Abstract
Mitochondrial ATP production is vital for meeting cellular energy demand at rest and during periods of high ATP turnover. We hypothesized that high-intensity interval training (HIT) would increase ATP flux in resting muscle (VPi→ATP) in response to a single bout of exercise, whereas changes in the capacity for oxidative ATP production (Vmax) would require repeated bouts. Eight untrained men (27 ± 4 yr; peak oxygen uptake = 36 ± 4 ml·kg−1·min−1) performed six sessions of HIT (4–6 × 30-s bouts of all-out cycling with 4-min recovery). After standardized meals and a 10-h fast, VPi→ATP and Vmax of the vastus lateralis muscle were measured using phosphorus magnetic resonance spectroscopy at 4 Tesla. Measurements were obtained at baseline, 15 h after the first training session, and 15 h after completion of the sixth session. VPi→ATP was determined from the unidirectional flux between Pi and ATP, using the saturation transfer technique. The rate of phosphocreatine recovery (kPCr) following a maximal contraction was used to calculate Vmax. While kPCr and Vmax were unchanged after a single session of HIT, completion of six training sessions resulted in a ∼14% increase in muscle oxidative capacity (P ≤ 0.004). In contrast, neither a single nor six training sessions altered VPi→ATP (P = 0.74). This novel analysis of resting and maximal high-energy phosphate kinetics in vivo in response to HIT provides evidence that distinct aspects of human skeletal muscle metabolism respond differently to this type of training.
Keywords: mitochondria, oxidative metabolism, phosphorus magnetic resonance spectroscopy, cycling exercise
a fundamental adaptation to endurance exercise training is an increased capacity to supply ATP via oxidative phosphorylation in skeletal muscle. This adaptation occurs in response to increased mitochondrial content and is associated with improved exercise capacity (31, 32). In contrast, little is known about the effect of exercise training on oxidative ATP production in resting muscle, which accounts for a significant portion of daily energy expenditure (55), and therefore may be important for overall metabolic health. Thus, while there is some evidence to suggest an association between mitochondrial content and ATP turnover in resting muscle (33, 57), it is not clear whether the exercise-induced adaptations responsible for increased oxidative capacity may also result in increased ATP production at rest. Furthermore, there is limited information on the scope and timing of exercise-induced adaptations in mitochondrial ATP production in vivo, which could have important implications for developing training interventions designed to enhance oxidative metabolism in resting and exercising skeletal muscle.
The effects of high-volume, endurance exercise training on oxidative metabolism in skeletal muscle were first demonstrated more than 40 years ago (31). More recently, studies from several laboratories have shown that low-volume, high-intensity, interval-style training (HIT) also is an effective approach for promoting mitochondrial biogenesis (9–11, 19, 21, 45, 62). In fact, studies have reported increased expression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), which is an important factor for coordinating the activation of genes encoding mitochondrial proteins, after a single session of exercise training (45, 46, 68). Similarly, some reports have shown that markers of in vitro oxidative capacity can be increased after a single session of high-intensity exercise (18, 63). Together, this group of studies has reformed the conventional notion that long-term, high-volume training interventions are required to stimulate mitochondrial biogenesis. However, despite numerous investigations of the molecular events accompanying HIT, the time course for functional changes in muscle oxidative capacity in vivo is still unclear.
Mitochondrial ATP production is vital for meeting cellular energy demand not only during periods of high ATP turnover but also in resting muscle (56). Because resting energy expenditure accounts for ∼60–75% of total daily energy expenditure, ATP flux in resting skeletal muscle may play an important role in body weight maintenance, as well as regulation of glucose and lipid metabolism (43, 50, 51, 69). Whereas numerous energy-consuming processes are active in resting tissue, protein synthesis is believed to account for a large portion of ATP turnover in resting skeletal muscle (55, 56). Various modes of exercise training have been shown to elevate rates of muscle protein synthesis and breakdown (4, 5, 52, 67), suggesting that exercise may result in elevated ATP demand in resting tissue, which presumably would be reflected by increased oxidative ATP flux.
The rate of ATP synthesis in resting tissue has been estimated using the saturation transfer experiment, which quantifies the rate of Pi incorporation into γ-ATP, i.e., VPi→ATP (2, 37, 50, 64). Notably, in part due to glycolytic contributions, VPi→ATP overestimates oxidative ATP synthesis in resting muscle compared with other measures of aerobic energy metabolism (20, 38), which may limit the ability of the saturation transfer experiment to detect changes in oxidative ATP production in resting muscle. Despite this potential limitation, several studies have shown elevated VPi→ATP in response to various physiological stimuli, e.g., hyperglycemia (44) and hyperinsulinemia (6, 51), suggesting that the saturation transfer technique is sensitive to changes in overall ATP turnover.
To date, only a few studies have investigated the effects of exercise training on ATP production in resting human muscle (37, 64). Kacerowsky-Bielesz et al. (37) showed that VPi→ATP in the gastrocnemius-soleus complex increased after only three sessions of moderate-intensity cycling, whereas Trenell et al. (64) showed no effects on VPi→ATP after 2 or 8 wk of daily walking. In a cross-sectional study, Befroy et al. (3) reported similar VPi→ATP in the calf muscles of untrained and endurance-trained young men, suggesting that oxidative ATP flux in resting muscle is not different in chronically trained muscle. One explanation for these contrasting results is that resting ATP turnover may increase transiently at the onset of training and then return to a steady-state rate in chronically trained muscle. Currently there are conflicting results in the literature regarding a potential relationship between resting and maximal rates of ATP synthesis in skeletal muscle (57, 64, 65). To date, no studies have examined the timing and magnitude of exercise-induced changes in both resting and maximal rates of oxidative ATP production.
The purpose of this study was to examine the acute and short-term effects of HIT on human skeletal muscle energetics in vivo using phosphorus magnetic resonance spectroscopy (31P-MRS). The vastus lateralis (VL) muscle of young, untrained men was studied before, during, and after a training period of high-intensity cycling exercise. We hypothesized that 1) a single session of HIT would increase VPi→ATP, with no change in oxidative capacity (Vmax), and 2) six bouts of HIT would increase Vmax with no further change in VPi→ATP. The results provide new and unique information regarding the time course and magnitude of changes in distinct aspects of oxidative metabolism in human skeletal muscle after HIT.
METHODS
Participants.
Eight healthy young males (27.0 ± 3.4 yr, 176.1 ± 6.9 cm; 83.0 ± 15.4 kg; means ± SE) volunteered for the study. A preliminary screening session was used to ensure that all participants: 1) had no history of neurological, pulmonary, cardiovascular, or metabolic disease; 2) were nonsmokers and able to undergo magnetic resonance procedures (no metal implants); 3) did not participate in any type of regular exercise; 4) did not take any medication or dietary supplements shown to affect muscle function or metabolism; and 5) had no first-degree relatives with Type 2 diabetes to eliminate potential differences in the metabolic response to exercise training. All experimental procedures and potential risks associated with the study were explained to the volunteers, who provided written informed consent, as approved by the institutional review boards at the University of Massachusetts, Amherst and Yale University School of Medicine.
Preparatory Procedures
Participants came into the laboratory for a preparatory session 7 to 14 days before the start of the training intervention. Measures of height, body mass, and blood pressure were obtained. Participants practiced maximal voluntary isometric contractions (MVICs) of the knee extensor muscles to familiarize them with the contraction protocol that would be used for muscle metabolic testing at Yale University. As described previously (41), participants were positioned supine on a patient bed, with the knee fixed at 35° from straight over a custom-built apparatus with a built-in strain gauge. The foot was held down with a cushioned strap placed over the ankle joint, which allowed for an isometric contraction of the knee extensor muscles and minimal movement of the limb. This setup matched the arrangement that was used in the 4-Tesla MR system at Yale University. Participants practiced MVICs (3–5 s duration, 2 min recovery between each) to ensure that these contractions could be performed consistently.
Next, participants performed an incremental exercise test to voluntary exhaustion on a mechanically braked cycle ergometer (828E, Monark, Vansbro, Sweden) to determine peak, whole body, oxygen consumption (V̇o2 peak). Respiratory gases from the expired air were measured using a metabolic cart (TrueOne 2400, ParvoMedics, Sandy, UT), and the highest value for oxygen consumption achieved over a 15-s collection period was used as V̇o2 peak. Subsequently, participants performed a 30-s sprint on the cycle ergometer (i.e., Wingate Test) to become familiarized with the training protocol. Three days after completing the final session of the training protocol, the participant returned to the laboratory to repeat the incremental V̇o2 peak test.
To quantify habitual physical activity level at baseline, participants were instructed in the use of a uniaxial accelerometer (GT1M, Actigraph, Pensacola, FL) (41). The accelerometer was worn at the waist for 7 consecutive days during all waking hours, while usual physical activity routines were maintained. Average daily counts were recorded to characterize the study group and confirm their relatively sedentary usual behavior.
Muscle Metabolic Testing
Participants were transported to the Magnetic Resonance Research Center at Yale University to determine the effects of HIT on skeletal muscle metabolism using 31P-MRS. Before and during these visits, participants were provided standardized meals, as described below. The muscle metabolic testing sessions were conducted three times: 1) 9 h before the first training session (baseline), 2) 15 h after the first training session (15 h post), and 3) 15 h after completing the sixth training session (2 wk post). Each testing session included measurements of 1) intracellular pH and concentrations of relevant energy metabolites, 2) unidirectional flux from Pi to ATP, 3) apparent longitudinal relaxation time of Pi (T1′), and 4) PCr recovery following a 24 s MVIC. Measurements of pH and metabolite concentrations in resting muscle and Pi→ATP flux were acquired after a 10-h overnight fast and lasted approximately 2 h. Participants were then given a light snack (10% of estimated daily energy expenditure) and allowed a short break outside the scanner before performing the muscle contraction and PCr recovery protocol.
All 31P-MRS measurements were performed on a 4-Tesla whole body system (Bruker Biospin, Ettlingen, Germany) and acquired with an MR probe (31P surface coil: 6 × 8 cm, 1H surface coil: 9 cm diameter) placed over the VL muscle of the dominant leg. To ensure consistent positioning of the MR probe across all testing sessions, the probe location was marked with a pen on the participant's thigh. The participant was positioned on the patient bed and centered in the magnet. A series of axial plane scout images were first acquired to ensure optimal positioning of the VL in the isocenter of the magnet. In addition, the image slice corresponding to the center of the MR probe was used for measuring VL cross-sectional area (cm2) using Image J software (http://rsb.nih.gov/ij/). Magnetic field homogeneity was optimized by localized shimming on the proton signal from tissue water using the 1H coil (41).
Intracellular pH and metabolites in resting muscle.
To ensure accurate estimation of intracellular 31P metabolites and pH in resting muscle, each testing session started with collection of a fully relaxed spectrum (repetition time = 35 s, 16 averages) acquired with an adiabatic excitation pulse and 90° flip angle. From the fully relaxed spectrum, concentrations of intracellular metabolites were determined based on the assumptions that total creatine ([PCr] + [creatine]) = 42.5 mM, and free creatine ≅ Pi in skeletal muscle (28, 48). To verify potential changes in metabolite concentrations, we also determined 31P metabolite ratios that provided insight about exercise-induced changes in the intracellular milieu with potential influence on enzymatic reactions in resting skeletal muscle. Intracellular pH was calculated based on the chemical shift between the Pi and PCr resonances (49). Because the concentration of ADP is too low to be detected in the spectrum, free intracellular [ADP] was calculated, according to the creatine kinase equilibrium:
with the assumption of equal concentrations of free creatine and Pi in skeletal muscle (48). The equilibrium constant of the creatine kinase reaction (kCK) was assumed to be 1.66·109 M−1 (66).
Resting oxidative ATP production.
The Pi→ATP rate in resting skeletal muscle (VPi→ATP) was determined using the 31P saturation transfer experiment (2, 7). Steady-state magnetization of Pi (M′) was measured in the presence of selective saturation of the γ-ATP resonance. A control spectrum was also acquired to yield equilibrium magnetization of Pi (M0) in the absence of γ-ATP saturation. To compensate for off-resonance effects of the saturation pulse, the control spectrum was acquired with the saturation pulse centered on a downfield frequency equidistant from Pi (Fig. 1). Additional parameters were 1-ms adiabatic half-passage excitation pulse (centered between Pi and γ-ATP), 15-s continuous low-power saturation pulse, sweep width of 10,000 Hz, 2,048 complex data points, 15-s repetition time, and 16 averages. Two sets of these paired 31P spectra were acquired and line-fit, and the results were then averaged.
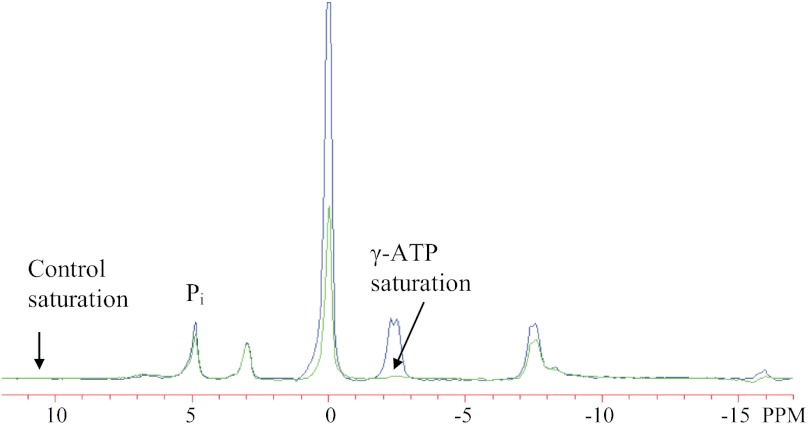
Fig. 1.
Phosphorus spectra acquired from vastus lateralis muscle during the saturation-transfer experiment. Selective γ-ATP saturation (arrow, right) is shown in green, with the control spectrum in blue. The control spectrum had selective saturation (arrow, left) at a downfield frequency equivalent to the frequency separation between γ-ATP and Pi. All other data acquisition parameters were the same in both conditions. The ratio of Pi magnetization with and without saturation (M′/M0) reflects the exchange of saturated spins from Pi to ATP and is used to calculate the unidirectional Pi→ATP flux.
The ratio of steady state relative to equilibrium magnetization of Pi under conditions of γ-ATP saturation (M′/M0) is given by the equation:
where k′ is a pseudo first-order rate constant describing the loss of Pi magnetization due to exchange of saturated spins between Pi and ATP, and T1′ is the apparent longitudinal relaxation time of Pi in the presence of continuous γ-ATP saturation. Calculation of T1′ was accomplished using a seven-point inversion recovery experiment (Sigmaplot, Systat Software) with multiple delays (0.05, 1.2, 3.1, 6, 10, 15, 20 s; 16 averages) between the adiabatic 180° inversion pulse and the 90° detection pulse (2). After determining T1′ and M′/M0, the exchange rate constant of Pi→ATP was calculated based on the equation:
and the unidirectional rate of oxidative ATP synthesis was calculated as the product of k′ and [Pi] (2, 7):
Maximal oxidative ATP production.
For the muscle contraction protocol, participants were positioned on the patient bed with the knee fixed at 35° of flexion over a custom-built apparatus, as described for the preparatory procedures and done previously (41). To standardize conditions, participants performed two brief “warm-up” MVICs (3–5 s duration, 2 min rest between each) before performing two 24-s MVICs contractions, separated by 10 min of recovery. The contraction duration of 24 s was selected as it has been shown to deplete PCr to ∼50–70% of resting levels in VL, without inducing significant acidosis (41, 42). The average of the two trials was calculated for each variable of interest. Participants were provided verbal encouragement and visual force feedback (from a series of light-emitting diodes) during all contractions. Free induction decays with 2,048 complex data points were acquired continuously for 11 min, with a nominal 60° hard pulse, 2 s repetition time, and spectral width of 8,000 Hz.
Spectral Analysis
All MRS data were processed using NUTS software (Acorn NMR, Livermore, CA). Free induction decays were zero filled to 16 k points and multiplied by an exponential factor corresponding to 10 Hz line-broadening before Fourier transformation. The 31P spectra were phased and baseline was corrected before peaks corresponding to PCr, Pi, and γ-ATP were fit to Lorentzian line shapes. All MRS analyses were performed by the same investigator who was blinded to the identity of the data. The data were averaged to yield 31P spectra with temporal resolution of 1 min at rest, 4 s during the MVIC, 8 s during first 5 min of recovery, and 30 s during last 5 min of recovery (Fig. 2).
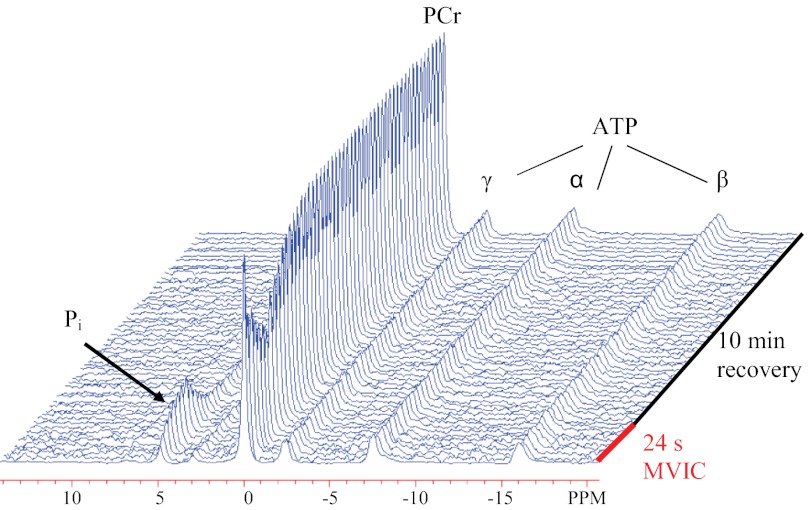
Fig. 2.
Stackplot of phosphorus spectra acquired from the vastus lateralis muscle of a representative subject during the muscle contraction protocol. The 24-s maximal voluntary isometric contraction depleted phosphocreatine (PCr) to ∼60% of resting concentration. The rate constant for PCr recovery (kPCr) was determined based on a monoexponential fit of the data during 10 min of recovery.
The recovery data, from the end of the 24-s MVIC through 10 min of recovery, were fitted with a three-parameter monoexponential equation to determine the rate constant kPCr:
where PCrend is the integral of PCr at the end of the MVIC, and ΔPCr is the difference in PCr between rest and end of the MVIC (41, 48). According to the linear model of muscle respiration (48), the maximal rate of oxidative ATP production can be estimated as the product of kPCr and resting concentration of PCr, using the following equation:
Dietary and Physical Activity Controls
Participants were instructed to maintain their usual diet and continue their regular physical activity routines during the study. In addition, dietary intake and physical activities were standardized before each muscle metabolic testing session to minimize potential confounding effects on our measures of muscle energetics. Specifically, the muscle metabolic testing sessions were performed at the same time of day across trials, and participants were asked to avoid strenuous activities (expect for the training session) for 48 h before each test session. Dietary intake before each study was controlled by providing participants with a standardized meal (30% of estimated daily energy expenditure) and a snack (10% of estimated daily energy expenditure) 11 and 10 h before the testing sessions, respectively. The snack was provided to minimize hunger during the MRS measures the next morning. Macronutrient composition (∼60% carbohydrate, ∼25% fat, ∼15% protein) was similar for all participants and consistent across trials. Daily energy expenditure was estimated based on the Harris-Benedict Equation (27) and multiplied by an activity factor of 1.5.
Training Protocol
The training protocol consisted of six sessions of cycling over a 2-wk period, with each training session performed on the same ergometer that was used for the V̇o2 peak test. Participants performed three training sessions per week, with a minimum of 36 h between sessions (week 1: Saturday, Tuesday, Friday; week 2: Monday, Wednesday, Friday). Each training session started with a 5 min warm-up at 60 W, including two brief sprints (4–5 s duration). This was followed by 4–6 bouts of 30 s “all-out” effort. The wheel resistance [7.5% of body mass, (11)] was adjusted manually by one of the investigators, and the participant was instructed to reach maximal effort within the time frame (∼2–3 s) required to arrive at the appropriate resistance. Each 30-s bout was followed by 4 min of active recovery, during which the participant pedaled against a light resistance to minimize venous pooling and any feeling of light-headedness (10). Consistent with previous studies using this HIT protocol, sessions 1 and 2 consisted of four bouts, the next two sessions comprised five bouts, and participants performed six bouts for sessions 5 and 6.
Because a primary objective of the study was to examine the effects of a single session of HIT on skeletal muscle energetics, the first training session was performed at the Magnetic Resonance Research Center at Yale University, where the metabolic testing was conducted. The subsequent five sessions were performed at the University of Massachusetts, Amherst. All training sessions were supervised by the same two investigators, who provided verbal encouragement, and continuously monitored heart rate and general well being of the participants.
Statistical Analyses
Comparison of V̇o2 peak, before and after training, was accomplished by Student's paired t-test. The effects of training on muscle metabolite levels and rates of skeletal muscle ATP synthesis were evaluated across the three testing sessions using mixed-model, repeated measures ANOVAs with unstructured covariance structure. Post hoc analyses were done using pairwise comparisons (Student's paired t-test), and exact P values were reported. Differences were considered significant when P < 0.05. All data are presented as means ± SE.
RESULTS
Participant Characteristics
Descriptive characteristics of the participants are presented in Table 1. The accelerometer data provide a measure of habitual physical activity, and the average daily counts reported are consistent with other studies of relatively sedentary young adults (40, 41). In agreement with these data, average V̇o2 peak was 35.8 ± 4.0 ml·min−1·kg−1 at baseline and improved to 39.3 ± 4.5 ml·min−1·kg−1 (P = 0.03) after completion of the 2-wk training period.
Table 1.
Group characteristics at baseline
| Age, yr | 27.0 ± 3.6 |
| Height, m | 1.76 ± 0.07 |
| Body mass, kg | 83.0 ± 15.4 |
| Systolic blood pressure, mmHg | 126.8 ± 4.9 |
| Diastolic blood pressure, mmHg | 77.5 ± 3.2 |
| Physical activity, daily counts/1,000 | 294.3 ± 37.1 |
Data are from 8 males and are expressed as means ± SE.
The total workload performed during the six training sessions was 431.4 ± 26.9 kJ, which is consistent with training data (∼225 kJ/wk) in a previous HIT study (10). In addition, mean (156.3 ± 1.0 beats/min, ∼86.2% of maximal) and peak (180.5 ± 1.3 beats/min, ∼99.5% of maximal) heart rate achieved during all thirty 30-s training bouts suggested maximal effort of the participants. Baseline cross-sectional area of the VL muscle (23.24 ± 2.47 cm2) was comparable to that reported by Hulmi et al. (34) in young men and did not change after the first (23.06 ± 2.13 cm2) nor the sixth training sessions (24.36 ± 2.21 cm2; P = 0.79).
Metabolite Levels in Resting Muscle
The effects of training on metabolite levels in resting muscle are summarized in Table 2. Intracellular [Pi] increased after the first training session (P = 0.005) and remained elevated at the end of the 2-wk protocol (P < 0.001), with no difference between the second and sixth training sessions (P = 0.28). Concomitantly, [PCr] decreased from baseline after the first and remained lower after the final training sessions. Intracellular pH in resting muscle increased after the first training session (P = 0.04) and was further elevated after 2 wk of training (P = 0.002). Cytosolic [ATP] was reduced after the first training session (P < 0.001), with a further decline after the sixth session (P < 0.001, Fig. 5). Relative to baseline, [ADP]free was lower after 2 wk of training (P = 0.02).
Table 2.
Metabolite levels and pH in resting muscle
| Baseline (1) | 15 h post (2) | 2 wk post (3) | P Value | |
|---|---|---|---|---|
| PCr, mM*† | 37.38 ± 0.22 | 36.77 ± 0.21 | 36.58 ± 0.21 | 0.001 |
| Pi, mM*† | 5.12 ± 0.22 | 5.73 ± 0.21 | 5.92 ± 0.21 | 0.001 |
| ATP, mM*†‡ | 8.87 ± 0.33 | 6.98 ± 0.29 | 6.06 ± 0.26 | <0.001 |
| ADP, μM† | 8.72 ± 0.69 | 7.88 ± 0.67 | 7.26 ± 0.60 | 0.07 |
| Pi/PCr*† | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | <0.001 |
| PCr/ATP*†‡ | 4.26 ± 0.17 | 5.34 ± 0.23 | 6.11 ± 0.26 | <0.001 |
| ADP/ATP, 103*† | 0.97 ± 0.05 | 1.12 ± 0.05 | 1.21 ± 0.05 | <0.001 |
| pHrest*†‡ | 7.07 ± 0.00 | 7.08 ± 0.01 | 7.09 ± 0.01 | 0.004 |
Data are from the vastus lateralis muscle of 8 males and are expressed as means ± SE. P values for main effects from repeated measures ANOVAs are shown, and statistical differences (P < 0.05) across time points from post hoc analyses are indicated by
(1 vs. 2),
(1 vs. 3),
(2 vs. 3).
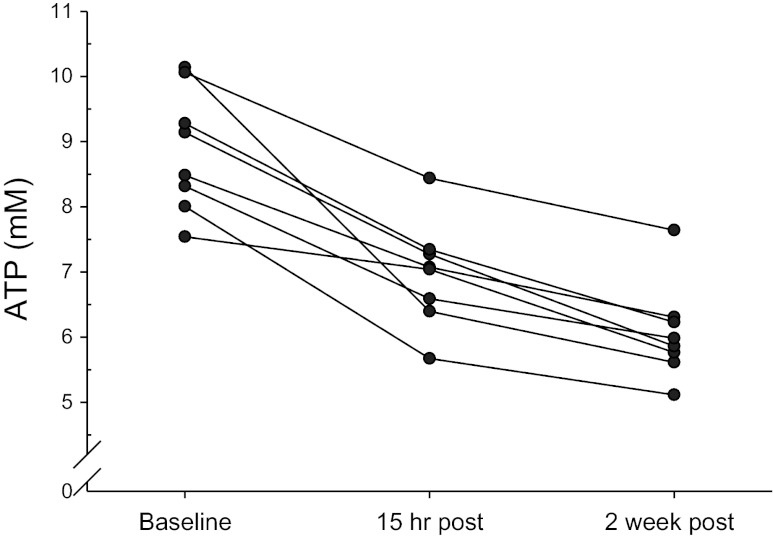
Fig. 5.
Individual values of [ATP] from the vastus lateralis muscle at baseline, 15 h after the first training session, and 15 h after the sixth training session. Cytosolic [ATP] was reduced from baseline after the first training session and further reduced after the sixth training session (see Table 2).
These changes in metabolite concentrations were reflected in the ratios of specific metabolite pairs. Specifically, the Pi/PCr ratio increased after the first training session (P = 0.005) and remained elevated after 2 wk of training (P < 0.001), with no difference between the second and sixth training session (P = 0.28). The ratio of PCr/ATP increased after the first training session (P = 0.002), with a further increase after the sixth session (P < 0.001). Finally, the ADP/ATP ratio increased after the first training session (P = 0.006) and remained elevated at the end of the 2-wk protocol (P < 0.001), with no difference between the second and sixth sessions (P = 0.09).
Pi→ATP Rate in Resting Muscle
The individual components contributing to the estimation of the unidirectional rate of ATP synthesis (i.e., VPi→ATP) in resting muscle are summarized in Table 3. Training had no effect on the pseudo-first-order rate constant (k′) for exchange from Pi→ATP (P = 0.22), VPi→ATP (P = 0.74, Fig. 3), or M′/M0 (P = 0.53). The apparent longitudinal relaxation time of Pi during γ-ATP saturation (T1′) increased after the first training session (P = 0.001) and remained elevated after 2 wk of training (P = 0.03). There was good agreement between the two measurements of k′ performed for each individual at each time point (coefficient of variation = 7.9%). Data in Table 3 and Fig. 3 are the average of these two measurements. Similarly, unpublished data from our laboratory on day-to-day variability of all components contributing to estimation of VPi→ATP show very good reproducibility, i.e., [Pi] = 4% (coefficient of variation), M′/M0 = 2%, T1′ = 6%, and VPi→ATP = 7%.
Table 3.
Variables from saturation transfer experiment
| Baseline (1) | 15 h post (2) | 2 wk post (3) | P Value | |
|---|---|---|---|---|
| M′/M0, % | 0.84 ± 0.01 | 0.85 ± 0.01 | 0.85 ± 0.01 | 0.53 |
| T1′, s*† | 4.23 ± 0.09 | 4.63 ± 0.09 | 4.57 ± 0.15 | 0.003 |
| k′, s−1 | 0.039 ± 0.003 | 0.032 ± 0.002 | 0.033 ± 0.001 | 0.22 |
| Pi, mM*† | 5.12 ± 0.22 | 5.73 ± 0.21 | 5.92 ± 0.21 | 0.001 |
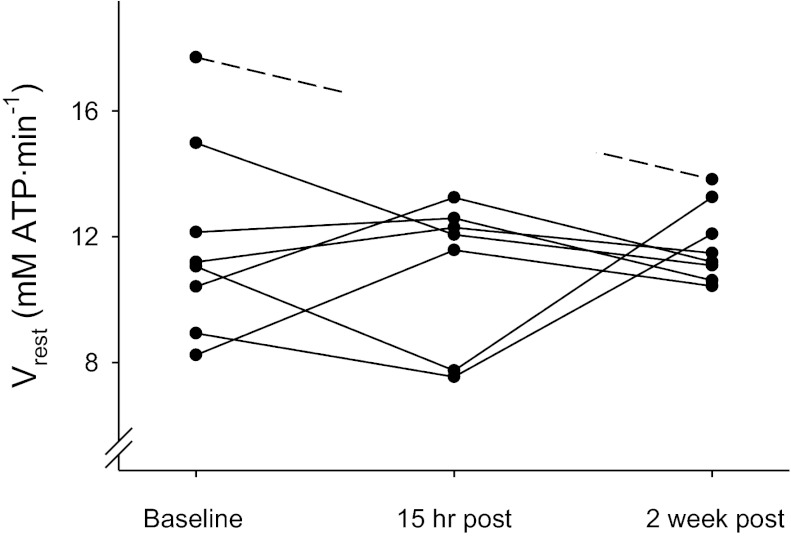
| Vrest, mM/min | 11.87 ± 1.14 | 11.01 ± 0.89 | 11.78 ± 0.43 | 0.74 |
Data are from the vastus lateralis muscle of 8 males (n = 7 for 15 h post, because of technical issues) and are expressed as means ± SE. M′/M0, ratio of Pi magnetization with and without selective saturation of γ-ATP; T1′, apparent longitudinal relaxation time of Pi with saturation of γ-ATP; k′, Pi→ATP exchange pseudo first-order rate constant; Vrest, resting Pi→ATP flux. P values are for main effect from repeated measures ANOVAs; post hoc statistical differences (P < 0.05) between 1 and 2 (
), and 1 and 3 (
) are indicated.
Fig. 3.
Individual values (average of 2 measurements) from the vastus lateralis muscle for unidirectional Pi→ATP flux (VPi→ATP) at baseline, 15 h after the first training session, and 15 h after the sixth training session. The 15-h data point for one participant (dashed line) is missing due to technical issues. Baseline VPi→ATP was not different from VPi→ATP at 15 h, or after 2 wk training (see Table 3), indicating that short-term, high-intensity interval training did not increase Pi→ATP flux in resting muscle.
Muscle Contraction Protocol and Oxidative Capacity
The muscle metabolic changes observed during the contraction protocol are presented in Table 4. As designed, the 24-s MVIC depleted PCr to ∼50–70% of resting concentration, and the level of depletion was not different across the three testing sessions (P = 0.47). Intracellular pH at the end of the 24-s MVIC was higher after the sixth training session compared with baseline (P < 0.001) and the first session (P = 0.009). During the initial part of recovery, pH continued to decline and the minimum pH reached during recovery was higher after 2 wk of training compared with baseline (P < 0.001).
Table 4.
Variables from the PCr recovery experiment
| Baseline (1) | 15 h post (2) | 2 wk post (3) | P Value | |
|---|---|---|---|---|
| PCrend, % of rest | 59.95 ± 3.02 | 59.75 ± 3.95 | 55.73 ± 3.15 | 0.47 |
| pHend*† | 7.07 ± 0.01 | 7.09 ± 0.02 | 7.12 ± 0.02 | <0.001 |
| pHmin* | 6.94 ± 0.02 | 6.96 ± 0.03 | 6.98 ± 0.02 | 0.005 |
| kPCr, s−1*† | 0.0288 ± 0.001 | 0.0278 ± 0.002 | 0.0326 ± 0.001 | 0.009 |
| Vmax, mM/min*† | 64.70 ± 2.97 | 61.31 ± 3.58 | 71.46 ± 3.12 | 0.02 |
Data are from the vastus lateralis muscle of 8 males and are expressed as means ± SE. PCrend, concentration of phosphocreatine at the end of the 24-s maximal voluntary isometric contraction; pHrest, intracellular pH at rest; pHend, pH at the end of the 24-s MVIC; pHmin, minimum pH attained during recovery; kPCr, rate of PCr recovery; Vmax, maximal capacity for oxidative phosphorylation. P values for main effects from repeated measures ANOVAs are shown and statistical differences (P < 0.05) between: 1 and 3 (
) and 2 and 3 (
) are indicated.
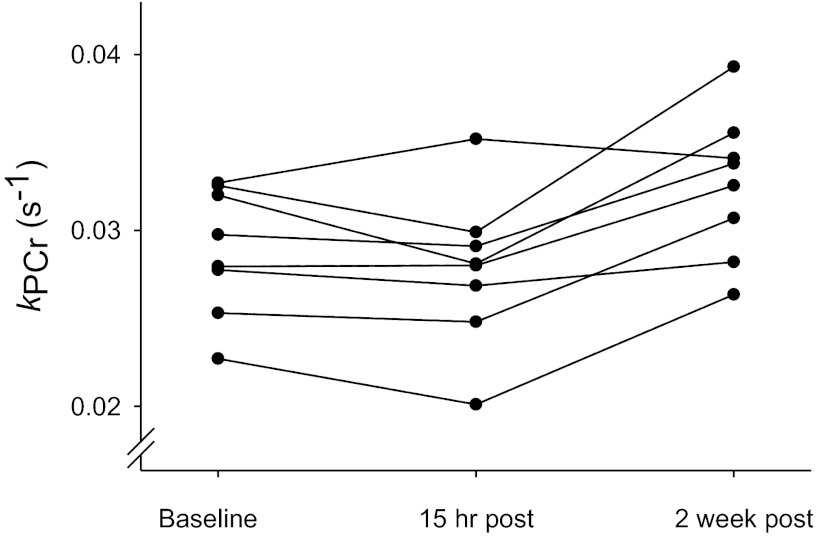
Figure 4 shows individual rate constants for PCr recovery (kPCr) acquired at baseline and after the first and sixth training sessions. The first training session had no effect on the rate of PCr recovery (P = 0.17), but kPCr increased by 14% after 2 wk of training (P = 0.001, Fig. 4 and Table 4). There was good agreement between the two measurements of kPCr performed for each individual at each time point (coefficient of variation = 9.0%). Consistent with the kPCr data, Vmax was increased after completion of the final training session (P = 0.004, Table 4). There were no correlations between kPCr or Vmax and V̇o2 peak at baseline (r2 ≤ 0.01, P ≥ 0.97), or between changes in kPCr or Vmax and changes in V̇o2 peak (r2 ≤ 0.11, P ≥ 0.49). While these observations suggest that V̇o2 peak was limited by central cardiovascular factors rather than muscle oxidative capacity, we cannot disregard the possibility that these results were influenced by the small ranges for these variables in the study group.
Fig. 4.
Individual values (average of 2 measurements) from the vastus lateralis muscle for the rate constant of phosphocreatine recovery (kPCr) at baseline, 15 h after the first training session, and 15 h after the sixth training session. Baseline kPCr was unchanged after the first training session but increased after the sixth training session (see Table 4), indicating that muscle oxidative capacity increased with short-term, high-intensity interval training.
DISCUSSION
We report here for the first time the scope and timing of the effects of HIT on bioenergetics of human skeletal muscle in vivo. As hypothesized, the rate of PCr recovery was unchanged after a single bout of interval training, whereas completion of six training sessions resulted in a 14% increase in muscle oxidative capacity. In contrast, neither a single nor six training sessions altered the unidirectional rate of ATP synthesis, rejecting our hypothesis about increased Pi→ATP rate in resting skeletal muscle following HIT. These results indicate that distinct aspects of muscle oxidative metabolism in humans respond differently to this type of training, such that in vivo oxidative capacity increases in response to short-term HIT, while resting VPi→ATP is unchanged.
Acute Effects of Exercise on Muscle Oxidative Capacity
The recent advances in our understanding of molecular mechanisms involved in exercise-induced cellular adaptations have provided some evidence indicating the potential for rapid activation of mitochondrial biogenesis after exercise training (1, 16, 32, 46, 68). While a recent study in humans reported increased expression of PGC-1α, mitochondrial protein content, and enzyme activities after four 30-s bouts of all-out cycling (45), the time course for the transfer of these molecular events into actual changes in the capacity for mitochondrial ATP production in vivo has not been determined. Tonkonogi and colleagues (63) showed that maximal ADP-stimulated respiration in skinned fibers from the VL was increased by ∼23% after a single session of high-intensity cycling, suggesting increased capacity for oxidative phosphorylation. To our knowledge, the present study provides the first investigation of the acute effects of a single bout of HIT on oxidative capacity in vivo. We showed no change in the rate of PCr recovery after the first training session (Fig. 4), indicating that the extent of remodeling accompanying a single session of HIT is not adequate to enhance the capacity for oxidative ATP production in vivo. Post hoc power calculations revealed that we were sufficiently powered to detect an ∼8% increase in kPCr. Thus we believe that the design and methodology used in the present study allowed us to detect physiologically meaningful changes in the rate of PCr recovery.
Effects of HIT on Muscle Oxidative Capacity
Exercise training protocols that consist of low-volume, high-intensity intervals have demonstrated potent effects on metabolic adaptations in skeletal muscle that resembles the adaptations associated with conventional, high-volume endurance training protocols (9–11, 21). In particular, protocols consisting of six sessions of 4–6 bouts of 30 s of maximal effort cycling have been shown to increase mitochondrial enzyme activity and protein content by 11–38% (9–11, 21), which is comparable with results from protocols consisting of 6–10 sessions of 2 h duration at 60–70% V̇o2 peak (13, 60). In agreement with these studies suggesting increased capacity for oxidative phosphorylation, we report a 14% increase in the rate of PCr recovery in VL after 6 sessions of HIT (Fig. 4), which suggests that the cumulative effects of repeated exercise sessions are required to promote an increased capacity for muscle oxidative ATP production in vivo.
Several investigators have used the PCr recovery method to examine the effect of endurance training on oxidative capacity in vivo (19, 36, 47). Rates of PCr recovery have been shown to increase by ∼30% in response to training protocols involving 12–24 wk of aerobic exercise (36, 47). Our result is consistent with a recent study reporting a similar increase in the rate of PCr recovery (14%) in the quadriceps muscles of young adults following the same 2-wk HIT protocol (19). A notable difference between our study and that of Forbes et al. (19) was the experimental approach used to deplete intracellular PCr levels for the oxidative capacity measure. We used a brief, maximal contraction to recruit all muscle fibers, without inducing acidosis. Consequently, our results of enhanced PCr recovery kinetics reflect global improvements in muscle oxidative capacity. In contrast, Forbes et al. (19) used moderate-intensity contractions of the quadriceps muscles, which presumably recruit a smaller portion of the muscle fibers (i.e., fibers innervated by low-threshold motoneurons), and therefore, provides a measure of oxidative capacity that reflects a selective population of muscle fibers. Despite the likely recruitment of different proportions of muscle tissue, the increase in the rate of PCr recovery following training was similar between studies, supporting the concept that HIT promotes mitochondrial adaptations in all muscle fibers (22).
Effect of a Single Bout of HIT on VPi→ATP
It is well established that protein turnover in skeletal muscle can remain elevated for up to 48 and even 96 h following a single bout of training (52, 53). Because these anabolic and catabolic processes are coupled to ATP hydrolysis, we hypothesized that VPi→ATP would be elevated 15 h after the first training session due to increased overall muscle ATP turnover. In contrast to our hypothesis, VPi→ATP remained unchanged, suggesting that ATP demand was not elevated after this type of exercise training, which is consistent with the fact that [ADP], a primary regulator of oxidative phosphorylation, was unchanged after the first session. Notably, Gibala et al. (23) showed that a single session of HIT was accompanied by activation of AMP-activated protein kinase (AMPK), while phosphorylation of protein kinase B/Akt tended to decrease in response to exercise. These results led the authors to suggest that HIT provides a concentrated stimulus for metabolic adaptations (i.e., mitochondrial protein synthesis) with no effect on muscle growth (i.e., myofibrillar protein synthesis) (23). Considering that mitochondrial protein represents a small fraction (∼10%) of the total muscle protein pool (15) and the notion that activation of AMPK conserves ATP by downregulating anabolic pathways (25), it is possible that low-volume HIT does not provide sufficient stimulus for increasing net muscle protein turnover or other processes coupled to ATP hydrolysis. To our knowledge, no studies have examined the effects of low-volume HIT on rates of muscle protein synthesis and breakdown. The 15-h time point was chosen to standardize conditions (i.e., meals and a 10-h overnight fast) before muscle metabolic testing and to minimize transitory effects (e.g., due to elevated temperature and altered hormone levels) of the preceding training session on muscle metabolism. Thus it is possible that a shorter time delay between exercise and muscle metabolic testing would have elicited a different response in VPi→ATP.
Effects of HIT on Vrest
As a surrogate for exercise training, chronic low-frequency stimulation of isolated cat gracilis muscle has been shown to increase resting and maximal oxygen consumption (33). Low-frequency stimulation also increased mitochondrial density, indicating that greater mitochondrial content may increase both resting and maximal oxygen consumption in skeletal muscle. Kacarowsky-Bielesz et al. (37) showed that three sessions of 30 min of moderate-intensity cycling (70% of V̇o2 max) resulted in increased VPi→ATP (∼18%) of the soleus-gastrocnemius complex of healthy men and women. Whereas this result suggests that a relatively small amount of exercise training elicits a higher rate of ATP synthesis in resting skeletal muscle, the study did not reveal whether increased VPi→ATP in resting muscle was accompanied by greater mitochondrial content or increased oxidative capacity. Our results suggest that low-volume HIT does not elevate VPi→ATP in resting VL muscle (Fig. 3), which is consistent with the results from Richards et al. (54) who reported unaltered whole body energy expenditure after an identical training protocol. In agreement with these results, Gibala and colleagues (23) provided evidence to suggest that myofibrillar protein synthesis was not stimulated after HIT, which is consistent with our result of unchanged VL CSA after completion of the 2-wk protocol. Despite an increase in oxidative capacity after the sixth training session, k′ and VPi→ATP remained unchanged. These results suggest that the muscular adaptations that occur in response to short-term HIT have differential effects on in vivo measures of resting and maximal rates of oxidative ATP synthesis in human skeletal muscle. Our results are informed by recent studies that have examined the effects of pronounced mitochondrial modifications on VPi→ATP in vivo. Increased expression of PGC-1α in mouse muscle has been shown to elicit a 2.4-fold increase in mitochondrial density but only a 50–60% increase in VPi→ATP (14). By inhibiting mitochondrial complex I in rat muscle, van den Broek et al. (65) used a different approach to investigate the link between mitochondrial function and muscle energetics in vivo. While complex I inhibition reduced the rate of PCr recovery by 46%, both k′ and VPi→ATP were unaffected by this type of intervention. Collectively, these results suggest that mitochondrial modifications, in the form of exercise training, transgenic manipulations, or dysfunction do not affect k′ and only have a small, if any, influence on VPi→ATP.
Effect of HIT on Muscle Energy Metabolites
Sustained or repeated bouts of maximal muscle activity result in metabolic perturbations that may alter the intracellular concentrations of energy metabolites in resting muscle (26, 29, 30, 61). Based on biopsy data, Gibala et al. (23) reported a ∼16% reduction in muscle [ATP] 3 h after a single session of HIT. Our study extends these findings by showing a similar reduction (∼21%) in intracellular [ATP] in vivo 15 h after the first HIT session (Table 2). Consistent with results from a biopsy study using an identical 2-wk HIT protocol (9), we observed lower intracellular [ATP] in resting muscle after the sixth training session (Table 2). Others have reported that muscle [ATP] remains depressed even after 7–8 wk of HIT (10, 26, 30, 61). High rates of ATP turnover, as occurred during each training session, result in elevated [AMP], increased flux through AMP deaminase, and thus accumulation of inosine monophosphate (IMP) (29, 30). Subsequent breakdown of IMP to inosine and hypoxanthine can cause a loss of adenine nucleotides from the muscle (29, 30). While restoration of purine nucleotide levels in the muscle is a slow process and can last several days (10, 29), lower [ATP] in response to HIT does not seem to be associated with muscle damage or decreased exercise performance (30, 61). This interpretation is supported by our observation of no change in muscle CSA in response to training indicating no evidence of muscle swelling in VL as would be expected in response to damage. Thus our results are consistent with previous in vitro studies and provide, for the first time, evidence of a loss of cytosolic ATP in vivo following a single and six sessions of HIT.
We calculated metabolite concentrations based on the assumption of stable concentration of total creatine (i.e., [PCr] + [Pi] = 42.5 mM). An alternate means of quantifying relative metabolite concentrations based on the assumption that [ATP] = 8.2 mM was not used because similar training protocols have resulted in a long-lasting decline in intracellular [ATP], with no effects on concentrations of total creatine (30, 61). Furthermore, there is no evidence to suggest that HIT would affect the stoichiometry between free creatine and inorganic phosphate. Therefore, the assumption of stable [total creatine] appears to be reasonable for quantification of metabolite levels in this type of training study.
Control of oxidative phosphorylation in skeletal muscle, particularly in vivo, appears to occur as a result of a combination of complex interactions (35, 58). It is generally understood that products of ATP hydrolysis (ADP, Pi) play important roles in regulating the rate of mitochondrial ATP synthesis. Consistent with the notion that [ADP] is a primary regulator of oxidative phosphorylation in vivo (7, 12, 17), our finding of a trend toward lower [ADP] in resting muscle (calculated based on the creatine kinase equilibrium) suggests that the stimulus for oxidative ATP synthesis is reduced after 2 wk of training, which could reflect lower ATP demand. It is possible, however, that the lower [ADP] following training reflected an increased sensitivity to ADP as a result of increased mitochondrial content (17), such that less ADP was required to achieve the same rate of muscle mitochondrial ATP production after training (17). Consistent with this interpretation, [ADP] in resting muscle and oxidative capacity were both unaltered 15 h after the first training session. However, this finding is in contrast to a previous study suggesting that increased sensitivity to ADP precedes changes in mitochondrial capacity (24). Notably, the progressive increase in [Pi] (Table 2) is consistent with in vitro (35) and modeling studies (35, 58) that have suggested an important role for this metabolite in establishing resting metabolic status. Overall, these data illustrate the intricacy of regulatory control of oxidative phosphorylation in vivo.
Saturation-Transfer Technique
Several studies have used the saturation transfer technique to assess mitochondrial ATP production under various conditions, which has led to the concept of VPi→ATP being an important variable in the context of mitochondrial function and health (37, 50, 51, 64). However, as recently reviewed by several research groups (20, 38), there are some limitations associated with using the saturation transfer technique to infer information about the rate of oxidative ATP production in resting skeletal muscle (38, 59, 65). First, the Pi→ATP rate can be influenced by ATP synthesis from nonoxidative sources. Whereas net ATP production from glycolysis is negligible in resting skeletal muscle, the glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase catalyze a coupled, near-equilibrium reaction that may contribute to the Pi→ATP exchange (8, 20, 59).
Second, particularly at low rates of respiration when ATP synthase operates near equilibrium (i.e., low ADP/ATP), mitochondrial Pi↔ATP exchange may contribute to the Pi→ATP rate, such that the unidirectional rate of Pi→ATP exchange exceeds net ATP synthesis (20, 39, 59). These additional sources of Pi→ATP exchange likely contribute to the apparent overestimation of oxidative ATP synthesis measured by the saturation transfer technique in comparisons with other measures (e.g., oxygen consumption) of oxidative energy metabolism in resting skeletal muscle (20, 38).
Unfortunately, we cannot decipher the contribution from glycolytic sources to our estimate of VPi→ATP. However, because energy intake and macronutrient composition were matched before each saturation transfer experiment, and the participants were fasted for 10 h before these experiments, we assume unaltered activation of glycolysis in resting muscle and therefore do not expect that contributions from glycolytic enzymes varied across time points. In contrast, we observed increased ADP/ATP (Table 2), which moves the ATP synthase reaction away from equilibrium, and presumably would result in reduced mitochondrial Pi↔ATP exchange and a lower k′ after training (20, 38). Thus, in addition to the aforementioned limitations of the saturation transfer experiment, it is possible that exercise-induced alterations in the intracellular metabolic environment constrain our ability to detect net changes in mitochondrial ATP turnover using the saturation transfer technique. While we have demonstrated very good reproducibility of the saturation transfer measure, additional studies are needed to clarify the determinants of the Pi→ATP rate and examine the influence of altered intracellular metabolic state on VPi→ATP. In the meantime, our results show that the Pi→ATP rate, which is often used as a measure of in vivo mitochondrial activity in resting skeletal muscle, is unchanged following HIT.
Perspectives and Significance
This study reveals that HIT promotes short-term (following 6 sessions) effects on the functional oxidative capacity of muscle in vivo but causes no acute (single session) effect. At the same time, the Pi→ATP rate in resting muscle was unchanged after single or multiple training sessions. These novel results indicate that repeated HIT sessions are required to promote the relatively rapid adaptations that are responsible for improved muscle oxidative capacity in vivo and suggest that the mechanisms that regulate adaptations to maximal muscle oxidative capacity are distinct from those regulating ATP synthesis in resting tissue. This study expands our understanding of muscle remodeling after HIT by providing evidence of the functional and temporal changes in human skeletal muscle energetics in vivo, which have implications for developing training interventions designed to improve skeletal muscle function and health.
GRANTS
This work was supported by NIH/NIA K02 AG023582, the Keck Foundation, and a UMass Graduate School Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.G.L., D.E.B., and J.A.K.-B. conception and design of research; R.G.L. performed experiments; R.G.L. analyzed data; R.G.L., D.E.B., and J.A.K.-B. interpreted results of experiments; R.G.L. prepared figures; R.G.L. drafted manuscript; R.G.L., D.E.B., and J.A.K.-B. edited and revised manuscript; R.G.L., D.E.B., and J.A.K.-B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the participants for their enthusiasm and perseverance throughout the study. We also thank Dr. John Buonaccorsi for statistical advice, Logan Maynard for help with data collection, and all other members of the Muscle Physiology Laboratory for help with various aspects of the project.
REFERENCES
- 1. Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Befroy DE, Falk PK, Rothman DL, Shulman GI. Assessment of in vivo mitochondrial metabolism by magnetic resonance spectroscopy. Methods Enzymol 457: 373–393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA 105: 16701–16706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273: E122–E129, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 55: 136–140, 2006 [PubMed] [Google Scholar]
- 7. Brindle KM, Blackledge MJ, Challiss RA, Radda GK. 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry 28:4887–4893, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Brindle KM, Radda GK. 31P-NMR saturation transfer measurements of exchange between Pi and ATP in the reactions catalysed by glyceraldehyde- 3-phosphate dehydrogenase and phosphoglycerate kinase in vitro. Biochim Biophys Acta 928: 45–55, 1987 [DOI] [PubMed] [Google Scholar]
- 9. Burgomaster KA, Heigenhauser GJ, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol 100: 2041–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol 98: 1985–1990, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Chance B, Leigh JS, Jr, Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci USA 82: 8384–8388, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chesley A, Heigenhauser GJ, Spriet LL. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am J Physiol Endocrinol Metab 270: E328–E335, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 105: 19926–19931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, Phillips SM, Hawley JA. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol 111: 1473–1483, 2011 [DOI] [PubMed] [Google Scholar]
- 16. De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab 294: E607–E614, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Dudley GA, Tullson PC, Terjung RL. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem 262: 9109–9114, 1987 [PubMed] [Google Scholar]
- 18. Fernstrom M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol 554: 755–763, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forbes SC, Slade JM, Meyer RA. Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Appl Physiol Nutr Metab 33: 1124–1131, 2008 [DOI] [PubMed] [Google Scholar]
- 20. From AH, Ugurbil K. Standard magnetic resonance-based measurements of the Pi–>ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles. Am J Physiol Cell Physiol 301: C1–C11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575: 901–911, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev 36: 58–63, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol 106: 929–934, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S, Farrance B. Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J Appl Physiol 72: 484–491, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Eager DM. Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol 89: 1793–1803, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Harris J, Benedict F. A Biometric Study of Basal Metabolism in Man. Washington D.C.: Carnegie Institute of Washington, 1919 [Google Scholar]
- 28. Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974 [PubMed] [Google Scholar]
- 29. Hellsten Y, Sjodin B, Richter EA, Bangsbo J. Urate uptake and lowered ATP levels in human muscle after high-intensity intermittent exercise. Am J Physiol Endocrinol Metab 274: E600–E606, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Hellsten-Westing Y, Norman B, Balsom PD, Sjodin B. Decreased resting levels of adenine nucleotides in human skeletal muscle after high-intensity training. J Appl Physiol 74: 2523–2528, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967 [PubMed] [Google Scholar]
- 32. Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol 59, Suppl 7: 5–18, 2008 [PubMed] [Google Scholar]
- 33. Hoppeler H, Hudlicka O, Uhlmann E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol 385: 661–675, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 37: 297–308, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Jeneson JA, ter Veld F, Schmitz JP, Meyer RA, Hilbers PA, Nicolay K. Similar mitochondrial activation kinetics in wild-type and creatine kinase-deficient fast-twitch muscle indicate significant Pi control of respiration. Am J Physiol Regul Integr Comp Physiol 300: R1316–R1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol 90: 1663–1670, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Kacerovsky-Bielesz G, Chmelik M, Ling C, Pokan R, Szendroedi J, Farukuoye M, Kacerovsky M, Schmid AI, Gruber S, Wolzt M, Moser E, Pacini G, Smekal G, Groop L, Roden M. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes 58: 1333–1341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kemp GJ. The interpretation of abnormal 31P magnetic resonance saturation transfer measurements of Pi/ATP exchange in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 294: E640–E642, 2008 [DOI] [PubMed] [Google Scholar]
- 39. LaNoue KF, Jeffries FM, Radda GK. Kinetic control of mitochondrial ATP synthesis. Biochemistry 25: 7667–7675, 1986 [DOI] [PubMed] [Google Scholar]
- 40. Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol Lond 583: 1093–1105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. In vivo oxidative capacity varies with muscle and training status in young adults. J Appl Physiol 107: 873–879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab 37: 88–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laurent D, Yerby B, Deacon R, Gao J. Diet-induced modulation of mitochondrial activity in rat muscle. Am J Physiol Endocrinol Metab 293: E1169–E1177, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Lim EL, Hollingsworth KG, Smith FE, Thelwall PE, Taylor R. Effects of raising muscle glycogen synthesis rate on skeletal muscle ATP turnover rate in type 2 diabetes. Am J Physiol Endocrinol Metab 301: E1155–E1162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R1303–R1310, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Mathai AS, Bonen A, Benton CR, Robinson DL, Graham TE. Rapid exercise-induced changes in PGC-1α mRNA and protein in human skeletal muscle. J Appl Physiol 105: 1098–1105, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de WT, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59: 572–579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer RA. A linear-model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254: C548–C553, 1988 [DOI] [PubMed] [Google Scholar]
- 49. Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem 248: 7276–7278, 1973 [PubMed] [Google Scholar]
- 50. Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2: e233, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Pikosky MA, Gaine PC, Martin WF, Grabarz KC, Ferrando AA, Wolfe RR, Rodriguez NR. Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J Nutr 136: 379–383, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Richards JC, Johnson TK, Kuzma JN, Lonac MC, Schweder MM, Voyles WF, Bell C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J Physiol 588: 2961–2972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rolfe DF, Brand MD. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol Cell Physiol 271: C1380–C1389, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77: 731–758, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Schmid AI, Schrauwen-Hinderling VB, Andreas M, Wolzt M, Moser E, Roden M. Comparison of measuring energy metabolism by different (31) P-magnetic resonance spectroscopy techniques in resting, ischemic, and exercising muscle. Magn Reson Med 67: 898–905, 2012 [DOI] [PubMed] [Google Scholar]
- 58. Schmitz JP, Jeneson JA, van Oorschot JW, Prompers JJ, Nicolay K, Hilbers PA, van Riel NA. Prediction of muscle energy states at low metabolic rates requires feedback control of mitochondrial respiratory chain activity by inorganic phosphate. PLos One 7: e34118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sheldon JG, Williams SP, Fulton AM, Brindle KM. 31P NMR magnetization transfer study of the control of ATP turnover in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 93: 6399–6404, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol 80: 2250–2254, 1996 [DOI] [PubMed] [Google Scholar]
- 61. Stathis CG, Febbraio MA, Carey MF, Snow RJ. Influence of sprint training on human skeletal muscle purine nucleotide metabolism. J Appl Physiol 76: 1802–1809, 1994 [DOI] [PubMed] [Google Scholar]
- 62. Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol 102: 1439–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Tonkonogi M, Walsh B, Tiivel T, Saks V, Sahlin K. Mitochondrial function in human skeletal muscle is not impaired by high intensity exercise. Pflügers Arch 437: 562–568, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Trenell MI, Hollingsworth KG, Lim EL, Taylor R. Increased daily walking improves lipid oxidation without changes in mitochondrial function in type 2 diabetes. Diabetes Care 31: 1644–1649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van den Broek NM, Ciapaite J, Nicolay K, Prompers JJ. Comparison of in vivo postexercise phosphocreatine recovery and resting ATP synthesis flux for the assessment of skeletal muscle mitochondrial function. Am J Physiol Cell Physiol 299: C1136–C1143, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Veech RL, Lawson JW, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. J Biol Chem 254: 6538–6547, 1979 [PubMed] [Google Scholar]
- 67. Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 282: 194–199, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86: 1423–1427, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]