Abstract
Unilateral ureteral obstruction (UUO), a widely used model of chronic kidney disease and congenital obstructive uropathy, causes proximal tubular injury and formation of atubular glomeruli. Because transforming growth factor-β1 (TGF-β1) is a central regulator of renal injury, neonatal and adult mice were subjected to complete UUO while under general anesthesia and treated with vehicle or ALK5 TGF-β1 receptor inhibitor (IN-1130, 30 mg·kg−1·day−1). After 14 days, glomerulotubular integrity and proximal tubular mass were determined by morphometry of Lotus tetragonolobus lectin distribution, and the fraction of atubular glomeruli was determined by serial section analysis of randomly selected individual glomeruli. Glomerular area, macrophage infiltration, fibronectin distribution, and interstitial collagen were measured by morphometry. Compared with placebo, inhibition of TGF-β1 by IN-1130 decreased apoptosis and formation of atubular glomeruli, prevented parenchymal loss, increased glomerular area and glomerulotubular integrity, and increased proximal tubule fraction of the adult obstructed kidney parenchyma from 17 to 30% (P < 0.05, respectively). IN-1130 decreased macrophage infiltration and fibronectin and collagen deposition in the adult obstructed kidney by ∼50% (P < 0.05, respectively). In contrast to these salutary effects in the adult, IN-1130 caused widespread necrosis in obstructed neonatal kidneys. We conclude that whereas IN-1130 reduces obstructive injury in adult kidneys through preservation of glomerulotubular integrity and proximal tubular mass, TGF-β1 inhibition aggravates obstructive injury in neonates. These results indicate that while caution is necessary in treating congenital uropathies, ALK5 inhibitors may prevent nephron loss due to adult kidney disease.
Keywords: proximal tubule, apoptosis, fibrosis, maturation, ureteral obstruction
chronic kidney disease is characterized by progressive renal parenchymal loss and interstitial fibrosis. Murine unilateral ureteral obstruction (UUO) is the most widely used animal model of renal disease, and renal fibrosis is typically utilized as a major end point when measuring the role of novel therapies or molecular pathways in genetically engineered animals (9). In contrast to chronic kidney disease in the adult human, in which hypertension and diabetes comprise the major etiologies, congenital abnormalities of the kidneys and urinary tract account for most renal failure in children, with obstructive nephropathy being the most frequent cause (11). In the past decade, models of UUO in fetal and neonatal animals have provided significant insight into the unique responses of the developing kidney to urinary tract obstruction (11).
Current therapies for slowing the progression of kidney disease are limited almost entirely to the treatment of hypertension and inhibition of the action of angiotensin II (38). Even with the most aggressive regimes and optimal patient adherence to treatment protocols, these approaches provide only partial protection from progressive nephron loss. Moreover, given the dependence of the developing and maturing kidney on an intact renin-angiotensin system, the administration of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers can actually aggravate progressive renal injury resulting from UUO in the neonate (6, 14).
We have recently adopted a new approach to the model of UUO in the mouse, shifting attention from the renal interstitium to the glomerulus and tubule. It appears that the magnitude of renal interstitial fibrosis resulting from UUO is largely overshadowed by proximal tubular injury, which leads to epithelial cell death and collapse of the glomerulotubular junction, with subsequent formation of atubular glomeruli (16, 17). A review of the literature reveals that atubular glomeruli are formed in a wide variety of renal disorders, including renal artery stenosis and renal allograft rejection, as well as diabetic, IgA, and membranous nephropathies (8).
While angiotensin serves as a primary target of progression of kidney disease, transforming growth factor-β1 (TGF-β1) is a major downstream mediator of renal injury stimulated by endogenous angiotensin II (38). A number of studies of UUO in mutant adult mice point to TGF-β as a key regulator of progressive pathologic changes (1). It is estimated that ∼10% of all genes undergo rapid changes in expression levels within only 4 h of TGF-β treatment (45). A new class of TGF-β1 receptor kinase (ALK5) inhibitors has been developed, which are highly specific and effective in suppressing renal TGF-β1 and Smad2/3 phosphorylation following 7 or 14 days of UUO in the rat (36).
TGF-β1 is a central regulator of renal tubular cell death as well as collagen deposition following UUO. We therefore decided to examine the role of inhibiting endogenous TGF-β1 through the administration of IN-1130 in the model of UUO in the mouse, comparing its effects on glomerular and proximal tubular injury as well as on interstitial collagen deposition. In addition, because of the unique responses of the developing kidney to obstructive injury, adult animals were compared with neonates. The results have potential implications for the investigation and treatment of progressive kidney disease in both early and adult life.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were maintained in standard breeding cages with the approval of the Animal Care and Use Committee of the University of Virginia. Neonates were kept with their mothers throughout the 21-day study. Animals were subjected to complete UUO within the first 36 h of life under general anesthesia with isoflurane and oxygen (5). Adult mice (6–8 wk of age) also underwent UUO and were studied at the same intervals following operation. Males and females were included in the study of neonates, while only males were used for studies of adults.
TGF-β1 receptor inhibition.
Based on previous studies of the use of IN-1130 in mice, a dose of 30 mg·kg−1·day−1 was selected (24, 32). Pharmacokinetic studies revealed that administration of IN-1130 increased plasma concentration for 60 min (area under the curve, intravenous: 1701 μg·min−1·ml−1 and oral: 152 μg·min−1·ml−1) (24). Concentrations of IN-1130 in kidney were 4 μg/g at 15 min, 3 μg/g at 30 min, and 0.7 μg/g at 60 min; tissue-to-plasma ratios in kidney were 1.5 at 30 min and 1.7 at 120 min. Based on these data, time release pellets (Innovative Research of America, Sarasota, FL), designed to release 30 mg·kg−1·day−1 IN-1130, were inserted into the peritoneum at the time of performing UUO. Pilot studies were performed to determine potential toxicity of this dose of IN-1130 in three mice 1–2 days of age, as described previously for neonatal rats (44). Two mice died within a week, and at the time of harvest 7 days after surgery, heart and lungs were found to be hypoplastic in the surviving animal. In a subsequent study, six neonatal mice underwent UUO and placement of intraperitoneal time release pellets at 7 days of age, with harvest of kidneys 7 days later. In three mice, pellets contained vehicle, and in the other three animals, pellets provided a continuous release of IN-1130 at 30 mg·kg−1·day−1. All animals survived to the end of the study, with no evidence of cardiac or pulmonary abnormalities. This was followed by an additional study of two groups of six neonatal mice that underwent UUO at 7 days, with one group receiving placebo and the other IN-1130 for the following 14 days. As in the prior study, all animals survived, with no evidence of cardiac or pulmonary abnormalities.
Twenty-one neonatal mice were subjected to UUO at 7 days of age and were studied 14 days later. Eleven mice received pellets containing vehicle, while the remaining 10 mice received pellets designed to release IN-1130 at a rate of 30 mg·kg−1·day−1 (36). Twenty adult mice were subjected to identical procedures, treated either with vehicle (10 animals) or IN-1130 (10 animals) by insertion of intraperitoneal pellets at the time of UUO, and studied 14 days later.
Tissue processing.
Renal tissue was harvested and fixed in 10% neutral buffered formalin, dehydrated and infiltrated with paraffin, and sectioned at 4-μm thickness. General structure was demonstrated by staining with the Masson trichrome procedure (HT-15 kit; Sigma-Aldrich, St. Louis MO). Glomerular area was determined in kidney sections stained with periodic acid-Schiff (PAS). Fragmented DNA was detected using Apoptag (Chemicon, Temecula, CA) with DAB development and methylene blue counterstaining, based on the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end labeling reaction (TUNEL; Ref. 19). Apoptotic and necrotic cells were both identified by TUNEL staining and were distinguished by their morphologic features. For immunohistochemistry, sections were pretreated with H2O2 in methanol and with avidin-biotin blocking kit (Vector Laboratories, Burlingame, CA). Monocytes and macrophages were identified by F4/80 immunostaining (no. ab6640; Abcam, Cambridge MA), and immunoreactive fibronectin was demonstrated by Abcam (no. ab6328). Collagen deposition was localized by picrosirius red (Polysciences, Warrington PA), which binds to types I, III, and IV collagen (21). Image analysis was used to quantitate glomerular area, proximal tubular area, and Sirius-positive area (ImagePro Plus 5.1; Media Cybernetics, Silver Spring, MD).
Morphometric determination of glomerular, tubular, and interstitial injury.
Glomerular area was determined in PAS-stained sections by calculating the mean of 50 glomeruli per section, identified in nonoverlapping fields at ×400 magnification. The number of apoptotic nuclei per field was counted in 25 high-power fields (×1,000) using TUNEL-stained slides. Apoptotic bodies were identified by their staining positivity with TUNEL and their morphological characteristics, including cell shrinkage and nuclear condensation. The fraction of F4/80, fibronectin, and Sirius-positive parenchyma was determined in 10 random fields alternating between cortical and medullary zones.
Identification and quantitation of normal glomerulotubular junctions and functional tubular mass.
Lotus tetragonolobus lectin (Vector Laboratories, Burlingame, CA) binds to mature proximal tubule epithelial cells in mouse kidney (5); kidney sections underwent proteinase K enzymatic digestion before exposure to biotinylated Lotus lectin (1:50) and development by the ABC-DAB regimen (Vector Laboratories Elite Standard ABC kit; liquid DAB substrate kit; Biogenex, San Ramon, CA). As recently reported (17), Lotus lectin staining can provide an index of proximal tubular mass. Accordingly, digital morphometry was used to measure the Lotus-positive fraction of renal parenchyma in kidneys from each group of mice. In addition, Lotus-stained material was utilized for clear identification of proximal tubules in cross section to determine average diameters; 50 such tubules were measured in each obstructed kidney of the adult animals subjected to 14 days of obstruction. Tubules were selected for measurement on the basis of lectin positivity and circular profile.
In addition to binding to proximal tubular cells in the mouse, Lotus tetragonolobus also binds to tall parietal epithelial cells surrounding the urinary pole of Bowman's capsule in this species (16). These Lotus lectin-positive cells, identical in histological and histochemical properties to proximal tubule cells, are normally found in ∼80% of adult male mouse glomeruli (16). However, following 14 days of ipsilateral UUO, the fraction of Lotus-positive glomeruli decreases to 20%, reflecting rapid progressive cell death at the glomerulotubular junction, which leads to separation of the glomerulus from the proximal tubule and the formation of atubular glomeruli (confirmed by serial section analysis; Ref. 16). Thus the fraction of Lotus-positive glomeruli in the adult mouse serves as a measure of the relative number of intact glomerulotubular junctions and integrity of the proximal tubular cells in this region. In the postnatal period, the fraction of Lotus-positive glomeruli increases steadily from 25% at 2 wk of age to 75% by 6 wk, reflecting glomerulotubular maturation from birth through adulthood (Forbes MS, unpublished observations). Measurement of the fraction of Lotus-positive glomeruli therefore can provide an index of maturation as well as of obstructive injury.
To validate the use of Lotus-positive glomeruli as an index of the fraction of normal glomerulotubular junctions, serial sections of 11 randomly selected glomeruli from the obstructed kidney of a placebo-treated adult mouse and 20 glomeruli from an IN-1130-treated mouse were stained with PAS-hematoxylin and examined. This procedure revealed the proportion of glomeruli with mild or severe atrophy of the glomerulotubular junction, as well as atubular glomeruli.
Statistical analysis.
Results were statistically analyzed for obstructed and contralateral kidneys using two-way ANOVA with age and IN-1130 treatment as variables followed by pairwise multiple comparison (Holm-Sidak method). Comparisons between left and right kidneys were performed using paired t-test. Data are summarized as means ± SE, and statistical significance was defined as P < 0.05.
RESULTS
Effects of TGF-β receptor inhibition on body weight and renal parenchymal mass following UUO.
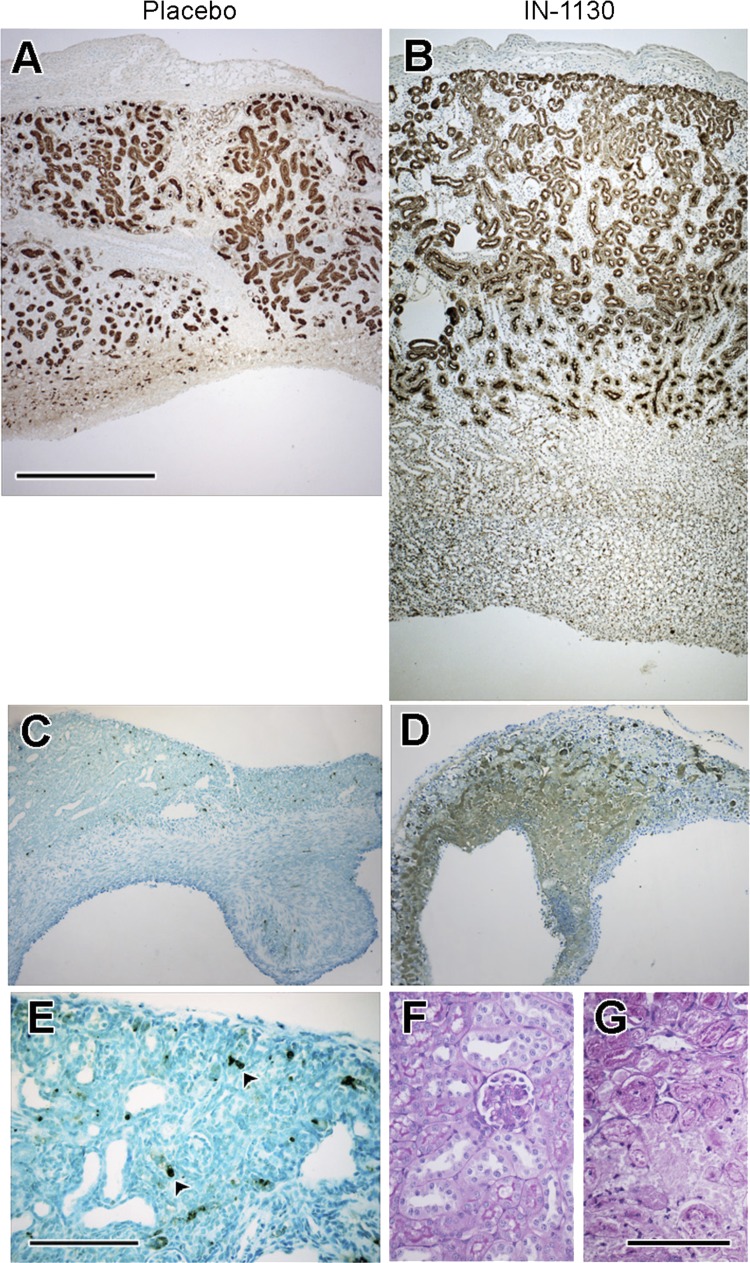
Body weight of placebo-treated adult mice (21.3 ± 0.4 g) was nearly threefold greater than that of neonatal animals (7.9 ± 0.4 g, P < 0.01). There was no effect of IN-1130 treatment on body weight of adult (21.8 ± 0.3 g) or neonatal mice (7.4 ± 0.4 g). Kidney weight was 2.5-fold greater in contralateral kidneys of adults compared with neonates, and there was no effect of IN-1130 on weight of the contralateral kidney regardless of age (Fig. 1A). Chronic UUO impaired growth of the obstructed neonatal kidney, and there was no significant effect of IN-1130 in neonates. However, while there was a 30% reduction in mass of the obstructed kidney in adult control mice, IN-1130 completely prevented loss of renal mass in adults (Fig. 1A). As shown in Fig. 2, A and B, IN-1130 administration resulted in the retention of greater parenchymal thickness of the adult obstructed kidney. In contrast, in the neonate, 4 of 10 obstructed kidneys of mice receiving IN-1130 developed large areas of necrotic parenchyma as revealed by TUNEL staining (Fig. 2, D and G), which damage was not observed in any of the placebo-treated animals (Fig. 2, C and E) or contralateral kidneys (Fig. 2F).
Fig. 1.
Effects of transforming growth factor-β1 (TGF-β1) receptor inhibition (administration of IN-1130 vs. placebo) and age (neonate vs. adult) on the renal response to unilateral ureteral obstruction (UUO). A: kidney weight. B: glomerular area. C: fractional proximal tubular area (Lotus lectin-positive fraction of renal parenchyma). D: fraction of apoptotic cells [terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end labeling reaction(TUNEL)-positive nuclei]. E: fraction of mature, intact glomerulotubular junctions (Lotus lectin-positive parietal Bowman's capsular epithelial cells). F: fraction distribution of F4/80-positive cells (macrophages). G: fractional distribution of fibronectin. H: fractional distribution of collagen (Sirius-staining parenchymal area). Black bars: obstructed kidney means ± SE; white bars: contralateral kidney. Statistical comparisons: two-way ANOVA for obstructed kidney (IN-1130 and age, variables): §IN-1130: significant effect of IN-1130; §age: significant effect of age; §IN-1130 * age: significant interaction of IN-1130 and age; pairwise multiple comparison (Holm-Sidak): *P < 0.05 vs. adult placebo. Two-way ANOVA for contralateral kidney: in A, B, C, and E, there was a significant effect of age; in D, F, G, and H, there was no effect of IN-1130 or age. Paired t-test, obstructed vs. contralateral kidney, all parameters were significantly different for all groups except contralateral kidney weight, adult IN-1130, which were not different (A).
Fig. 2.
Effects of TGF-β1 receptor inhibition (administration of IN-1130 vs. placebo) on renal parenchyma of the obstructed adult (A and B) and neonatal (C–G) kidney. A. Lotus lectin-stained adult placebo-treated kidney with parenchymal thinning and reduced proximal tubular mass. B: Lotus lectin-stained adult IN-1130-treated kidney shows superior preservation of parenchyma and proximal tubular mass. C: TUNEL-stained neonatal placebo-treated kidney with scattered apoptotic TUNEL-positive nuclei. D: TUNEL-stained neonatal IN-1130-treated kidney with widespread cell death throughout the medulla. E: higher magnification of neonatal placebo-treated kidney shown in C, apoptotic nuclei indicated by arrowheads. F and G: periodic acid-Schiff (PAS)-stained neonatal kidneys. F: contralateral unobstructed kidney demonstrates normal morphology with blue-staining tubular nuclei. G: obstructed kidney reveals collapsed tubules lacking distinct nuclei, characteristic of necrotic degeneration. Bar in A = 500 μm and applies to A–D; bar in E = 100 μm; bar in G = 100 μm and applies to F and G.
Effects of TGF-β receptor inhibition on glomerular and proximal tubular mass following UUO.
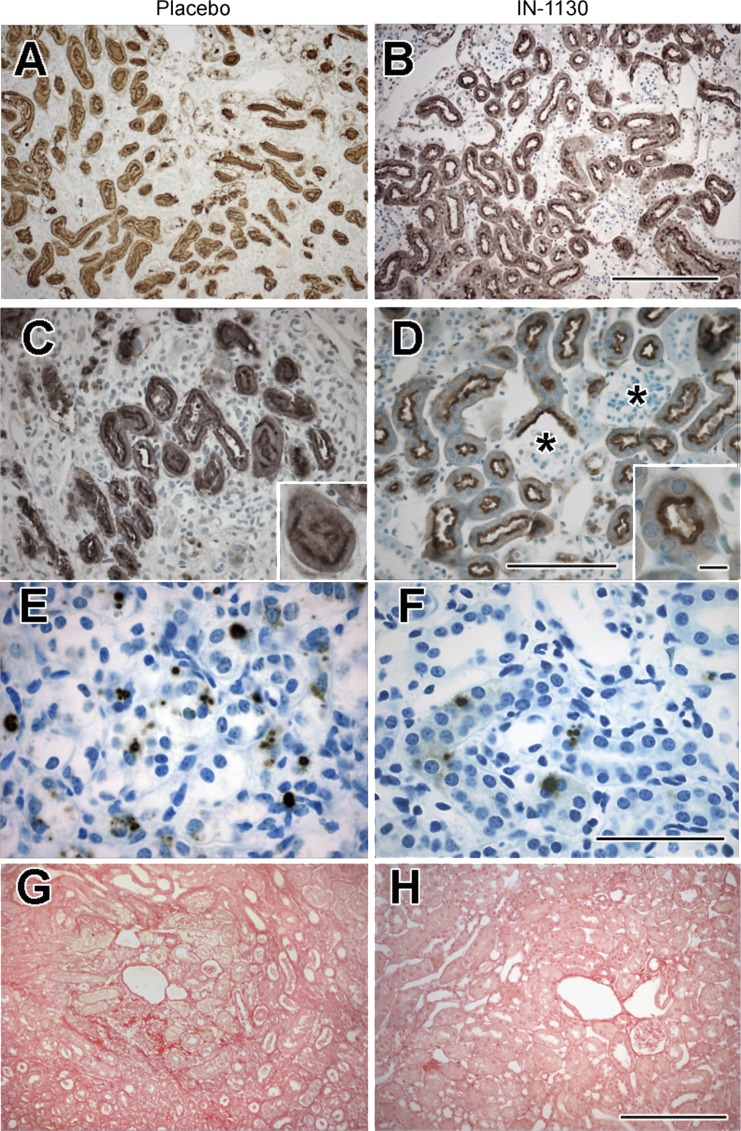
Glomerular cross-sectional area of contralateral kidneys doubled with age and was not affected by IN-1130, while glomerular area of obstructed neonatal kidneys was reduced by 45% despite treatment with IN-1130 (Fig. 1B). In contrast, the glomerular area of adult obstructed kidneys was 26% greater in mice treated with IN-1130, although remaining significantly lower than that of contralateral kidneys (Fig. 1B). Glomeruli in placebo-treated adult-obstructed kidneys appeared more crowded as well as being smaller than those of mice treated with IN-1130 (Fig. 3, A and C; B and D, respectively). Proximal tubular integrity was better preserved in the obstructed kidney of drug-treated adult mice (Figs. 2, A and B, and 3, A–D). Proximal tubular diameter in adult obstructed kidneys was 27.1 ± 1.0 μm for placebo-treated mice, and 30.1 ± 1.5 μm for IN-1130-treated animals (P = not significant). However, tubules in obstructed kidneys of adult mice receiving IN-1130 were characterized by taller Lotus-positive proximal tubular cells compared with placebo (Fig. 3, C and D, insets), and necrotic proximal tubules were more typically found after placebo treatment. Fractional proximal tubular mass of the contralateral kidney increased with age by 44% (Fig. 1C). Growth of proximal tubules in the obstructed neonatal kidney was profoundly suppressed by UUO, with an 80% reduction regardless of treatment with IN-1130 (Fig. 1C). In contrast, fractional proximal tubular mass was reduced by 70% in placebo-treated adult obstructed kidneys but was reduced by only 50% in IN-1130-treated adult mice (Fig. 1C).
Fig. 3.
Effects of TGF-β1 receptor inhibition (administration of IN-1130 vs. placebo) on cellular response of the adult obstructed kidney. A–D: Lotus lectin staining of proximal tubules, showing preservation of proximal tubular morphology in IN-1130 group (B and D) compared with placebo (A and C). Identification of Lotus-positive Bowman's capsules of glomeruli showing glomeruli with contiguous Lotus-positive epithelial cells in IN-1130 group (*). Insets: normal Lotus staining pattern concentrated in proximal tubular cell apices in the IN-1130 group (D) but a collapsed proximal tubule profile in the placebo group. E and F: TUNEL-staining nuclei in tubular cells showing reduced apoptosis in IN-1130 group. G and H: distribution of Sirius red staining showing decreased fractional collagen distribution in IN-1130-treated group. Scale bars (bar for IN-1130 groups in B, D, F, and H applies also to placebo groups in A, C, E, and G): A, B, G, and H = 200 μm; C, D, E, and F = 100 μm; C and D, inset = 10 μm.
Effects of TGF-β receptor inhibition on proximal tubular injury.
Compared with the contralateral kidney, UUO induced a marked increase in tubular apoptosis in the obstructed kidney regardless of age, with a greater increase in adults compared with neonates (Fig. 1D). While IN-1130 had no significant effect on tubular apoptotic cell fraction in neonates, there was a 37% decrease in tubular apoptosis in obstructed adult kidneys of mice receiving IN-1130 (Figs. 1D, and 3, E and F). The fraction of glomeruli with positive Lotus lectin capsular staining nearly doubled in contralateral kidneys of adult compared with neonatal mice; this is a reflection of normal maturation. Comparing the fraction of Lotus-negative glomeruli of obstructed kidneys to age-matched contralateral kidneys provides a measure of lost glomerulotubular integrity (glomeruli attached to atrophic proximal tubules or separated altogether from proximal tubules; Ref. 16). There was a marked reduction in the fraction of Lotus-positive glomeruli in obstructed compared with contralateral kidneys regardless of age. While there was no effect of IN-1130 on the fraction of Lotus-positive glomeruli in obstructed neonatal kidneys, there was a greater increase in the fraction of intact glomerulotubular junctions in the obstructed kidney of adult mice receiving IN-1130 (Fig. 1E).
The effects of IN-1130 on morphology of the glomerulotubular junction were examined by serial sectioning of 11 randomly selected glomeruli from a placebo-treated adult mouse, and compared with those from an IN-1130 treated mouse (Fig. 4). Glomeruli were categorized as atubular (no connection to the proximal tubule in any serial section; Fig. 4A), severely atrophic (glomerulus connected to a collapsed proximal tubule), mildly atrophic (glomerulus connected to a patent atrophic proximal tubule), or normal (Fig. 4B). Fractional distribution of severity of glomerulotubular junction injury was markedly affected by treatment with IN-1130: there were <10% normal glomeruli but 27% atubular glomeruli in placebo-treated mice, compared with 50% normal glomeruli and no atubular glomeruli in those receiving TGF-β1 receptor inhibitor (Fig. 4B). Notably, the fraction of Lotus-positive glomeruli in each mouse paralleled morphologic data from detailed serial sectioning (Fig. 4B).
Fig. 4.
A: demonstration of atubular glomeruli. Serial sections were performed in 11 glomeruli randomly selected from PAS-stained sections of a placebo-treated adult mouse and 20 glomeruli from a mouse treated with IN-1130. Shown are serial 4-μm sections of a representative atubular glomerulus (green, *) from a placebo-treated animal. Glomerulus is first identified in the second section of the leftmost column and can be traced from top to bottom in subsequent columns, disappearing in the section in the bottom right corner. There is no tubular connection of this glomerulus visible in any of the sections. B: classification of glomerulotubular injury. Following serial sectioning, the 31 glomeruli (11 placebo and 21 IN-1130) were identified as severely atrophic (collapsed glomerulotubular junction), mildly atrophic (atrophic tubular cells with patent glomerulotubular junction), or normal (tall tubular epithelial cells with preserved brush border and patent glomerulotubular junction). Representative sections of each of these types of glomerulotubular junctions are shown. C: fractional distribution of morphology of glomerulotubular junction in placebo and IN-1130-treated mice. In the placebo-treated animal, <10% of glomeruli were normal and nearly 30% were atubular. Following treatment with IN-1130, 50% of glomeruli were normal, and there were no atubular glomeruli. Comparison of serial-section data with Lotus lectin-positive fractions for the same animals demonstrates close correlation of Lotus-positive glomeruli with the fraction of normal glomerulotubular junctions as validated by serial sections.
Effects of TGF-β receptor inhibition on interstitial injury.
The fraction of renal parenchyma occupied by macrophages was very low in both kidneys of neonates and was unaffected by IN-1130 (Fig. 1F). By contrast, UUO induced significant interstitial macrophage infiltration in the adult obstructed kidney, which was reduced by 50% during treatment with IN-1130 (Fig. 1F). Fibronectin expression was greater in neonates than adults and was further stimulated by UUO regardless of age (Fig. 1G). Treatment with IN-1130 reduced fractional immunoreactive fibronectin only in adult mice. Fractional interstitial collagen was <1% in contralateral kidneys regardless of age (Fig. 1H). This fraction increased to ∼5% in the obstructed kidney of both neonatal and adult mice receiving placebo. IN-1130 significantly reduced fractional collagen to 3% in the adult (Fig. 3, G and H), whereas there was no significant effect of IN-1130 in the neonate (Fig. 1H).
DISCUSSION
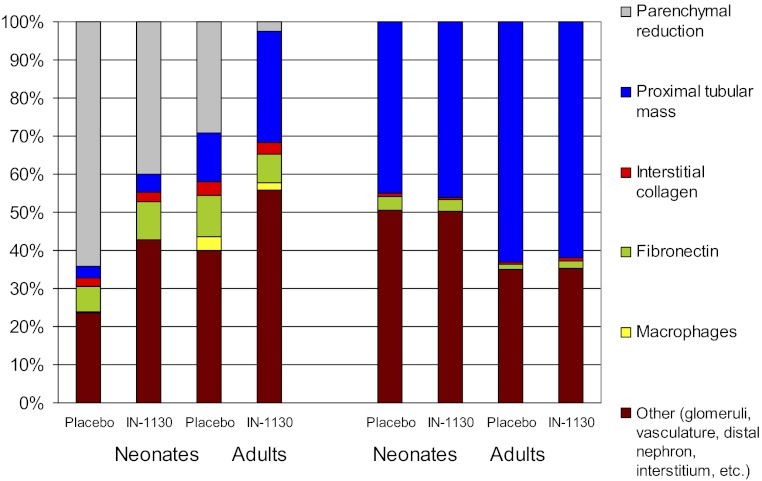
The majority of reports of murine UUO as a model of progressive renal disease have focused on renal interstitial responses and the deposition of intersitial collagen as end points to determine the efficacy of therapeutic interventions (9). In this study, a selective TGF-β1 receptor inhibitor, IN-1130, is shown to reduce cell death, thereby decreasing glomerular and proximal tubular atrophy and preventing the formation of atubular glomeruli in adult mice subjected to UUO. As confirmed by serial sections, and because the fraction of Lotus lectin-negative-staining glomeruli in the obstructed kidney is a surrogate for glomerulotubular injury (16), these results point to TGF-β as an important cytokine in this pathway to nephron loss. Compared with its effects on nephron integrity, the impact of IN-1130 on interstitial collagen accumulation in the obstructed kidney is modest. The morphometric determinations of proximal tubules and collagen (Fig. 1, C and H) describe fractional parenchymal contribution for each parameter by obstructed and contralateral kidneys. To relate the effects of age and UUO on the obstructed vs. the intact kidney, the fractional areas of Lotus-staining proximal tubules and Sirius-staining collagen were factored by the ratio of obstructed to contralateral kidney weight (Fig. 5). In the contralateral kidney, proximal tubular mass increases with age and is not affected by IN-1130. Proximal tubular mass is markedly reduced by UUO, but the impact is greater on the neonate than the adult. In placebo-treated adults, the diminished complement of proximal tubules is not only quantitative, but qualitative, since many tubules exhibit evidence of atrophy or cell death, thus effectively reducing the functional nephron population even further.
Fig. 5.
Effects of TGF-β1 receptor inhibition (administration of IN-1130 vs. placebo) and age (neonate vs. adult) on renal compartmental response to UUO in obstructed and contralateral kidneys. Parenchymal mass of the obstructed kidney is calculated as the ratio of obstructed to contralateral kidney weight, and the difference between kidneys is shown as “parenchymal reduction.” Functional mature proximal tubular mass is determined from the fractional Lotus lectin-staining contribution, which is reduced in the contralateral neonatal vs. adult kidney due to immaturity of the neonatal nephron. IN-1130 increased fractional proximal tubular mass by 16% in obstructed adult kidneys but by 2% in obstructed neonatal kidneys. There was <3% effect of IN-1130 on collagen in obstructed kidneys regardless of age. Macrophage contribution increased only in obstructed kidneys of adult mice and was decreased by IN-1130. In contralateral kidneys, there was no effect of IN-1130 on fractional contribution of proximal tubules or on any of the remaining parameters.
Activation of inflammatory signals and macrophage recruitment in the obstructed kidney are suppressed by conditional deletion of TGF-β1-activated kinase-1 in adult mice subjected to UUO (33). Inhibition of this pathway by IN-1130 in the present study would account for a reduction in macrophage accumulation in the obstructed kidney. A decrease in macrophage infiltration, in turn, would reduce proximal tubular cell death (29). The lack of effect of IN-1130 on neonatal UUO may be due in part to a maturational reduction in macrophage response.
In embryonic development, undifferentiated mesenchyme expresses fibronectin but no type IV collagen (15). However, with nephron induction and maturation, fibronectin largely disappears, and basement membrane-specific components such as type IV collagen predominate (15). In addition to its role in early nephron development, fibronectin is important in the renal response to injury and is necessary for fibroblasts to form collagen matrix (42). In response to UUO, renal fibronectin increases severalfold but decreases from 15 to 8% of the parenchymal area in the obstructed kidney in adult mice treated with IN-1130. The lack of interstitial fibronectin response to IN-1130 in obstructed neonatal kidneys suggests that signaling mechanisms for this glycoprotein change with maturation. Although collagen is increased by UUO in all mice, the decrement induced by IN-1130 in adults is <3% of the parenchymal area. In contrast, IN-1130 increased the proximal tubular contribution to the parenchymal area by 16% in adult kidneys (Fig. 5). These results reveal that endogenous TGF-β1 has a major role in mediating proximal tubular injury in addition to promoting interstitial fibrosis in the UUO model.
The increase in TGF-β1 expression by proximal tubular cells is significantly greater than that from other sources following UUO and leads to an increase in the expression of tubular TGF-β receptors I and II (18, 41). Following release of UUO, renal TGF-β distribution is decreased compared with the persistently obstructed kidney but remains greater than that of the normal kidney (10). Renal TGF-β1 released following UUO triggers tubular apoptosis, mediated by DAP-kinase through the activation of Smads 2, 3, and 4 (18, 23). Many factors in the obstructed kidney trigger tubular cell apoptosis, including tumor necrosis factor-α (TNF-α), Fas, p53, caspases, and ceramide. Because it promotes tubular cell death by increasing proapoptotic and decreasing survival signals, TGF-β1 plays a central role in apoptotic injury (35). In addition to myofibroblast transformation, TGF-β1 also is said to contribute to phenotypic transformation of tubular epithelial cells to fibroblasts (epithelial-mesenchymal transition), although the role of such a process is controversial (22, 26). This leads to the production of fibrogenic cytokines (including TGF-β1 and tumor necrosis factor-α) that contribute to interstitial fibrosis (10). The effects of IN-1130 on renal interstitial collagen deposition are mediated by the balance of collagen deposition and degradation: additional studies will be needed to determine which mechanism is dominant.
In contrast to these salutary effects of IN-1130 on the obstructed adult kidney, TGF-β1 receptor inhibition is injurious to the developing neonatal kidney. In the obstructed kidney of the neonatal rat subjected to UUO, renal TGF-β1 expression increases linearly through the first month of life, in contrast to a decrease in the normal kidney (12). Microarray analysis has revealed changes in renal gene expression: renal TGF-β increases following 12 days of complete UUO in the neonatal rat, as well as in rats with spontaneous congenital partial UUO studied at 32–35 days of age (39, 40). Apoptosis is present in human fetuses and infants with lower tract obstruction (37). In the multicystic kidney, an extreme form of obstructive nephropathy resulting from ureteral atresia, TGF-β is also increased as described following UUO in the neonatal rat (30, 40).
Knockout of TGF-β1 in the mouse results in 60% intrauterine mortality (28). The remaining offspring have normal kidneys at birth but develop a rapid wasting syndrome and die of severe multiorgan inflammation by 3–4 wk of age (27). In mice, receptor-activated Smads (1, 2, 3, 5, and 8), Smad4, and the inhibitory Smads (6 and 7) are expressed in the kidney from embryonic day 12 until the end of nephrogenesis at postnatal day 15 (43). Infusion of TGF-β1 neutralizing antibody in 3- to 5-day-old rats delays glomerular capillary formation and inhibits endothelial fenestration in developing glomeruli (31). These results indicate that members of the TGF-β superfamily and the Smad transcription factors play a role in normal nephrogenesis and maturation and that strategies to block its actions in the obstructed kidney must take this into account.
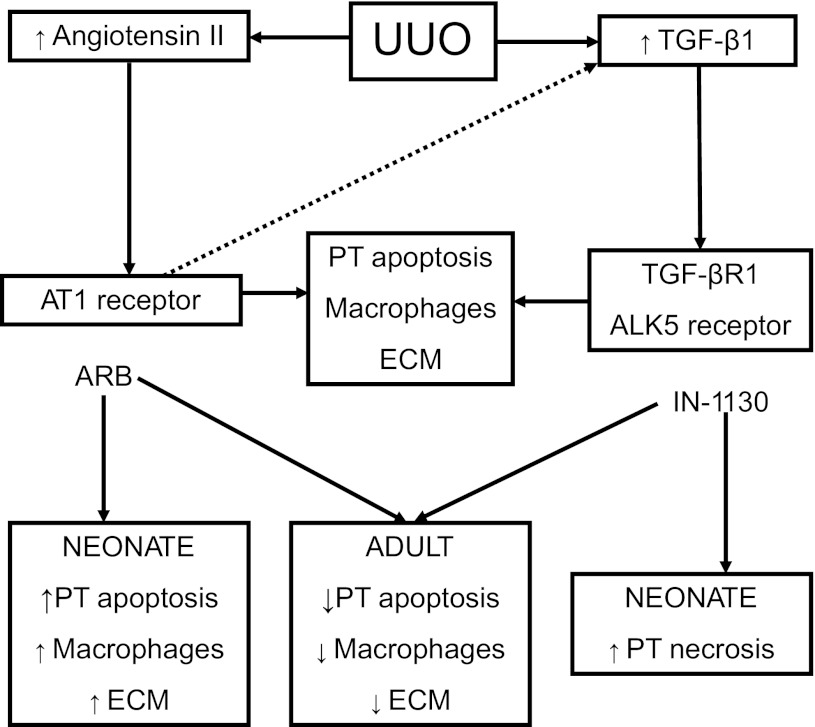
Since ALK5 is not involved in metanephric development, IN-1130 would be unlikely to have a significant impact on the normally maturing kidney (13). In the present study, the lack of effect of IN-1130 on the contralateral neonatal kidney is consistent with this prediction. In contrast, TGF-β1 receptor inhibition was associated with increased tubular cell death in the neonatal obstructed kidney, revealing a compounding effect of obstructive injury with immaturity. This response is similar to the injurious effects of angiotensin inhibitors in the neonate compared with the salutary effects on adult obstructed kidneys (4, 6, 14). As shown in Fig. 6, UUO stimulates renal production of angiotensin II, which, through binding to the AT1 receptor, induces proximal tubular apoptosis, intersitial macrophage infiltration, and extracellular matrix accumulation (38). Chronic UUO also stimulates TGF-β1 production (largely as a result of activation by angiotensin), which induces the same pattern of injury. Inhibition of either AT1 receptors with angiotensin receptor blockers or of TGF-βR1 receptors with IN-1130 reduces obstructive renal injury in the adult kidney. However, inhibition of angiotensin (14) or TGF-β1 in the neonate exacerbates obstructive injury. Lessons learned from renal injury produced by fetal exposure to maternal use of angiotensin inhibitors (7) should guide the clinical development of TGF-β inhibitors to slow the progression of renal disease.
Fig. 6.
Scheme demonstrating relationship between activation of angiotensin and TGF-β1 by UUO in the obstructed kidney of adult and neonatal mice. Many studies have demonstrated a central role for endogenous angiotensin II and transforming growth factor-β1 in the progression of renal injury resulting from UUO. Activation of the AT1 receptor, in turn, upregulates TGF-β1 activity (dashed line). Proximal tubule (PT) apoptosis, interstitial macrophage infiltration, and increase in extracellular matrix result from stimulation of AT1 and TGF-βR1 receptors. Interruption of signaling by angiotensin receptor blockers (ARB) or TGF-βR1 inhibitors (IN-1130) can reduce progressive injury in adult models of UUO. However, both angiotensin and TGF-β play a role in normal renal development and maturation, and their inhibition can lead to renal maldevelopment. Previous studies have shown that angiotensin inhibition can aggravate renal injury in neonatal models of UUO, and the present study demonstrates an injurious effect of TGF-β1 inhibition as well. ECM, extracellular matrix.
Of potential significance, TGF-β1 is an important cytokine responsible for wound healing and many facets of regeneration and remodeling following organ injury (25). There are a number of parallels between the cellular pathways involved in morphogenesis and those that are recalled to orchestrate tissue repair (34). Following ischemic renal injury, TGF-β plays a role in maintaining and remodeling the tubular basement membrane by enhancing synthesis of the extracellular matrix (2). TGF-β1 expression increases primarily in the regenerating tubules of the outer medulla, particularly along flattened cells lining dilated tubules (3). In this regard, TGF-β may be modulating the attachment of regenerating epithelial cells to the tubular basement membrane by regulating integrin subunits (20). It is likely that such salutary roles for TGF-β1 are more important in the neonatal than the adult obstructed kidney.
In conclusion, TGF-β1 mediates proximal tubular atrophy and cell death leading to the formation of atubular glomeruli resulting from UUO in the adult mouse. In addition to obstructive nephropathy, this pathway to nephron loss has been shown in patients with diabetes, IgA nephropathy, renal allograft rejection, and renal artery stenosis (8). Whereas therapies to slow progression of renal disorders have largely focused on intersitial fibrosis, proximal tubular injury should also be considered as a major target for early intervention. Inhibition of TGF-β type I receptor kinase may prove clinically effective in the preservation of functionally intact nephrons in adult chronic kidney diseases. Since in neonatal mice TGF-β1 receptor inhibition failed to attenuate obstructive injury and actually increased cell death in the developing obstructed kidney, caution is indicated for pediatric use.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-83372 and a grant from the University of Virginia Children's Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.I.G., B.A.T., M.S.F., D.-K.K., and R.L.C. conception and design of research; C.I.G., B.A.T., M.S.F., and L.N.S. performed experiments; C.I.G., B.A.T., L.N.S., and R.L.C. analyzed data; C.I.G., M.S.F., L.N.S., and R.L.C. interpreted results of experiments; C.I.G., M.S.F., and R.L.C. prepared figures; C.I.G., B.A.T., M.S.F., D.-K.K., and R.L.C. edited and revised manuscript; C.I.G., B.A.T., M.S.F., L.N.S., D.-K.K., and R.L.C. approved final version of manuscript; R.L.C. drafted manuscript.
ACKNOWLEDGMENTS
This work was presented in part (abstracts published) at the annual meeting of the American Society of Nephrology, Philadelphia, PA, November 2011, and the annual meeting of the Pediatric Academic Societies, Boston, MA, April, 2012.
REFERENCES
- 1. Bascands JL, Schanstra JP. Obstructive nephropathy: insights from genetically engineered animals. Kidney Int 68: 925–937, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basile DP, Martin DR, Hammerman MR. Extracellular matrix-related genes in kidney after ischemic injury: potential role for TGF-beta in repair. Am J Physiol Renal Physiol 275: F894–F903, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Basile DP, Rovak JM, Martin DR, Hammerman MR. Increased transforming growth factor-β1 expression in regenerating rat renal tubules following ischemic injury. Am J Physiol Renal Fluid Electrolyte Physiol 270: F500–F509, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Beharrie A, Franc-Guimond J, Rodriguez MM, Au J, Zilleruelo G, Abitbol CL. A functional immature model of chronic partial ureteral obstruction. Kidney Int 65: 1155–1161, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Cachat F, Lange-Sperandio B, Chang AY, Kiley SC, Thornhill BA, Forbes MS, Chevalier RL. Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int 63: 564–575, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen CO, Park MH, Forbes MS, Thornhill BA, Kiley SC, Yoo KH, Chevalier RL. Angiotensin converting enzyme inhibition aggravates renal interstitial injury resulting from partial unilateral ureteral obstruction in the neonatal rat. Am J Physiol Renal Physiol 292: F946–F955, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chevalier RL. Mechanisms of fetal and neonatal renal impairment by pharmacologic inhibition of angiotensin. Curr Med Chem 19: 4572–4580, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 19: 197–206, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Chevalier RL, Kim A, Thornhill BA, Wolstenholme JT. Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int 55: 793–807, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Chevalier RL, Thornhill BA, Forbes MS, Kiley SC. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol 25: 687–697, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Chung KH, Chevalier RL. Arrested development of the neonatal kidney following chronic ureteral obstruction. J Urol 155: 1139–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Clark AT, Young RJ, Bertram JF. In vitro studies on the roles of transforming growth factor-pi in rat metanephric development. Kidney Int 59: 1641–1653, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Coleman CM, Minor JJ, Burt LE, Thornhill BA, Forbes MS, Chevalier RL. Angiotensin AT1 receptor inhibition exacerbates renal injury resulting from partial unilateral ureteral obstruction in the neonatal rat. Am J Physiol Renal Physiol 293: F262–F268, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Ekblom P. Formation of basement membranes in the embryonic kidney: an immunohistological study. J Cell Biol 91: 1–10, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forbes MS, Thornhill BA, Chevalier RL. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: a new look at an old model. Am J Physiol Renal Physiol 301: F110–F117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forbes MS, Thornhill BA, Minor JJ, Gordon KA, Galarreta CI, Chevalier RL. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiol Renal Physiol 303: F120–F129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukuda K, Yoshitomi K, Yanagida T, Tokumoto M, Hirakata H. Quantification of TGF-β1 mRNA along rat nephron in obstructive nephropathy. Am J Physiol Renal Physiol 281: F513–F521, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heino J, Ignotz RA, Hemler ME, Crouse C, Massague J. Regulation of cell adhesion receptors by transforming growth factor-beta. J Biol Chem 264: 380–388, 1989 [PubMed] [Google Scholar]
- 21. Hironaka K, Sakaida I, Uchida K, Okita K. Correlation between stellate cell activation and serum fibrosis markers in choline-deficient l-amino acid-defined diet-induced rat liver fibrosis. Dig Dis Sci 45: 1935–1943, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jang CW, Chen CH, Chen CC, Chen J, Su YH, Chen RH. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol 4: 51–58, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Kim YW, Kim YK, Lee JY, Chang KT, Lee HJ, Kim DK, Sheen YY. Pharmacokinetics and tissue distribution of 3-{[5-(6-methylpyridin-2-yl)-4-(quinoaxalin-6-yl)-1H-imidazol-2-yl]methyl}benzamide; a novel ALK5 inhibitor and a potential anti-fibrosis drug. Xenobiotica 38: 325–339, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Kitamura M, Fine LG. Evidence for TGF-deta-mediated “defense” of the glomerulus: a blackguard molecule rehabilitated? Exp Nephrol 6: 1–6, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 121: 468–474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 90: 770–774, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kulkarni AB, Ward JM, Yaswen L, Mackall CL, Bauer SR, Huh CG, Gress RE, Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am J Pathol 146: 264–275, 1995 [PMC free article] [PubMed] [Google Scholar]
- 29. Lange-Sperandio B, Fulda S, Vandewalle A, Chevalier RL. Macrophages induce apoptosis in proximal tubule cells. Pediatr Nephrol 18: 335–341, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Liapis H, Doshi RH, Watson MA, Liapis A, Steinhardt GF. Reduced renin expression and altered gene transcript profiles in multicystic dysplastic kidneys. J Urol 168: 1816–1820, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Liu A, Dardik A, Ballermann BJ. Neutralizing TGF-β1 antibody infusion in neonatal rat delays in vivo glomerular capillary formation. Kidney Int 56: 1334–1348, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Luo J, Ho PP, Buckwalter MS, Hsu T, Lee LY, Zhang H, Kim DK, Kim SJ, Gambhir SS, Steinman L, Wyss-Coray T. Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J Clin Invest 117: 3306–3315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma FY, Tesch GH, Ozols E, Xie M, Schneider MD, Nikolic-Paterson DJ. TGF-β1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol 300: F1410–F1421, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development 131: 3021–3034, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Jr, Felsen D. Antibody to transforming growth factor-β ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Moon JA, Kim HT, Cho IS, Sheen YY, Kim DK. IN-1130, a novel TGF-beta type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int 70: 1234–1243, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Poucell-Hatton S, Huang M, Bannykh S, Benirschke K, Masliah E. Fetal obstructive uropathy: patterns of renal pathology. Pediatr Dev Pathol 3: 223–231, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 17: 2985–2991, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Seseke F, Thelen P, Ringert RH. Characterization of an animal model of spontaneous congenital unilateral obstructive uropathy by cDNA microarray analysis. Eur Urol 45: 374–381, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Silverstein DM, Travis BR, Thornhill BA, Schurr JS, Kolls JK, Leung JC, Chevalier RL. Altered expression of immune modulator and structural genes in neonatal unilateral ureteral obstruction. Kidney Int 64: 25–35, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Sutaria PM, Ohebshalom M, McCaffrey TA, Vaughan ED, Felsen D. Transforming growth factor-beta receptor types I and II are expressed in renal tubules and are increased after chronic unilateral ureteral obstruction. Life Sci 62: 1965–1972, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha11beta1 and alpha2beta1. J Biol Chem 277: 37377–37381, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Vrljicak P, Myburgh D, Ryan AK, van Rooijen MA, Mummery CL, Gupta IR. Smad expression during kidney development. Am J Physiol Renal Physiol 286: F625–F633, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Yoo KH, Thornhill BA, Chevalier RL. Angiotensin stimulates TGF-β1 and clusterin in the hydronephrotic neonatal rat kidney. Am J Physiol Regul Integr Comp Physiol 278: R640–R645, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci USA 98: 6686–6691, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]