directed against platelet receptors were added to the platelet suspension 20 min before incubation with the endothelial cells. Antibodies raised against human CD41 (Glycoprotein IIb, Pharmingen) and CD42a (GP1bα, Pharmingen) were used at final concentrations of 5 and 10 μg/ml. Arg-gly-asp (RGD) peptide (A8052, Sigma) was used at 50 and 100 μg/ml and thrombin (Sigma, UK) at a concentration of 0.5 U/ml. Antibodies and blocking peptides directed against endothelial cell receptors were added to cultured endothelium or liver sections 20 min before addition of platelets. Antibody raised against human αvβ3 (Chemicon International) was used at concentrations of 5, 10, and 15 μg/ml, whereas fibrinogen binding peptide (Sigma) was added at concentrations of 50 and 100 μg/ml. To activate platelets in vitro, samples of freshly prepared platelets were treated with 0.5 U/ml thrombin (Sigma) for 20 min, washed, and then incubated on tissue sections as normal.
ELISA to detect chemokine expression.
Production of CCL2 (monocyte chemoattractant protein-1) and CXCL8 (IL-8) by HSEC in response to binding of platelets was determined using a capture ELISA (R&D Systems) according to the manufacturer's instructions. Confluent monolayers of HSEC grown in 24-well plates were incubated with control serum-free media or platelets (0.05 × 108-0.1 × 109/ml) for 4 h. Cell-free supernatants were collected and stored at −80°C until ELISA development.
Measurement of endothelial NF-κB activation.
To determine whether binding of platelets to HSEC induced activation of NF-κB, we used a commercially available ELISA-based reporter assay (NF-κB p50 Transfactor Kit, Clontech) according to the manufacturer's instructions. HSEC were exposed to 1 × 108 platelets/ml or media alone for 10 min before trypsinization and generation of nuclear and cytoplasmic extracts. Protein concentration of extracts was calculated using a micro Lowry assay (Sigma) according to manufacturer's instructions. Binding of NF-κB in nuclear extracts to consensus sequences in DNA immobilized to the ELISA plate was quantified colorimetrically, and signals were compared with binding to scrambled DNA sequences. Stimulation of HSEC with TNF-α was used as a positive control.
Statistical analysis.
For functional data, standard error was used to express variation about the mean, and an unpaired Student's t-test was used to assess significance. A value of P < 0.05 was classified as significant for all investigations. To measure statistical significance of differentially expressed genes between the endothelial sources in our SAGE analysis, two methods were employed. First, the SAGEmap algorithm was employed that computes a Bayesian posterior distribution for a fold ratio changed in gene expression between two groups of SAGE libraries (38). Second, a recently published likelihood ratio test was also used with a False Discovery Rate procedure to find significantly differentially expressed genes between the two groups of endothelial SAGE libraries (23).
RESULTS
SAGE analysis of sinusoidal endothelium shows reduced expression of vWF, CD31, and αv integrin by hepatic endothelium.
To compare the expression of potential platelet-binding receptors on hepatic and other endothelial cells, genes differentially expressed in liver endothelium compared with pooled data from kidney, breast, lung, and aortic endothelium, SAGE libraries (55, 65) (Table 1) were analyzed from the Gene Expression Omnibus database (GEO, Ref. 45). This analysis generated a list of 1,535 genes with significant differential expression using a FDR Q value ≤0.05 and a posterior probability of ≥0.8. Expression of characteristic sinusoidal endothelial phenotypic indicators CD32b (FCGR2B), LYVE-1 (LYVE1), CLEVER-1 (STAB1), and the mannose receptor (MRC1) (35) was increased in hepatic compared with nonhepatic endothelium (Table 2), confirming the sinusoidal origin of the hepatic endothelium. We then focused on genes implicated in platelet adhesion and showed significantly reduced expression of vWF, the αv integrin subunit, and CD31 by sinusoidal endothelium (Table 2) compared with the nonhepatic cells. ICAM-1, E-, and P-selectin were not differentially expressed between unstimulated liver endothelium and other endothelium in the SAGE library analyses. Supplemental Table S1 shows the full results of the SAGE analyses, listing all genes that were significantly differentially expressed; 864 genes were enriched in liver endothelium vs. other endothelial cell types, and 671 genes were enriched in other endothelial cells vs. liver endothelium. Supplemental material is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website.
Table 2.
Differentially expressed genes of interest
| Gene | Product | Q-value | Up/down regulated in Liver | Posterior Probability | Liver Count | Other Count | Tag | Score of Mapping |
|---|---|---|---|---|---|---|---|---|
| LYVE1 | Lymphatic vessel endothelial hyaluronan receptor 1 | 0 | up | 1 | 13 | 1 | GAGATTCTCA | 5018064 |
| CD36 | CD36 molecule (thrombospondin receptor) | 0 | up | 1 | 25 | 0 | TCTAGCCACT | 4015038 |
| FCGR2B | Fc fragment of IgG, low affinity IIb, receptor (CD32) | 0.0001 | up | 0.99 | 9 | 0 | TAAATCTATA | 3016558 |
| MRC1 | Mannose receptor, C type 1 | 0.001 | up | 0.98 | 14 | 5 | TGCAGAAGAA | 4007016 |
| STAB1 | Stabilin 1 | 0.0496 | up | 0.86 | 6 | 1 | CACAGGGAGG | 2002514 |
| VCAM1 | Vascular cell adhesion molecule 1 | 0.0519 | up | 0.88 | 8 | 3 | GTACGGAGAT | 5012058 |
| ITGAV | Integrin, alpha V (vitronectin receptor, α polypeptide, antigen CD51) | 0 | down | 1 | 2 | 77 | TAACTTGTGA | 4060121 |
| PECAM1 | Platelet/endothelial cell adhesion molecule (CD31 antigen) | 0.0212 | down | 0.88 | 4 | 57 | CCTGTAGTAT | 3015037 |
| vWF | von Willebrand factor | 0 | down | 1 | 4 | 345 | TTCTGCTCTT | 2062153 |
Unstimulated platelets bind to human liver endothelial cells.
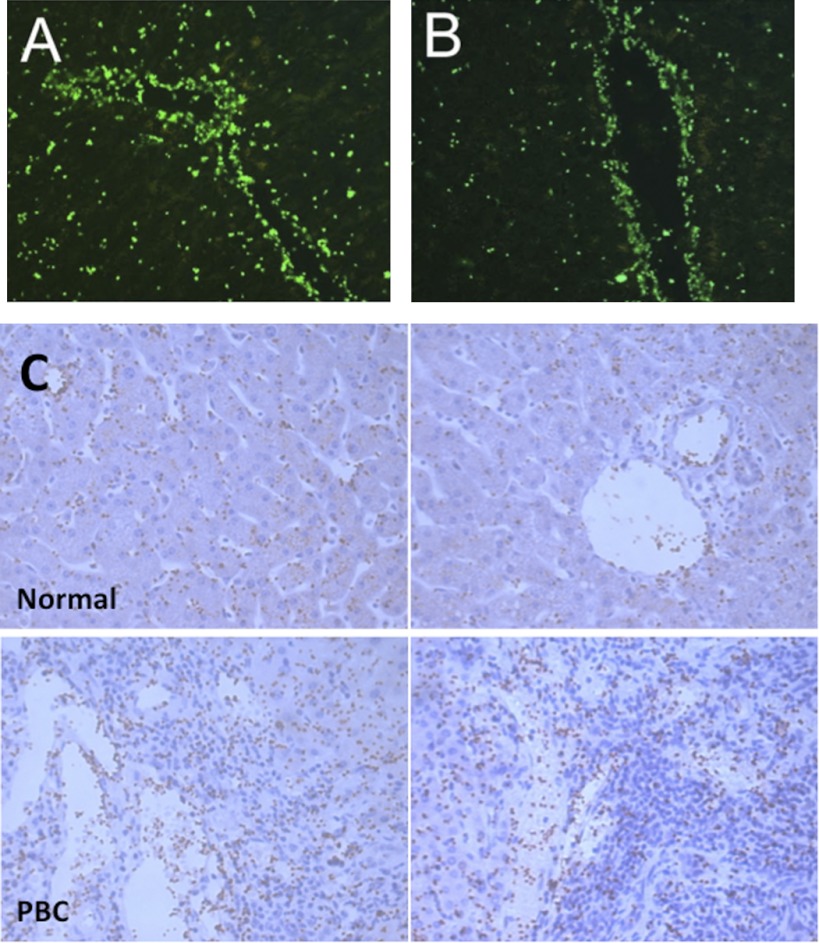
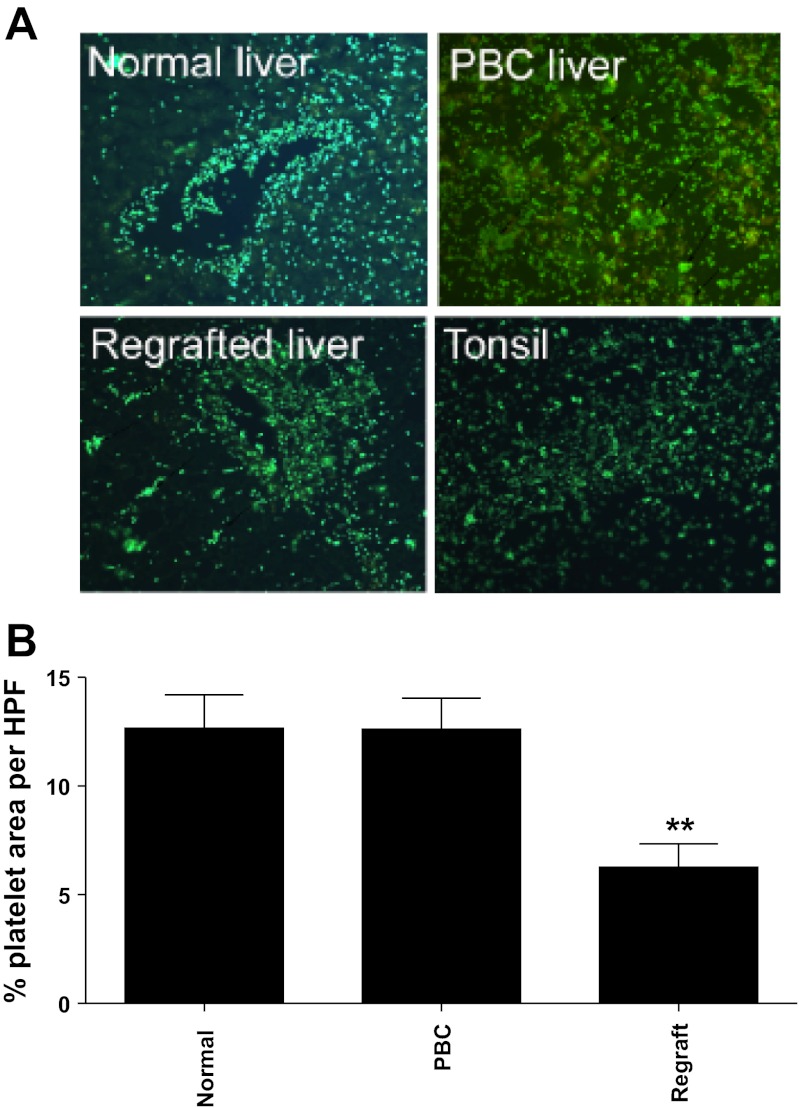
Unstimulated human platelets bound to vessels within fresh snap-frozen liver tissue. Figure 1, A and B, shows binding of fluorescently labeled platelets to liver sinusoids and vessels with minimal adhesion to epithelial cells or stroma. The adherent platelets were localized to vascular endothelial cells in portal and central vessels and were observed on the majority of such vessels in the liver sections studied. Platelets also bound to hepatic sinusoids (see Fig. 1). Additional immunohistochemical detection methods confirmed that platelet binding localized to vascular structures in the portal tracts and to sinusoidal endothelium in both normal and disease liver sections (see Fig. 1C) with increased numbers of platelets binding to vessels in inflamed portal areas in disease. To show that the staining was due to the addition of exogenous platelets, we stained the sections with an antibody against the platelet receptor CD41. No staining for CD41 was detected on normal sinusoidal or vascular endothelium in the absence of exogenous platelets (not shown). We compared platelet binding to liver tissue from patients with inflammatory liver disease including PBC, ALD, and chronic allograft rejection using sections of tonsil as controls (Fig. 2A). The pattern of binding to chronically diseased liver was not significantly different to that seen in normal tissue (Fig. 2B) although adherent platelets tended to aggregate into clumps on diseased tissue (see arrows Fig. 2A), suggesting that components of the diseased liver sections are acting as a stimulus for platelet activation. We also noted that there was a tendency for reduced platelet binding to acutely injured liver in patients with graft failure (regrafted tissue in Fig. 2B).
Fig. 1.
Resting platelets bind to vessels in human liver tissue sections. A and B: data from Stamper-Woodruff adhesion assays used to detect binding of exogenously added platelets to tissue. Adherent platelets were fluorescently labeled with antibody raised against CD41 to visualize binding. 2 representative fields of view captured using fluorescent microscopy with Axiovision software (Zeiss) are shown. Original magnification ×400. C: representative images from Stamper-Woodruff adhesion assays using normal and primary biliary cirrhosis (PBC) liver specimens. Binding of platelets was visualized by indirect immunohistochemical labeling of antibody raised against CD41 and diaminobenzidine substrate. Sections were counterstained with hematoxylin, and adherent platelets are stained brown. 2 representative fields of view from each donor captured using bright-field microscopy with Axiovision software (Zeiss) are shown. Original magnification ×400.
Fig. 2.
Adherent platelets localize to vascular structures in liver and bind to normal and diseased tissue. A: data from Stamper-Woodruff adhesion assays used to detect binding of exogenously added platelets to tissue. Adherent platelets were fluorescently labeled with antibody raised against CD41 to visualize binding. The figure shows representative fluorescent microscopic images from 3 experiments performed using indicated tissue samples from different donors. B: 6 random nonoverlapping views per sample centered on vascular structures at ×200 magnification were quantified using threshold analysis and calculation of % area occupied by platelets with ImageJ software (rsbweb.nih.gov/ij/). Data represent means ± SE % area coverage with platelets on indicated liver types per high-powered field (HPF). ANOVA indicated no significant difference in amount of platelet binding to normal and PBC tissue, whereas adhesion to regrafted tissue was significantly reduced (**P < 0.01 for both).
Adhesion of platelets to liver endothelial cells is integrin dependent.
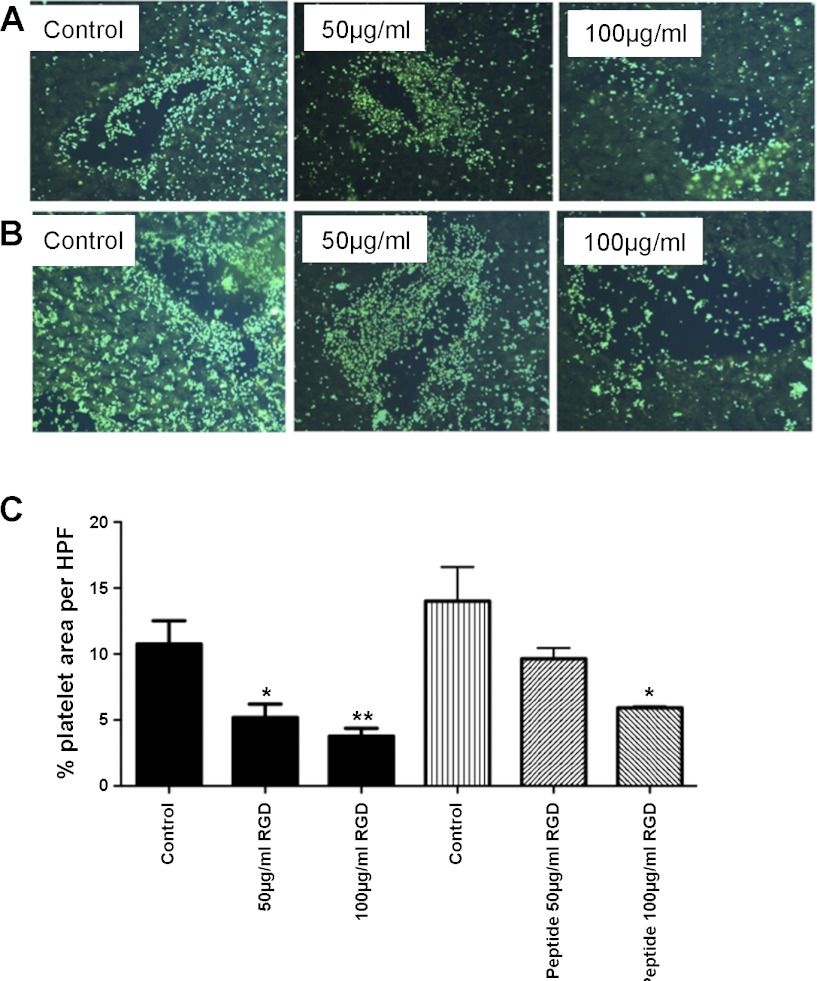
Incubation of platelets for 20 min with RGD-containing peptides resulted in a greater than 50% dose-dependent reduction in binding to both vascular and sinusoidal endothelium, demonstrating the involvement of integrin-mediated adhesion (Fig. 3, A and C). We next used specific monoclonal antibodies raised against either the CD41 α chain of the αIIbβ3 integrin or glycoprotein Ibα (GP1bα, CD42) in the tissue-binding assay. Antibody raised against CD41 had a small inhibitory effect on platelet binding that was less than that observed with RGD peptide; CD42 antibody had no detectable effect (not shown). Because platelets can bind intact endothelium via fibrinogen (44) on the endothelial surface (6), we tested the effect of a fibrinogen-binding inhibitory peptide in the tissue-binding assay. This peptide markedly reduced platelet binding, particularly to the vascular endothelium to a similar level as that seen with RGD (Fig. 3, B and C). A blocking antibody against integrin αvβ3, which is expressed by platelets and can bind fibrinogen (19), had no effect in the tissue-binding assay.
Fig. 3.
Platelet adhesion to liver tissue sections is integrin dependent and is inhibited by pretreatment with fibrinogen-binding peptide. A and B: representative fields of platelet binding to normal liver in the absence (control) or presence of indicated concentrations of either arg-gly-asp (RGD) peptide (A) or Fb-binding peptide (B). C: quantitative data generated using ImageJ to calculate % area of HPF occupied by platelets in 4 fields of view from 3 different normal liver samples. Data represent means ± SE % area coverage, and ANOVA indicated significant inhibition in the presence of RGD peptide and the highest dose of FB-binding peptide (*P < 0.05, **P < 0.01).
Thus the adhesion experiments using human liver tissue sections suggested that CD41 and fibrinogen were the main factors involved in the adhesion of resting platelets to hepatic endothelial cells. Because of the difficulty in quantifying adhesion to endothelium within tissue sections and the fact that adhesion in vivo occurs under conditions of blood flow, we used flow-based binding assays to isolated human hepatic endothelial cells to pursue these studies further.
Resting and activated platelets adhere to cultured human hepatic endothelial cells under static conditions.
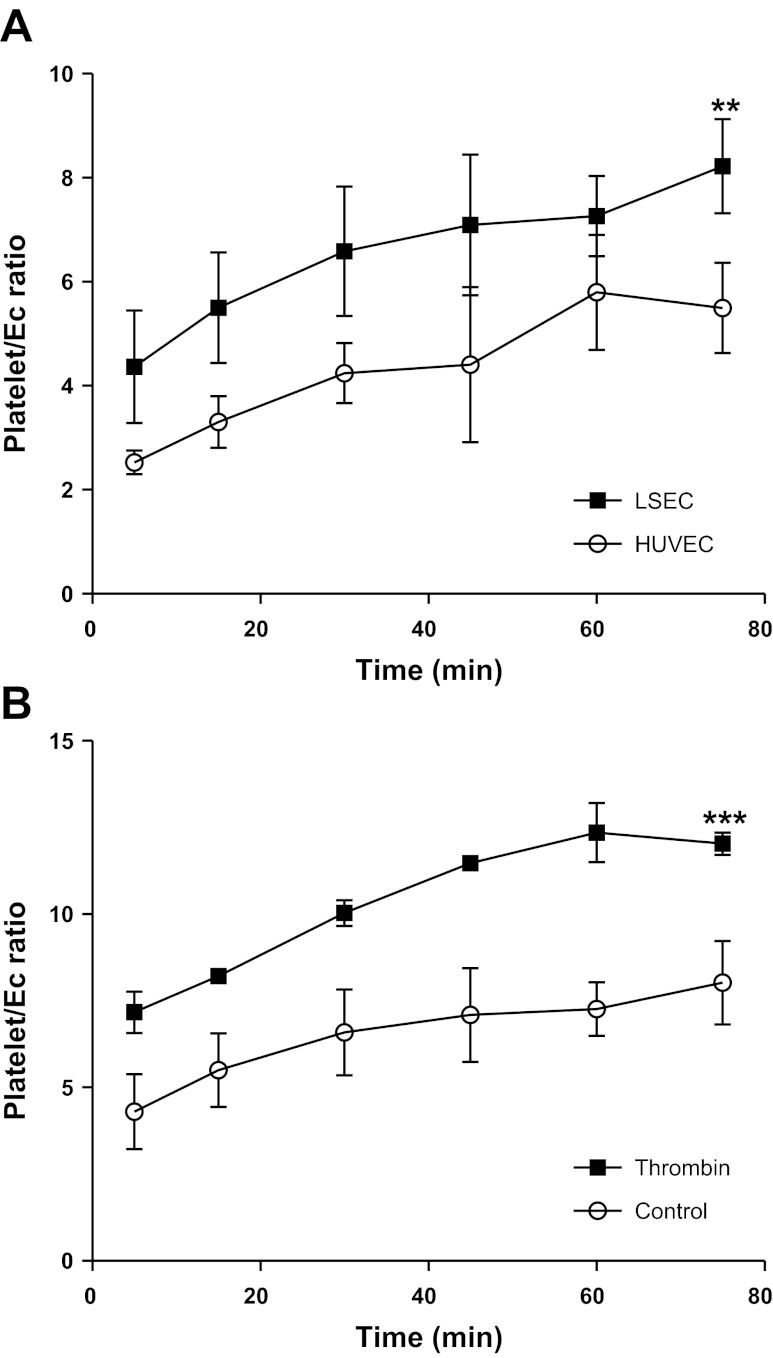
We compared the binding seen to HSEC with that observed with HUVEC, which are a frequently used as a model endothelium in experimental studies. Phase-contrast video microscopy was used to calculate the number of platelets binding to each endothelial cell (platelet/EC ratio). Figure 4A shows that platelet adhesion to both endothelial cell lines examined increased with time. Platelet binding was observed after 5-min coincubation with endothelial cells and doubled over the subsequent 75-min incubation. Human liver endothelial cells bound significantly more platelets at all time points studied per endothelial cell compared with HUVEC (Fig. 4). We then used thrombin to activate the platelets and found that this significant activation doubled the number of adherent platelets (Fig. 4B). However, it is likely that we underestimated the number of adherent platelets that bound following thrombin treatment because activation induced platelet aggregation, making the counting of individual platelets difficult.
Fig. 4.
Resting and activated platelets bind to isolated, cultured endothelial cells under static conditions. Platelet binding was assessed using phase-contrast microscopy. At least 10 fields of view were counted to calculate the average number of platelets binding per endothelial cell (Platelet/EC ratio). A: data generated experiments where resting platelets were allowed to bind to hepatic sinusoidal endothelial cells (HSEC) or human umbilical vein endothelial cells (HUVEC) for up to 75 min. ANOVA revealed significantly increased binding to HSEC vs. HUVEC (**P < 0.001). B: adhesion of both resting and thrombin activated (0.5 U/ml thrombin pretreatment) platelets to HSEC and ANOVA indicated a significant stimulatory effect (***P < 0.0001). All data are means ± SE of 3 replicate experiments.
Adhesion of platelets to endothelial cells under physiological shear stress.
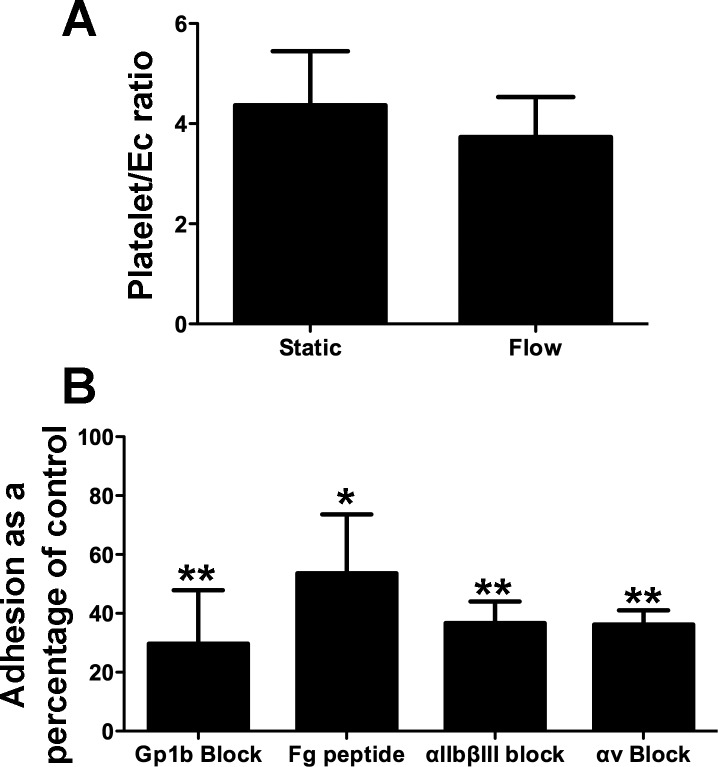
We next set up experiments to model platelet interactions with liver endothelium under conditions of shear stress. This is important because, in the static assays, platelets settled on endothelial cells in the absence of blood flow, and this may have permitted interactions, which would not occur under flow in vivo. We used flow rates that correspond to those observed in hepatic sinusoids and post capillary venules. When 1 × 108/ml platelets were perfused over HSEC at 0.1 Pa, binding events per endothelial cell were equivalent to those observed in static assays (Fig. 5A). Use of predetermined optimal concentrations of RGD peptide and blocking antibodies revealed that the adhesive event was mediated at least in part by the integrins αIIbβ3 and Gp1b (Fig. 5B). An important difference compared with the static assays was the role we found for the integrin αvβ3 under flow.
Fig. 5.
Integrin-dependent binding of platelets bind to resting endothelial cells under conditions of shear stress. Platelets were perfused over endothelial cells for 5 min at a wall shear stress of 0.1 Pa. Nonadherent platelets were then washed free of the system before assessment of the platelet/EC ratio as before. A: binding of resting platelets from matched donors under static or flow conditions. Data represent means ± SD of 2 replicate experiments with different platelet donors. B: binding of platelets per endothelial cell expressed as a percentage of control binding. Platelets were perfused over endothelial cells for 5 min at a wall shear stress of 0.1 Pa in the absence or presence of fibrinogen-binding peptide (100 μg/ml, Fg peptide) or blocking monoclonal antibodies raised against αIIbβ3 integrin (10 μg/ml), Gp1b (10 μg/ml), or αvβ3 integrin (av Block, 10 μg/ml). Data represent means ± SE of 4 replicate experiments. All reagents significantly inhibited adhesion compared with untreated controls (ANOVA, *P < 0.05 or **P < 0.01).
Binding of platelets to HSEC induces activation of NF-κB and release of CCL2 and CXCL8 and promotes adhesion of neutrophils and lymphocytes.
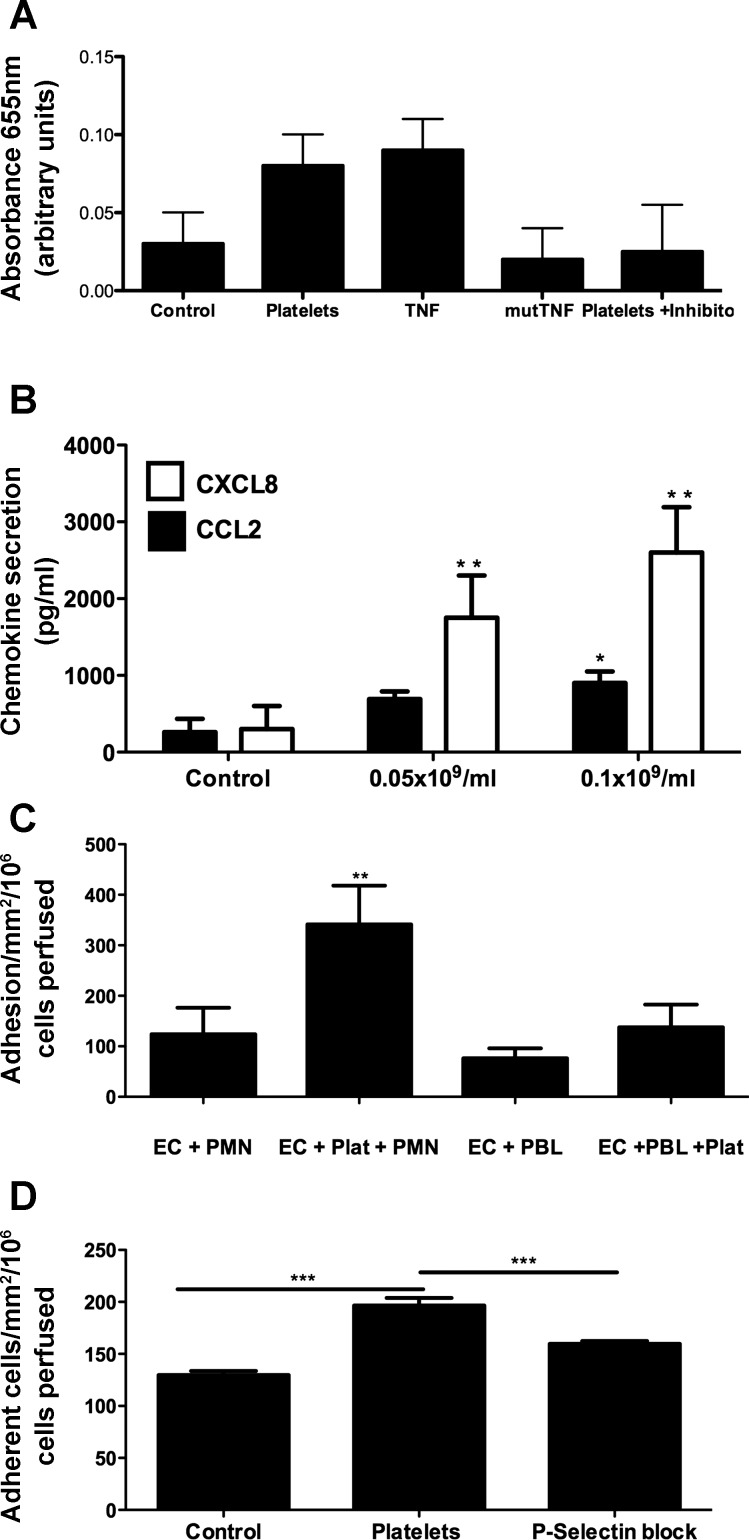
Finally, we sought to determine the proinflammatory consequences of platelet binding to HSEC. Figure 6A shows that brief interactions between platelets and HSEC from two independent donors are sufficient to cause activation of NF-κB and translocation of p50 to the endothelial cell nucleus, as detected by increased binding to target DNA. Signal was comparable to that generated following activation of cells with TNF-α, and specificity was confirmed by decreased signal in the presence of a competitive inhibitor of NF-κB binding and to a mutated consensus sequence. Endothelial activation resulted in an increase in secretion of CCL2 and CXCL8 in response to binding of platelets, which was dependent on the numbers of platelets applied (Fig. 6B). Finally, we used the flow-based adhesion assay to show that when platelets were prebound to HSEC monolayers, increased numbers of lymphocytes, and particularly neutrophils were recruited by the endothelial cells (Fig. 6C). Use of a selective inhibitor of P-selectin confirmed that, at least in part, the enhanced adhesion of neutrophils was dependent on P-selectin expression by the adherent platelets.
Fig. 6.
Binding of platelets to HSEC induces activation of NF-κB and release of CCL2 and CXCL8 and promotes P-selectin-dependent adhesion of neutrophils and lymphocytes. A: colorimetric quantification of NF-κB activation was performed using a commercially available ELISA (NF-κB p50 Transfactor Kit, Clontech) according to manufacturer's instructions. Nuclear extracts were generated from endothelial cells (2 independent donors) following stimulation with platelets (1 × 108/ml, 10 min) or TNF-α (10 ng/ml, 30 min) or unstimulated controls. Binding of NF-κB p50 to consensus or scrambled (mut) DNA was quantified. A competitive inhibitor supplied in the kit (platelets+inhibitor) was used to confirm specificity of signal. Data represent means ± SD absorbance at 655 nm. Statistical analysis was not performed, as samples from only 2 donors were tested. B: production of CXCL8 and CCL2 was determined using commercially available ELISA kits (R+D Systems HS800 and DCP00, respectively) according to manufacturer's instructions. Supernatants were collected from unstimulated EC or cells incubated with indicated numbers of platelets for 4 h. Data represent means ± SE chemokine production (pg/ml) in triplicate samples with 2 HSEC donors. *P < 0.05, **P < 0.01. C and D: platelets were perfused over endothelial cells for 5 min at a wall shear stress of 0.1 Pa. Nonadherent platelets were then washed free of the system before perfusion of 1 × 106/ml freshly isolated neutrophils (PMN) or peripheral blood lymphocytes (PBL) for 5 min (C). Leukocyte adhesion was counted and expressed as adherent cells/mm2/106 cells perfused on EC alone (EC+) or EC with preimmobilized platelets (EC+Plat). Data represent means ± SE from 4 replicate experiments with independent blood donors, and paired t-test indicated significant enhancement of neutrophil adhesion to HSEC in the presence of platelets **P < 0.01. D: binding of neutrophils to endothelium alone (control) endothelium and immobilized platelets (platelets) or endothelium and immobilized platelets pretreated with P-selectin inhibitor (P-selectin block, KF 38789, 10 μM). Paired t-test indicated significant enhancement of neutrophil adhesion to HSEC in the presence of platelets and significant inhibition by KF38789, ***P < 0.001 for both.
DISCUSSION
Platelets promote liver injury in models of hepatitis, and antiplatelet therapy is under investigation for treatment of chronic diseases such as nonalcoholic steatohepatitis (18). However, platelets also serve key hemostatic roles (51) and help neutrophils clear pathogens during sepsis (43). In addition, they modulate fibrogenesis (66) and secrete serotonin and other mediators that support liver regeneration (10, 39). Despite their important multiple roles in liver injury and inflammation, little is known about the mechanisms by which platelets bind to the hepatic vasculature.
Sinusoidal endothelial cells have a unique phenotype, characterized by a lack of Wiebel Palade bodies and consequent failure to express P-selectin and low levels of vWF even in response to inflammation (1, 20, 35, 68, 70) in their mature differentiated state. This combined with the relatively modest shear rate within the sinusoids provides a unique environment for platelet and leukocyte adhesion. Studies suggest that platelet activation is important for adhesion to both sinusoidal and postsinusoidal vessels and that platelet aggregation and perfusion failure can be improved by inhibiting Rho kinase (37). In most vascular beds expression of P-selectin is important for leukocyte adhesion, but the lack of P-selectin expression by hepatic sinusoids (68) means it is less likely to be important within the liver. Thus we used human hepatic sinusoidal endothelial cells and ex vivo human tissue to determine the molecular regulation of platelet:HSEC binding and the possible proinflammatory consequences of this interaction.
We found that resting platelets can bind to both vascular and sinusoidal vessels in normal liver tissue. We observed more binding to vascular endothelium in the portal tract compared with binding to sinusoids. Previous studies in liver ischemia/reperfusion (58, 59, 73) have reported platelet sequestration on periportal and midzonal sinusoidal endothelium (11, 57), but other studies in cholestatic liver injury report adhesion within both sinusoids and postsinusoidal vessels (37) consistent with the binding we observed to PBC tissue (Figs. 1 and 2). The absence of physiological shear stress in the tissue-binding experiments allows cells to settle on the vessels without the requirement for capture and removes the effect of shear stress on subsequent adhesive interactions with endothelium, leading us to study interactions using cultured human sinusoidal endothelial cells under physiological shear stress.
Resting platelets bound readily to primary hepatic sinusoidal endothelial cells under flow, confirming that platelets are able to bind to endothelium in the microvascular sinusoidal circulation despite low expression of some important adhesion receptors. The molecular basis of binding involves integrins because we could block it with RGD peptides or anti-integrin antibodies. RGD peptides have previously been shown to block platelet adhesion to human saphenous vein cells and HUVEC in vitro (6, 40, 53). However, in these investigations, either the platelets or the endothelial cells were activated before the adhesive event. To study interactions between resting platelets and endothelium, we used theophylline to maintain platelets in a quiescent state (28) and then used thrombin to study activated cells. Thrombin activation led to increased adhesion to endothelial cells and to a degree of platelet aggregation comparable to the aggregated platelet phenotype seen when platelets bound inflamed liver tissue. Activation of adherent platelets has also been observed in vivo following liver transplantation (52), suggesting that factors in inflamed liver can activate platelets upon contact (16). This may also happen in liver disease because, although circulating platelets in cirrhotic patients are functionally normal, serum from such patients contains elevated levels of vWF and promotes platelet binding to collagen (41). Our studies on liver tissue binding suggest that fibrinogen within the liver microenvironment may promote platelet activation and adhesion in situ. Fibrinogen has roles in both platelet adhesion and aggregation, and it is transiently released from activated platelets from where it can bind to the platelet surface via interactions with αIIbβ3 integrin (50, 67). Fibrinogen can also accumulate on the surface of endothelial cells following reperfusion injury where it can act as a focus for the adhesion of platelets (44), consistent with our findings that adhesion of platelets to vessels in liver sections was significantly reduced in the presence of a fibrinogen blocking peptide. The peptide also blocked adhesion under flow to HSEC but had little effect on binding to HUVEC. Endothelial cells within tissue will have been exposed to fibrinogen in circulating blood and exposure is increased during inflammation and liver disease (42, 64); thus fibrinogen may have been deposited on the hepatic endothelial cells in contrast to those cultured in normal serum in vitro.
Although the results of the static adhesion assays yield important insights into the mechanisms of platelet binding to endothelium, interactions in vivo occur under the constraints of blood flow, and we thus established a more physiological shear stress-dependent assay to model the in vivo environment and used it to demonstrate that resting platelets can indeed bind to unstimulated endothelium from flow. Approximately six platelets bound to each endothelial cell, and, although this appears to be a relatively low level of coverage, it has been demonstrated that coverage of less than 1% of the endothelial cell surface with adherent platelets significantly enhances neutrophil adhesion (28). Thus equivalent levels of platelet binding in the liver in vivo could precipitate leukocyte recruitment into grafted or injured tissue. Use of RGD peptide and blocking antibodies against the integrins Gp1b, αIIbβ3, and endothelial αvβ3 revealed that these receptors all contributed to platelet adhesion under shear stress. Platelet Gp1bα binds vWF and can operate at high levels of shear stress such as those found in larger arteries (54). In addition, signaling through this receptor can also activate platelet β3 integrins. The platelet receptor GpIb is composed of four different polypeptide chains (GpIbα, GpIbβ, GpIX, and GpV); GpIα contains the binding sites for vWF (54, 69), as does the integrin αIIbβ3 (21) although this interaction requires platelet activation. Thus the Gp1b-dependent platelet adhesion to HSEC was interesting in the context of low levels of vWF expression (21). The expression of vWF by HSEC increases as cells dedifferentiate in culture (35), and deposition on endothelial cells (5) may provide sufficient protein to support platelet interactions. However, the low shear stress used for our experiments and evidence that knockout mice deficient in both fibrinogen and vWF still demonstrate normal platelet deposition and thrombus formation in response to experimental vessel injury (47) suggest minimal contribution to initial adhesion. In addition, it has been demonstrated that some endothelial cells can express the GP1bα receptor (69), which can interact with activated platelets. It is possible, therefore, that unbound Gp1b antibody perfused with the platelet solution may interact with endothelial Gp1b.
We also demonstrated roles for the integrins αIIbβ3 and αVβ3 in platelet adhesion to hepatic endothelial cells under low shear conditions consistent with evidence from other microvascular beds (reviewed in Ref. 48). Previous studies using human saphenous vein endothelial cells have shown that thrombin-treated platelets use αIIbβ3 to interact with activated endothelial cells (40), but ours is the first demonstration of a role for this receptor in the adherence of platelets to unstimulated hepatic endothelia. αvβ3 integrin is expressed on platelets and endothelial cells (3, 15), where it mediates RGD-dependent adhesion to fibrinogen, fibronectin, vitronectin, and vWF (4). Our SAGE analysis and previous reports (7, 56) suggest that αvβ3 is expressed at low levels on sinusoidal endothelial cells, but it is clearly present at a sufficient concentration to support platelet adhesion in vitro.
Finally, we studied the functional consequences of platelet:HSEC binding for leukocyte recruitment by studying lymphocyte and neutrophil adhesion to platelet-bound endothelial cells under flow. We found that platelet binding triggered NF-κB-dependent secretion of the chemokines CXCL8 and CCL2, which could promote neutrophil and lymphocyte adhesion, respectively (22). Parallel hepatic NF-κB p50 and p65 activation occurs in liver injury (74), and sinusoidal endothelial cell p65 nuclear translocation leads to chemokine secretion (75). Our enhanced activation of NF-κB p50 in nuclear extracts from HSEC stimulated by platelet binding supports the role of this transcription factor in sinusoidal chemokine release and inflammation. Hence, platelet binding enhanced leukocyte adhesion to unstimulated hepatic sinusoidal endothelium, which express basal levels of VCAM-1 and ICAM-1 but no P-selectin in culture (Table 2). Our inhibitor data supports the idea that adherent platelets may provide a P-selectin bridge for leukocyte adhesion (11, 52, 72) although other adhesion receptors likely also play a role. Release of chemokines by activated endothelium or presentation of platelet-derived chemokine then permits immune cell integrin activation and binding to VCAM-1 and ICAM-1.
In conclusion, we have demonstrated that resting platelets can adhere to human hepatic endothelial cells using the integrins αIIbβ3 and αVβ3 under physiological shear stress. Our findings explain how platelets bind to hepatic vessels and suggest that such interactions promote immune cell recruitment by triggering chemokine secretion and providing an adhesive substrate for circulating leukocytes. These findings provide further evidence that antiplatelet therapy might modify liver injury in inflammatory liver disease (18, 61).
GRANTS
Work in our laboratory is funded by grants from The Medical Research Council and The Wellcome Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.F.L. and D.H.A. conception and design of research; P.F.L. and J.M.H. performed experiments; P.F.L. and J.M.H. analyzed data; P.F.L., J.M.H., and R.B. interpreted results of experiments; P.F.L. prepared figures; P.F.L. drafted manuscript; P.F.L. and D.H.A. edited and revised manuscript; P.F.L., J.M.H., R.B., and D.H.A. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to our surgical colleagues and patients at the Queen Elizabeth Hospital in Birmingham for providing access to their tissue samples.
REFERENCES
- 1. Adams DH, Hubscher SG, Fisher NC, Williams A, Robinson M. Expression of E-selectin and E-selectin ligands in human liver inflammation. Hepatology 24: 533–538, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6: 205–217, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Beacham DA, Tran LP, Shapiro SS. Cytokine treatment of endothelial cells increases glycoprotein Ib alpha-dependent adhesion to von Willebrand factor. Blood 89: 4071–4077, 1997 [PubMed] [Google Scholar]
- 4. Bennett JS, Chan C, Vilaire G, Mousa SA, DeGrado WF. Agonist activated àvá3 on platelets and lymphocytes binds to the matrix protein osteopontin. J Biol Chem 272: 8137–8140, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Boerma MKJ, van Loenen M, Klein HR, Bart CI, Zurcher C, Wondergem J. Increased deposition of von Willebrand factor in the rat heart after local ionizing irradiation. Strahlenther Onkol 180: 8, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bombeli T, Schwartz B, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GpIIbIIIa-dependent bridging mechanism and novel roles for endothelial ICAM-1,àvá3 integrin and Gp1bà. J Exp Med 187: 329–339, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burt AD, Le Bail B, Balabaud C, Bioulac-Sage P. Morphologic investigation of sinusoidal cells. Semin Liver Dis 13: 21–38, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 190: 255–266, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol Gastrointest Liver Physiol 272: G1195–G1200, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Clavien PA. Liver regeneration: a spotlight on the novel role of platelets and serotonin. Swiss Med Wkly 138: 361–370, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Cywes R, Mullen JB, Stratis MA, Greig PD, Levy GA, Harvey PR, Strasberg SM. Prediction of the outcome of transplantation in man by platelet adherence in donor liver allografts. Evidence of the importance of prepreservation injury. Transplantation 56: 316–323, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Cywes R, Mullen JB, Stratis MA, Greig PD, Levy GA, Harvey PR, Strasberg SM. Prediction of the outcome of transplantation in man by platelet adherence in donor liver allografts. Evidence of the importance of prepreservation injury. Transplantation 56: 316–323, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Day CP. Who gets alcoholic liver disease: nature or nurture? J R Coll Physicians Lond 34: 557–562, 2000 [PMC free article] [PubMed] [Google Scholar]
- 14. Diacovo TG, Catalina MD, Siegelman MH, von Andrian UH. Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J Exp Med 187: 197–204, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eliceiri BP, Cheresh DA. The role of àv integrins during angiogenesis : insights into potential mechanisms of action and clinical development. J Clin Invest 103: 1227–1230, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Etingin OR, Silverstein RL, Hajjar DP. Von Willebrand factor mediated platelet adhesion to virally infected endothelial cells. Proc Natl Acad Sci USA 90: 5153–5156, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, Mendenhall CL, The VACG. Pathology of alcoholic liver disease. Semin Liver Dis 13: 154–169, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Fujita K, Nozaki Y, Wada K, Yoneda M, Endo H, Takahashi H, Iwasaki T, Inamori M, Abe Y, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nagashima Y, Nakajima A. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut 57: 1583–1591, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Gawaz M, Neumann FJ, Dickfield T, Reininger A, Adelsberg H, Gebhardt A, Schomig A. Vitronectin receptor (avb3) mediates platelet adhesion to the lumenal aspect of endothelial cells. Implications for reperfusion in acute myocardial infarction. Circulation 96: 1809–1818, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Gerlach JC, Zeilinger K, Spatkowski G, Hentschel F, Schnoy N, Kolbeck S, Schindler RK, Neuhaus P. Large-scale isolation of sinusoidal endothelial cells from pig and human liver. J Surg Res 100: 39–45, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Goto S, Salomon DR, Ikeda Y, Ruggieri ZM. Characterisation of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J Biol Chem 270: 23352–23361, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391: 591–594, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Herbert JM, Stekel D, Sanderson S, Heath VL, Bicknell R. A novel method of differential gene expression analysis using multiple cDNA libraries applied to the identification of tumour endothelial genes. BMC Genomics 9: 153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hubscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology 49: 450–465, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol Gastrointest Liver Physiol 260: G355–G362, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52: 2745–2756, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenne CN, Wong CH, Petri B, Kubes P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS One 6: e25109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirton CM, Nash GB. Activated platelets adherent to an intact endothelial cell monolayer bind flowing neutrophils and enable them to transfer to the endothelial surface. J Lab Clin Med 136: 303–313, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Lal A, Lash AE, Altschul SF, Velculescu V, Zhang L, McLendon RE, Marra MA, Prange C, Morin PJ, Polyak K, Papadopoulos N, Vogelstein B, Kinzler KW, Strausberg RL, Riggins GJ. A public database for gene expression in human cancers. Cancer Res 59: 5403–5407, 1999 [PubMed] [Google Scholar]
- 30. Lalor P, Nash GB. Adhesion of flowing leucocytes to immobilized platelets. Br J Haematol 89: 725–732, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Lalor PF, Adams DH. Adhesion of leucocytes to sinusoidal endothelial cells under conditions of shear stress. In: Cells of the Hepatic Sinusoid, edited by Wisse E, Knook DL, deZanger R, Arthur MJP. Leiden, The Netherlands: The Kupffer Cell Foundation, 2001, p. 99–101 [Google Scholar]
- 32. Lalor PF, Adams DH. Lymphocyte homing to allografts. Transplantation 70: 1131–1139, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Lalor PF, Curbishley SM, Adams DH. Identifying homing interactions in T-cell traffic in human disease. Methods Mol Biol 616: 231–252, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol 169: 983–992, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Lalor PF, Lai WK, Curbishley SM, Shetty S, Adams DH. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J Gastroenterol 12: 5429–5439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lalor PF, Sun PJ, Weston CJ, Martin-Santos A, Wakelam MJ, Adams DH. Activation of vascular adhesion protein-1 on liver endothelium results in an NF-kappaB-dependent increase in lymphocyte adhesion. Hepatology 45: 465–474, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Laschke MW, Dold S, Menger MD, Jeppsson B, Thorlacius H. The Rho-kinase inhibitor Y-27632 inhibits cholestasis-induced platelet interactions in the hepatic microcirculation. Microvasc Res 78: 95–99, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Lash AE, Tolstoshev CM, Wagner L, Schuler GD, Strausberg RL, Riggins GJ, Altschul SF. SAGEmap: a public gene expression resource. Genome Res 10: 1051–1060, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Li JM, Podolsky RS, Rohrer MJ, Cutler BS, Massie MT, Barnard MR, Michelson AD. Adhesion of activated platelets to venous endothelial cells is mediated via GpIIbIIIa. J Surg Res 61: 543–548, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 44: 53–61, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Luyendyk JP, Mackman N, Sullivan BP. Role of fibrinogen and protease-activated receptors in acute xenobiotic-induced cholestatic liver injury. Toxicol Sci 119: 233–243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost 6: 415–420, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Massberg S, Enders G, Matos FC, Tomic LI, Leiderer R, Eisenmenger S, Messmer K, Krombach F. Fibrinogen deposition at the postischaemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood 94: 3829–3838, 2002 [PubMed] [Google Scholar]
- 45. NCBI Gene Expression Omnibus Database (GEO). http://www.ncbi.nlm.nih.gov/projects/geo/
- 46. NCBI SAGE genet to tag mapping ftp site. ftp://ftp.ncbi.nlm.nih.gov/pub/sage/mappings
- 47. Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest 106: 385–392, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ni H, Freedman J. Platelets in hemostasis and thrombosis: role of integrins and their ligands. Transfus Apher Sci 28: 257–264, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Pak S, Kondo T, Nakano Y, Murata S, Fukunaga K, Oda T, Sasaki R, Ohkohchi N. Platelet adhesion in the sinusoid caused hepatic injury by neutrophils after hepatic ischemia reperfusion. Platelets 21: 282–288, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Peerschke EI. Adhesive protein expression on thrombin-stimulated platelets ; time-dependent modulation of anti-fibrinogen, -fibronectin, and -von Willebrand factor antibody binding. Blood 79: 948–953, 1992 [PubMed] [Google Scholar]
- 51. Pereboom IT, Lisman T, Porte RJ. Platelets in liver transplantation: friend or foe? Liver Transpl 14: 923–931, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Porte RJ, Blauw E, Knot E, de Maat MP, de Ruiter C, Minke Baaker C, Terpstra OT. Role of the donor liver in the origin of platelet disorders and hyperfibrinolysis in liver transplantation. J Hepatol 21: 592–600, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Reininger AJ, Agneskirchner J, Bode PA, Spannagl M, Wurtzinger LJ. C7E3 fab inhibits low shear flow modulated platelet adhesion to endothelium and surface adsorbed fibrinogen by blocking platelet GpIIbIIIa as well as endothelial vitronectin receptor. Thromb Haemost 83: 217–223, 2000 [PubMed] [Google Scholar]
- 54. Ruggieri ZM. Old concepts and new developments in the study of platelet aggregation. J Clin Invest 105: 699–701, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saha S, Sparks AB, Rago C, Akmaev V, Wang CJ, Vogelstein B, Kinzler KW, Velculescu VE. Using the transcriptome to annotate the genome. Nat Biotechnol 20: 508–512, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Scoazec JY, Feldmann G. The cell adhesion molecules of hepatic sinusoidal endothelial cells. J Hepatol 20: 296–300, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Shi J, Kokubo Y, Wake K. Expression of P-Selectin on hepatic endothelia and platelets promoting neutrophil removal by liver macrophages. Blood 92: 520–528, 1998 [PubMed] [Google Scholar]
- 58. Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Platelets inducer sinusoidal endothelial cell apoptosis upon reperfusion of the cold ischemic rat liver. Gastroenterology 118: 183–191, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Synergism betewwn platelets and leukocytes in inducing endothelial cell apoptosis in the cold ischemic rat liver: a Kupffer cell mediated injury. FASEB J 15: 1230–1232, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Stamper HB, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high endothelial venules. J Exp Med 144: 828–833, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sullivan BP, Wang R, Tawfik O, Luyendyk JP. Protective and damaging effects of platelets in acute cholestatic liver injury revealed by depletion and inhibition strategies. Toxicol Sci 115: 286–294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tabuchi A, Kuebler WM. Endothelium-platelet interactions in inflammatory lung disease. Vascul Pharmacol 49: 141–150, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Takasaki S, Hano H. Three-dimensional observations of the human hepatic artery (Arterial system in the liver). J Hepatol 34: 455–466, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med 22: 1354–1358, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Velculescu VE, Zhang L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science 270: 484–487, 1995 [DOI] [PubMed] [Google Scholar]
- 66. Watanabe M, Murata S, Hashimoto I, Nakano Y, Ikeda O, Aoyagi Y, Matsuo R, Fukunaga K, Yasue H, Ohkohchi N. Platelets contribute to the reduction of liver fibrosis in mice. J Gastroenterol Hepatol 24: 78–89, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest 100: 2085–2093, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest 99: 2782–2790, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu G, Essex DW, Meloni F, Takafuta T, Fujimura K, Konkle BA, Shapiro S. Human endothelial cells in culture and in vivo express on their surface all four components of the glycoprotein Ib/IX/V complex. Blood 90: 2660–2669, 1997 [PubMed] [Google Scholar]
- 70. Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, Hultenby K, Christensson B, Ericzon BG, Holgersson J, Sumitran-Holgersson S. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol 163: 1275–1289, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xu XD, Ueta H, Zhou S, Shi C, Koga D, Ushiki T, Matsuno K. Trafficking of recirculating lymphocytes in the rat liver: rapid transmigration into the portal area and then to the hepatic lymph. Liver Int 28: 319–330, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Yadav S, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischaemic mouse liver. Hepatology 29: 1494–1502, 1999 [DOI] [PubMed] [Google Scholar]
- 73. Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology 29: 1494–1502, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.